Abstract
The brain, endocrine, and immune systems are inextricably linked. Immune molecules have a powerful impact on neuroendocrine function, including hormone-behavior interactions, during health as well as sickness. Similarly, alterations in hormones, such as during stress, can powerfully impact immune function or reactivity. These functional shifts are evolved, adaptive responses that organize changes in behavior and mobilize immune resources, but can also lead to pathology or exacerbate disease if prolonged or exaggerated. The developing brain in particular is exquisitely sensitive to both endogenous and exogenous signals, and increasing evidence suggests the immune system has a critical role in brain development and associated behavioral outcomes for the life of the individual. Indeed, there are associations between many neuropsychiatric disorders and immune dysfunction, with a distinct etiology in neurodevelopment. The goal of this review is to describe the important role of the immune system during brain development, and to discuss some of the many ways in which immune activation during early brain development can affect the later-life outcomes of neural function, immune function, mood and cognition.
Keywords: microglia, cytokines, chemokines, cognition, hippocampus, Toll-like Receptors, infection, sensitive periods
INTRODUCTION
Interactions among the brain, endocrine, and immune systems are now well accepted. Though immune processes within the brain are not identical to those occurring in the periphery, the brain has resident immune cells, namely microglia, which produce cytokines and other inflammatory molecules in response to disturbances in homeostasis in a manner similar to peripheral immune cells. Other central nervous system (CNS) cells, including perivascular macrophages, astrocytes, endothelial cells, oligodendrocytes, and neurons also produce cytokines and chemokines and express their receptors, during normal brain function as well as in response to injury, infection, or illness. In addition to resident immunocompetent cells, there are multiple pathways by which peripherally-derived immune factors can affect the brain, and in turn by which the brain can impact peripheral immune responses. These include the autonomic nervous system (ANS), activation of the “stress axis” (the hypothalamic-pituitary-adrenal (HPA) axis), and cytokines, chemokines, and leukocytes that travel or signal across the (BBB). The neural and hormonal pathways by which the neuroendocrine and immune systems interact and communicate during infection or illness have been extensively reviewed [27, 61, 75, 83, 141, 199, 231, 238, 289]. The primary goal of this review is to discuss the role of immune molecules, primarily cytokines and chemokines, in normal brain development, and to highlight the mechanisms by which early-life events may alter this normal developmental trajectory via their specific impact on cytokine expression, and thereby impact later-life neuroendocrine-behavioral interactions.
NEUROENDOCRINE-IMMUNE COMMUNICATION
Beyond its traditional role in host defense, the immune system is now considered a diffuse sensory organ, which works in concert with the neuroendocrine system to achieve and maintain homeostasis throughout the entire body [138, 296]. Immunocompetent cells are located throughout virtually every organ of the body, including the brain and other endocrine tissues, and sophisticated interactions occur among these cells, via hormones, neurotransmitters, and soluble protein messengers called cytokines. Cytokines have autocrine and paracrine actions within local tissues, as well as hormonal actions via their release into the blood stream and subsequent signaling to the CNS [55, 81]. Many cytokine families have been identified, including the tumor necrosis factors (TNF), interferons (IFN), interleukins (IL-1 through 26), transforming growth factor (TGF), colony-stimulating factors (CSF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), macrophage inflammatory proteins (MIP), macrophage migration inhibitory factors (MIF), and insulin-like growth factors (IGF). An equally lengthy list of chemokines has been described, a subset of cytokines that mediate cell adhesion, chemotaxis, and leukocyte trafficking. Chemokines are separated into four categories including the C-C chemokines, the C-X-C chemokines, the C chemokines, and one CX3C chemokine [57]. Chemokines tend to be classified by their structural characteristics more than their function. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and hormones such as leptin, prolactin, and growth hormone have also been characterized for their cytokine-like functions [123, 162, 234]. Cytokines are generally described for their pro-inflammatory versus anti-inflammatory roles during the course of an immune response; however, many cytokine actions are pleiotropic and depend highly on the cytokine environment around them. In addition, there are a number of endogenous cytokine receptor antagonists (e.g., IL-1ra) that are released in conjunction with cytokines, which modulate their production and function.
Cytokines and the Brain
Cytokines have neuromodulatory properties within the brain during infectious and inflammatory processes; however, they are also constitutively expressed in healthy brain tissue and regulate such homeostatic mechanisms and behaviors as sleep, memory, and metabolism [89, 154, 296, 316]. One of the best-described observations demonstrating the profound effects cytokines can have on brain function is in the expression of sickness behavior [72–74]. Sick animals exhibit several well-characterized behavioral changes, including reductions in food and water intake, activity, exploration, increased sleep, and reduced social and sexual interactions [124]. These so-called sickness behaviors are not mediated by the infectious pathogens themselves, but rather they are a critical component of the immune response orchestrated by the immune system via the release of cytokines [72–74]. Cytokines induce physiological (e.g., fever) and behavioral changes via their actions within the brain [72]. As a result, preventing the synthesis of cytokines, or the binding of cytokines to their receptors within the brain prevents the expression of sickness behavior even in the presence of a peripheral immune challenge [72, 212]. Conversely, administering individual cytokines directly into the brain, in the absence of a peripheral infection, will induce sickness behavior [74]; e.g., direct injection of IL-1β into the ventricles of the brain induces the full range of sickness behaviors [6]. Numerous studies have now demonstrated that these behaviors, rather than being pathological consequences of infection, are organized, adaptive strategies that are critical to host survival [46, 74]. As such, sickness behavior reflects an overall change in the motivational/behavioral state of the individual that is organized by the nervous, endocrine, and the immune systems.
Cytokine receptors have been characterized in microglia, astrocytes, neurons, endothelial cells, and oligodendrocytes, ubiquitously throughout the CNS of mammals, although relative densities for individual receptors vary by brain region (see [81, 243, 267, 291, 294] for review). Cytokines are produced within the brain by numerous cell types in response to virtually any perturbation of CNS homeostasis, including trauma, stroke, ischemia, neurodegeneration, or infection [15, 133, 244]. Cytokines are also produced within the brain in response to peripheral cytokine production or infectious stimuli, indicating that cytokine signals are transmitted from the periphery into the brain. This transmission may occur via several routes, including neurotransmission following cytokine binding to their receptors on vagal afferents [5, 72, 112, 145]; signaling across the BBB -- e.g., via endothelial cells, astrocytes, and microglia within the BBB that recapitulate the immune signal from the periphery by secreting their own cohort of cytokines into the brain [264, 293, 305]; crossing into the brain at circumventricular organs where the BBB is permeable or leaky (e.g., area postrema [311]); or finally, active transport across the BBB by specialized transporters [16, 18]. These routes of transmission have been extensively reviewed [16, 18, 74, 167, 183, 230, 237, 310]. We touch on aspects of brain development that significantly impact these transmission pathways, and the implications for long-term function, in subsequent sections of this review.
EARLY-LIFE PROGRAMMING OF BRAIN AND BEHAVIOR BY IMMUNE DISRUPTION
In recent years, scientists and medical professionals have identified similarities between sickness behaviors caused by an acute illness and the behaviors expressed by individuals with certain neuropsychiatric disorders (Figure 1) [74, 76]. In particular, the behavioral and physiological symptoms of depression are strikingly similar to the list of sickness behaviors described above including decreased food intake, decreased activity, increased sleep disturbances, and decreased social/sexual interactions; suggesting that many psychiatric disorders may involve a dysregulation of immune function even in the absence of an overt immune challenge [71, 211]. However, depression is not the only psychiatric disorder with a well-described link to immune dysregulation. These include schizophrenia, anxiety and stress disorders, major depressive disorder, autism, and learning disabilities [134, 201, 232, 263]. For instance, individuals with schizophrenia have abnormal levels of IL-1β, IL-6, growth factors (e.g. BDNF), and neuregulin within the brain and body [190, 191, 200], indicating that these individuals have not only abnormal immune function but also differences in proteins critical for synapse formation and function within the brain. Individuals with posttraumatic stress disorder (PTSD) have increased levels of circulating inflammatory markers, increased reactivity during skin antigen tests, lower T cell counts, and increased global methylation of immune genes [25, 210, 265]. Individuals with autism spectrum disorders and Rett syndrome have altered cytokine profiles circulating within the periphery, low immunoglobulin levels, and altered T cell activation [12, 13, 25, 113, 210, 265].
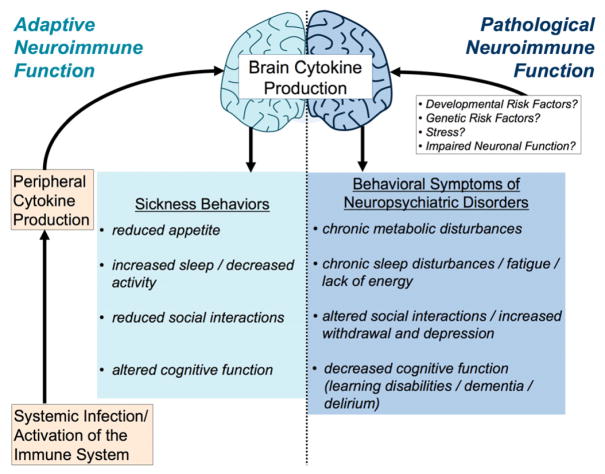
Figure 1. Adaptive and pathological neuroimmune function similarly increases brain cytokine production and influences behavior.
Systemic infection produces a peripheral cytokine response, which in turn produces a cytokine response in the brain. Cytokines within the brain induce a well-characterized set of adaptive behaviors that are intended to help fight infection, including reduced appetite (food and water intake), increased sleep and decreased overall activity, reduced social interactions, and altered cognitive function. Many neuropsychiatric or mood disorders exhibit a similar set of behavioral symptoms that have become prolonged or exaggerated, including chronic metabolic disorders or decreased appetite, chronic sleep disturbances/fatigue, altered social interactions, withdrawal/depression, and decreased cognitive function (e.g. learning disabilities, dementia, and delirium). Not surprisingly, many neuropsychiatric disorders are also associated with altered immune/neuroimmune function.
Notably many of these neuropsychiatric disorders also have a known or suspected developmental origin. The developing brain is exquisitely sensitive to both endogenous and exogenous signals that may significantly alter the developmental trajectory of cells, neural circuits, and associated behavioral outcomes. Indeed, increasing evidence suggests diverse experiences during the pre- or postnatal period, including maternal stress, nutrition, trauma, or infection, may profoundly modulate or “program” developing neural circuits, with the result that adult outcomes including behavior are significantly and often permanently affected [39, 79, 90, 207].
A link between perinatal infection and neuropsychiatric disorders may have first been proposed in 1891, when Thomas Clousten suggested there might be an infectious origin to what he described as “adolescent insanity”. Since then, many researchers have noted the strong relationship between early-life infection and the later-life onset of schizophrenia [99, 178]. The specific mechanisms by which infections may lead to psychopathology include direct infection of the developing fetus and subsequent abnormal neural development, the generation of auto-antibodies by the mother that subsequently react with fetal neural tissue, and alterations in cytokine production, which may be an underlying component of all three mechanisms [220]. Elevated levels of pro-inflammatory cytokines generated by the maternal or fetal immune system have been associated with abnormal fetal brain development and an increased risk of neurodevelopmental disorders [56, 179, 216, 290]. For instance, concentrations of IL-1β, IL-6, and TNFα are elevated in infants with severe perinatal complications or bacterial meningitis [184, 194], and concentrations strongly correlate with the occurrence of neurological sequelae. Similarly, increased levels of IL-6 in amniotic fluid, the result of increased inflammation from bacterial infection in late pregnancy, correlates significantly with increased rates of mortality and brain injury [317]. There are several reports in humans that maternal influenza infection induces cytokine synthesis via the activation of the maternal immune system, the fetal immune system, and the placenta, each of which has been linked to increased risk of schizophrenia in the offspring [52, 130, 186, 290]. Recent advances in maternal and perinatal medicine have greatly increased survival rates among mothers suffering infections or trauma in developed countries; however, these studies establish that infections, despite the low risk of death, nonetheless leave an enduring mark [33]. In an effort to determine the mechanisms underlying such changes, a number of animal models of early-life immune activation have been developed and characterized.
Animal Models of Early-Life Immune Activation
Polyinosinic:polycytidylic acid(Poly IC)
Poly IC is a synthetic double-stranded RNA molecule that is commonly used as a viral mimetic. Poly IC is recognized by the pattern recognition receptor, toll-like receptor (TLR) 3, which specifically recognizes double stranded RNA, the genetic information for many viruses [3]. Perinatal poly IC exposure causes a robust febrile response, a profound increase in cytokine production, and HPA axis activation [95, 185, 245]. Based on the significant link between maternal influenza virus and the increased risk of schizophrenia in human offspring, several researchers have studied the long-term consequences of perinatal poly IC exposure on physiology and behavior in rodent models. Treatment of newborn rat pups with poly IC has significant effects on adult immune responses, including an attenuated febrile response and an exaggerated corticosterone response to an adult immune challenge [85]. Similar to behavioral symptoms seen in individuals with schizophrenia and autism, offspring that were prenatally treated with poly IC display significant deficits in pre-pulse inhibition acoustic startle response, decreases in exploratory behavior in both open-field and novel-object tests, and decreases in social interactions as adults [136, 179–181, 217, 262]. Cognitive impairments resulting from prenatal immune activation include deficits in reversal learning of a previously learned task [122]. In addition, rats or mice exposed to poly IC prenatally have an impaired ability to ignore irrelevant environmental stimuli, one of the central deficits in humans with schizophrenia. This is manifested in humans and rodents as a lack of latent inhibition. Latent inhibition is a phenomenon whereby repeated pre-exposure to an inconsequential stimulus (such as a tone) can decrease the capacity for that same stimulus to signal significant consequences later [322, 323]. Notably many of these behavioral deficits caused by early-life poly IC exposure can be reversed by acute administration of antipsychotic (clozapine and chlorpromazine) and psychomimetic drugs such as ketamine [262], which is why this animal model is heralded as a strong model for schizophrenia.
Lipopolysaccharide (LPS)
LPS, the cell wall component of gram-negative bacteria, has been used to mimic infection in many animal studies because it initiates a well-characterized immune response via the activation of TLR4. Within the immature rat brain, LPS induces a rapid and robust increase in cytokine expression characterized by a robust increase in the expression of many cytokines and chemokines, including IL-1β, IL-6, TNFα, CXCL1, CXCL2, CXCL10, CCL2 and CCL7, among others, as well as a marked increase in circulating corticosterone [94, 114, 206, 259, 290, 300]. Treatment of either pregnant dams or neonatal pups with high doses of LPS is linked to overt white matter damage, decreased oligodendrocyte development, hypo-myelination of neurons [56, 87, 216], and enhanced behavioral pain responses in adulthood [132]. Lower doses of LPS given during the perinatal period also induce a number of long-term changes in the brain, both biochemical and behavioral.
Interestingly, prenatal exposure to LPS causes a decrease in stress responsivity and a blunted immune response to subsequent immune challenges during the neonatal period in rats [131, 187]. In contrast, treatment of pups postnatally with LPS causes an increase in circulating corticosterone following a stressor, and a decrease in the expression of sickness behaviors and fever following an adult immune challenge [84, 85, 298, 299]. Rats exposed to endotoxin postnatally also show changes in behavior in adulthood such as increased anxiety, and exaggerated acoustic startle responses [299, 300]. Based on both models of LPS administration during the perinatal period (pre vs. postnatal), it is apparent that the time point at which the immune challenge occurs is critically important for determining the long-term effects on neuroimmune function and behavior.
Escherichia coli (E. coli)
E. coli is a primary cause of infection in low-birth weight premature infants in the US, and infections in premature infants are associated with significant delays or alterations in neurodevelopment [1, 274]. We have extensively characterized the impact of neonatal E. coli infection in rats on later-life brain and behavior [32, 34–38, 41, 42, 44, 45, 307]. Infection of rat pups on postnatal day (P)4 (relatively comparable to a preterm human) with E. coli markedly increases circulating cytokines in the periphery (IL-1β, IL-6, TNFα) and increases circulating corticosterone for at least 48 hours after infection [35]. Within the brain, neonatal E. coli infection increases IL-1β mRNA and protein at 24 h [35], as well as the expression of a host of other genes important for IL-1β signaling, including Caspase 1 (which cleaves IL-1β into its active form), IL-18, and the IL-1 type 1 receptor [257]. As adults, rats infected neonatally with E. coli exhibit a number of physiological and behavioral changes, including vulnerability to cognitive impairments, sensitized fever to an LPS challenge, decreased corticosterone responses to a stressor, and decreased social interactions [32, 35, 41, 42] (Figure 2).
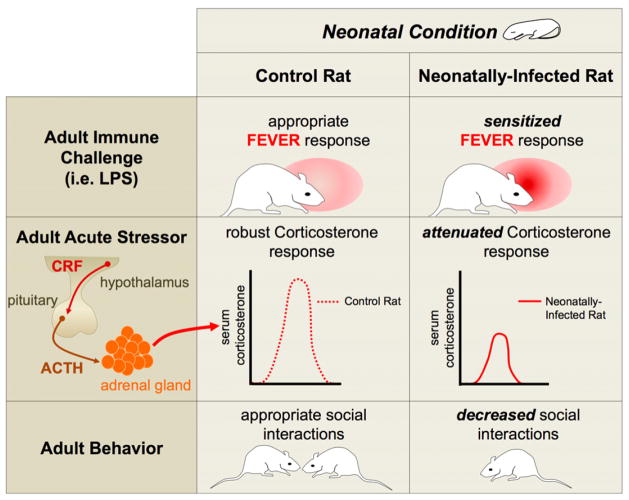
Figure 2. Neonatal infection in male rats produces a number of long-term physiological and behavioral changes.
Neonatally-infected rats exhibit a sensitized fever response following an adult immune challenge, such as LPS, when compared to control rats [41]. Neonatally-infected rats have an attenuated corticosterone response to an acute stressor, when compared to control rats [42]. Neonatally-infected rats also exhibit decreased social interactions with other rats when compared to control rats [42].
Early-Life Immune Activation and Later-Life Cognition
The impact of an early-life immune challenge has been well characterized for later-life cognitive abilities, and we focus on that literature here. Cytokine receptors are distributed throughout the brain, but the hippocampus has one of the highest densities of microglia and receptors for IL-1β, a cytokine that has been well characterized in relation to cognition [69, 255]. The hippocampus is a brain region critical for learning and memory, as well as a host of other behaviors important for survival such as emotion. Notably, the hippocampus is also particularly vulnerable to damage from events that occur during development or in adulthood, such as chronic/severe stress, epilepsy, stroke, hypoxia/ischemia, or cardiac arrest [100, 225, 248, 249]. Thus, one might predict that the developing hippocampus may also be particularly vulnerable to early-life immune activation.
There are two potential pathways by which perinatal immune activation may influence neural function and its associated behavioral outcomes such as learning and memory: 1) early-life immune activation could permanently alter or disrupt the development of neural pathways important for learning and memory, or, 2) early-life immune activation could re-program adult immune function, affecting how an adult responds to a subsequent immune challenge via either prolonged or exaggerated pro-inflammatory cytokine production or decreased anti-inflammatory regulation, which would indirectly impair the neural processes important for cognition (Figure 3).
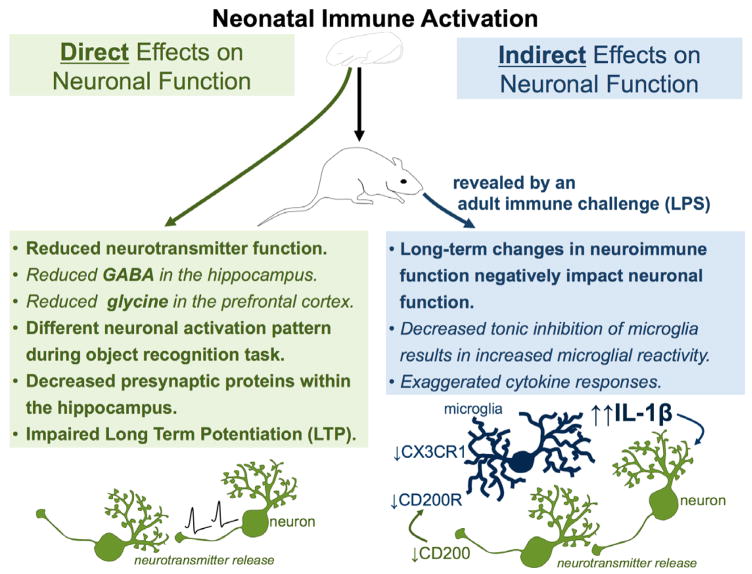
Figure 3. Neonatal immune activation can have direct long-term effects on neuronal function or indirect long-term effects on neuronal function via alterations in neuroimmune function.
Neonatal immune activation directly affects neuronal function by reducing neurotransmitter function (including GABA in the hippocampus and glycine in the prefrontal cortex), decreasing the expression of presynaptic proteins in the hippocampus, inhibiting long-term potentiation, and producing a differential neuronal activation pattern during a learning task such as the novel object recognition task. Neonatal immune activation indirectly alters neuronal function by producing long-term changes in neuroimmune function that in turn negatively impact neuronal function. Decreased tonic inhibition of microglia via altered expression of neuronal inhibitory signals, including fractalkine (via its receptor CX3CR1) and CD200, also results in exaggerated cytokine responses, which impact neuronal function.
Long-Term Consequences of Early-Life Immune Activation: Direct Effects on Cognition
Many labs have investigated the direct effects of perinatal immune activation on the neural circuits underlying cognition (option #1 above), and have demonstrated that perinatal immune activation can significantly affect the development of specific neural processes such as neurotransmission and synaptic plasticity important for learning and memory [43, 140, 205, 208, 308, 313]. Prenatal exposure to Poly IC causes reduced basal neurotransmission of dopamine and glutamate, as well as reduced levels of the inhibitory transmitter GABA, within the hippocampus, and reduced glycine within the prefrontal cortex, a brain region critical for working memory. The authors of these studies attribute the cognitive/behavioral inflexibility of these offspring exposed prenatally to Poly IC to the observed changes in neurotransmitter function [43]. Maternal immune activation using Poly IC also causes a reduced frequency and increased amplitude of miniature excitatory postsynaptic potentials within the hippocampus of the offspring, indicative of significant differences in baseline glutamatergic transmission [140]. There is a distinctly different pattern of neuronal activation within the hippocampus of prenatally infected rats during an object recognition task, indicating that not only is neurotransmission different but the overall function of the neuronal circuit is significantly altered [140]. Lastly, maternal Poly IC treatment also causes decreases in pre-synaptic proteins within the hippocampus of offspring suggesting differences in synapse number; these offspring also have impaired long-term potentiation (LTP), a process of synaptic strengthening important for learning, within the CA1-CA3 pathway of the hippocampus [205].
Long-Term Consequences of Early-Life Immune Activation: Indirect Effects on Cognition
Other experiments have been designed to explicitly test option #2 above; i.e. whether early-life immune activation can alter the adult neuroimmune response to a subsequent immune challenge thereby indirectly affecting the neural circuitry underlying learning and memory. To test this possibility, rats infected neonatally with E. coli or its vehicle were tested in a modified version of contextual fear conditioning known as the context pre-exposure task [24]. This paradigm assesses the rat’s memory for a recently explored context. In this task, when a rat is placed into a specific context and immediately shocked, it displays little or no conditioned fear (freezing) to the context. This absence of fear to the context is thought to occur because the animal does not have the opportunity to sample the environment and thus store a representation of its features (a hippocampal dependent process) prior to an immediate shock [246, 247]. If, however, a rat is pre-exposed to the context for several minutes the day before, an immediate shock the following day will produce substantial freezing on a subsequent test day [88, 247, 303].
Using this paradigm, adult rats from each neonatal treatment group (control vs. E. coli infection) received no injection, saline, or a low dose of LPS (which by itself does not typically cause memory impairments) immediately following the context pre-exposure. If the adult LPS challenge causes an exaggerated or prolonged immune response in neonatally infected rats that would interfere with learning the context, then only rats that experience the combination of a neonatal infection and LPS after context pre-exposure would display impaired memory. However, if neonatal E. coli infection directly alters the development of neural pathways that support memory formation, one would predict that neonatally infected rats should display impaired memory regardless of the adult immune challenge. Results demonstrated that only rats that experienced the combination of neonatal infection and subsequent LPS exposure displayed impaired memory for the explored context [35]. In contrast, neonatally infected rats that did not receive an adult immune challenge at the time of context pre-exposure did not exhibit memory deficits. Taken together, these data support the hypothesis that neonatal immune activation increases the risk of cognitive deficits indirectly, via long-term programming of neuroimmune responses that subsequently interfere with the cellular processes of learning and memory. Interestingly, these data are in good accord with the “two-hit hypothesis” of schizophrenia, which posits that the combination of an underlying vulnerability (likely instantiated early in life) plus a later-life (typically young adult) precipitating event (e.g., stress, infection) is required for the manifestation of the illness [59, 148, 150, 173, 217]. From these data, one might ask: (1) How is the adult immune response different in an animal exposed to an early-life immune challenge; and (2) How does this impact behavior?
Cytokines and Neural Function
A growing body of evidence implicates a role for cytokines in normal, non-pathological, synaptic plasticity mechanisms within the brain and associated learning and memory behaviors [174]. TNFα is important for activity-dependent synaptic scaling within the hippocampus [26, 270]. Moreover, TNFα, as well as multiple interleukins (e.g., IL-6, IL-1, IL-10) and prostaglandins can markedly impact cognitive function, primarily memory (reviewed in [316]). Similarly, IL-6 has been implicated in LTP maintenance [14]. High frequency stimulation in the hippocampus increases IL-6 mRNA expression [142]. Treatment of rat hippocampal neuronal cultures with IFNγ during the peak of synaptogenesis reduces spontaneous excitatory-postsynaptic currents (EPSCs) and increases spontaneous inhibitory PSCs several weeks later [51].
IL-1β in particular has been well characterized for its role in cognition. IL-1β is induced within the hippocampus in response to fear conditioning [115], and in hippocampal slices during the induction of LTP. However, IL-1β is also necessary for the maintenance of LTP of hippocampal synapses [241, 269]. Mice lacking endogenous IL-1β or its receptor exhibit markedly impaired hippocampal-dependent learning and memory, and similarly transgenic over-expression of the endogenous IL-1 receptor antagonist (IL-1ra) impairs LTP as well as hippocampal-dependent memory in the water maze and fear-conditioning paradigms [115, 268, 269].
In contrast to these data, exaggerated IL-1β within the brain is also strongly associated with memory impairment. IL-1β is very tightly regulated during the course of the immune response within the normal brain. Patients with AIDS-related dementia, cancer, chronic inflammatory diseases (e.g., Alzheimer’s), or autoimmune diseases often exhibit exaggerated levels of IL-1β co-occurring with cognitive impairment [103, 118, 182]. Exogenously applied IL-1β inhibits LTP within hippocampal slices [67, 147], and similarly, a systemic injection of a high dose of LPS inhibits LTP of the hippocampal perforant pathway in vivo [292]. This effect is blocked by inhibition of Caspase-1, the enzyme necessary for cleaving IL-1β into its biologically active form [292], suggesting that IL-1β is the mechanism by which peripheral LPS administration inhibits LTP in vivo. Rats injected with high levels of IL-1β directly into the dorsal hippocampus also display memory impairments [23, 228]. These data suggest that cytokines, in particular IL-1β, are necessary for normal cognitive function, specifically influencing the synaptic mechanisms underlying learning and memory within the hippocampus. Moreover, the physiological level of IL-1β within the hippocampus is critically important, as too little or too much IL-1β can equally impair learning and memory, consistent with a hormesis function observed for many hormones and biochemical events within the body and brain [116, 171].
Based on the critical role for IL-1β in normal learning processes, and the learning and memory deficits seen in neonatally infected rats following an adult immune challenge, subsequent experiments have explored whether IL-1β production within the hippocampus is exaggerated or prolonged in adult rats infected neonatally with E. coli. The expression of IL-1β and its associated family of proteins is relatively low in the normal healthy brain. IL-1β protein within the hippocampus of neonatally infected rats is not detectable and not significantly different than control rats in the absence of an adult immune challenge, indicating that neonatal infection does not result in chronically elevated IL-1β levels within the brain [35]. However, in response to LPS treatment, neonatally infected rats exhibit a prolonged and exaggerated expression of IL-1β protein specifically within the hippocampus and the adjacent parietal cortex [35]. At the messenger RNA level, the genes encoding for IL-1β, IL-1β converting enzyme (caspase-1), and the IL-1β type I receptor, are all significantly elevated following LPS treatment within the hippocampus of neonatally-infected rats compared to controls. In addition, mRNA for the anti-inflammatory IL-1ra is not significantly elevated in neonatally infected rats above controls [37]. Thus, early-life infection appears to program a shift in the neuroimmune system towards an exaggerated pro-inflammatory response, specifically an exaggerated increase in IL-1β within the hippocampus, during the course of an immune challenge in the adult brain. Based on these data and the literature suggesting that the level or duration of IL-1β is important for learning and memory, one might hypothesize that this exaggerated IL-1β response within the hippocampus of neonatally infected rats could impair learning of the context in the fear conditioning task. In support of this hypothesis, blocking the synthesis of IL-1β using a caspase-1 inhibitor at the time of LPS administration completely prevents the contextual memory deficit described above in neonatally infected rats [35]. Thus, exaggerated IL-1β within the hippocampus in response to an immune challenge can critically interfere with memory formation and, moreover, the risk for dysregulation of immune responses and associated behavioral deficits is capable of being programmed by early-life immune activation.
Interim Conclusions
The accumulated evidence from each of the described models of perinatal immune activation (Poly IC, LPS, and E. coli) provides interesting interpretations of the “big picture.” Although the mechanism of action underlying distinct immune challenges may be different (TLR3 vs. TLR4 activation), the end results of early-life immune activation share similarities. First, all models of perinatal immune activation produce a robust increase in cytokine expression at the time of the challenge. Thus, increased cytokine production during the perinatal period emerges as a common mechanism by which many of these long-term changes in neuroimmune function and behavior may be programmed for the life of the individual. Notably, while researchers often use LPS as an immune challenge to mimic the effects of E. coli, the two stimuli can elicit very distinct cytokine profiles and behavioral outcomes in adulthood. Namely, E. coli produces a robust yet pathway-specific increase in gene expression (focused on the IL-1β family of proteins) following infection, whereas LPS produces a robust yet very broad cytokine and chemokine response following administration [257]. Therefore, the identification of common cytokines that are elevated within the developing brain by these distinct immune challenges may guide future research aimed at understanding how the incidence of seemingly disparate infections and injuries in humans has been positively linked with the incidence of neuropsychiatric disorders and cognitive impairment.
A second similarity among disparate perinatal immune challenges is their induction of long-term changes in behavior in adulthood. Some of these appear direct, and others indirect via long-term changes within the immune system as discussed previously. However, it should be noted that baseline cytokine expression was never analyzed in the adult offspring exposed to Poly IC prenatally. An alternate conclusion for the described alterations in those animals is that perinatal immune activation alters the baseline function or expression of neuroimmune molecules (even in the absence of an adult immune challenge), and in so doing indirectly affects the endpoints measured, including basal neurotransmission, LTP, and synapse formation within the adult hippocampus.
MECHANISMS UNDERLYING ENDURING CONSEQUENCES OF EARLY-LIFE IMMUNE CHALLENGE
Microglia are the innate immune cells of the brain. They are rapid responders to any disruption of homeostasis or immune challenge, and are major producers of cytokines, chemokines, and other neuromodulators within the brain. They express a wide number of surface and nuclear receptors, including those for complement proteins (CD11b), cytokines and chemokines (e.g., TNF, CCL2), as well as major histocompatibility (MHC) molecules, immunoglobulins, toll-like-receptors (TLRs), cell adhesion molecules, and many others (see [105, 123] for review). Microglia in the normal adult brain have a resting, highly ramified morphology, with low levels of “activation markers” (e.g., CD11b, MHC II) on the cell surface. However, the term “activation” marker is somewhat of a misnomer, given that resting microglia are by no means dormant or inactive. Increasing evidence suggests a role for microglia in normal synaptic plasticity mechanisms within the adult brain, including interactions with extracellular matrix composition and geometry, and dendritic spine remodeling and elimination [287, 288]. These cells are very dynamic, even when resting [204], and continually survey their microenvironments by extending and contracting processes into nearby synapses, with a frequency that is activity-dependent [286, 297]. For instance, they sample individual synapses more frequently following visual stimulation, or in response to injury, and are likely responsible for synapse removal via phagocytosis [286]. Microglia have receptors for multiple neurotransmitters and neuromodulators, including those important for learning and memory (e.g., ATP, norepinephrine, glutamate) [227], suggesting a rapid and direct role for these cells in normal cognition. Many excellent reviews have been published on microglia function and biology, which goes beyond the scope of the current manuscript to extensively review here [105, 123, 275, 276, 278].
Glial Priming
Microglia are an excellent candidate for inducing long-term changes within the brain, because these cells have the capacity to become and remain chronically sensitized or “primed” [283]. In response to injury or immune stimulation, microglia up-regulate a number of these surface receptors, including those for complement proteins, MHC II (important for antigen presentation), and cytokines, which in turn initiate both repair and cytotoxic processes via interactions with numerous other CNS cell types (e.g., astrocytes, neurons) [242]. Microglial priming has been implicated in Alzheimer’s, Parkinson’s, and Huntington’s diseases, as well as in normal aging [22, 32, 68, 96, 110, 165, 221]. The characteristics of priming are not well defined, though primed glia have been characterized in situ by an activated morphology with enlarged cell bodies and short, thick processes. An important feature of priming is that these cells do not constitutively over-produce pro-inflammatory mediators within the brain. Rather, the pro-inflammatory response produced by primed glia to a subsequent challenge (e.g., systemic infection) is significantly exaggerated when compared to resting/quiescent glia that receive the same challenge [223]. Thus, it is hypothesized that primed glia adopt a prolonged sensitized state, presumably following initial activation by insult or injury [224, 277]. We hypothesize based on our research that the subsequent challenge (i.e., “second hit”) may occur temporally quite distant from the initial precipitating event; however, in general the dynamics of priming are relatively unknown. Note that there is also evidence that microglia become dystrophic with age in contrast to sensitized, which exhibit stripped or disembodied processes and are impaired in their normal homeostatic functions [277, 279]. In either case, because microglia are believed to be long-living cells, glial pathology has the capacity to significantly alter neural function and behavior, perhaps over the entire lifespan.
Though the priming literature has largely been focused on microglia (similar to macrophages in the peripheral immune system [144, 170, 209]), there is some evidence that astrocyte function is altered by a prior inflammatory challenge; for instance, astrocytes cultured with discrete cytokines (IL-1β or TNFα), or with microglia-conditioned media exhibit sensitized responses to subsequent TLR2 ligands [126]. Moreover, rats given kainic acid (KA) on P15 exhibit a lower threshold to induce seizures following a subsequent exposure to KA on P45, along with long-term increases in the astrocyte markers GFAP and S100B, in addition to greater microglial activation [266].
The concept of glial priming is striking in its similarity to the pattern of cytokine expression and cognitive impairment in adult rats infected on P4 with E. coli, in which a sensitized IL-1β response and memory deficit is only observed following the subsequent challenge later in life. Indeed, we now have strong evidence that early-life infection with E. coli leads to long-term sensitization/priming of microglia within the brain. The microglial surface antigens CD11b and MHC II are markedly increased within the hippocampus in response to infection on P4, and this increase is sustained into adulthood [35, 37]. In contrast, there were no acute or long-term changes in basal GFAP expression following neonatal infection. However, increased microglial marker expression could indicate an increase in cell number, as opposed to a change in their reactivity, consistent with the definition of “priming”. Notably, by adulthood the total number of microglia, astrocytes, & neurons within the hippocampus does not differ as a consequence of neonatal treatment. However, the morphology of microglia in neonatally-infected rats is very different in adulthood; cells are larger and amoeboid-like, with thicker processes, consistent with priming [45]. This difference is apparent basally, indicating a longterm effect of the infection alone. More recently we have confirmed and extended this characterization of glia using flow cytometric analysis of rapidly isolated microglia from rats in each condition. To do this, rats from each neonatal treatment were injected as adults with saline or LPS, and the hippocampus was collected 24 h later. Microglia were rapidly isolated [97, 128], and stained with an APC-conjugated CD11b antibody. Cell size (forward light scatter) and CD11b+ expression were assessed using flow cytometry. Again, total cell number did not differ, whereas microglia were both larger and exhibited increased CD11b+ expression on a per cell basis in neonatally-infected rats [307], consistent with our previous in situ findings [45].
Microglia are the source of exaggerated IL-1β in neonatally-infected rats
These collective data suggest that changes in the function of microglia, rather than simply changes in number, underlie their increased reactivity in neonatally-infected rats. Consistent with this interpretation, rapid isolation and separation of microglia (CD11b+ cells) from other CNS cell types (CD11b− cells; e.g., astrocytes, neurons) using magnetically activated cell sorting (MACS®) for analysis of IL-1β expression indicates that microglia are the sole source of exaggerated IL-1β in neonatally-infected rats within the brain. Most exciting, these experiments also revealed that CD11b+ cells are the source of IL-1β during hippocampal-dependent learning. As described above, systemic infection with E. coli on P4 leads to marked hippocampus-dependent memory impairment in adulthood, but only if these animals receive a low dose LPS challenge after learning. The impairment is causally linked to exaggerated CNS IL-1β production, as preventing IL-1β synthesis within the brain completely prevents the memory impairment [35]. Curiously, LPS 24 h prior to learning also impairs long-term contextual memory in neonatally-infected but not control rats despite the fact that hippocampal IL-1β is largely undetectable in both groups by 24 h after an LPS injection [35, 38]. These data led us to test the hypothesis that learning itself induces IL-1β protein within the hippocampus, which is differentially modulated in NI rats. To test this, rats treated with PBS or E. coli on P4 were injected with saline or LPS as adults, and fear conditioned 24 h later. IL-β was detectable at low but physiological levels within the hippocampus 2 h after contextual fear conditioning in both groups of rats [307]. In contrast, IL-1β was not detected within the cortex, confirming that IL-1β is significantly and selectively elevated within the hippocampus at the time of context learning. Importantly, IL-1β was undetectable within the hippocampus following either exposure to the context (with no shock) or footshock alone, suggesting that IL-1β is only synthesized within the hippocampus during a learning experience. Most importantly, this learning-induced increase in hippocampal IL-1β levels was significantly exaggerated in neonatally-infected rats that were previously injected with LPS [307] (Figure 4).
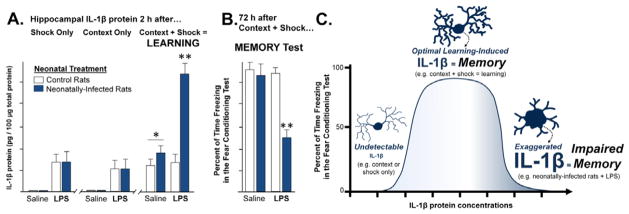
Figure 4. Learning increases IL-1β protein within hippocampal microglia, which is modulated by neonatal infection.
(A) Neonatally-infected rats and controls were treated in adulthood with saline or lipopolysaccharide (LPS) 24 h prior to either a learning experience (fear conditioning, consisting of 2 min context exploration followed by a footshock), or a control procedure which consisted of footshock only (without context exploration) or context exposure only (no footshock). IL-1β protein was measured in the hippocampus of separate groups of rats from each neonatal condition, 2 h after each of these conditions (shock alone, context alone, or context + shock/fear learning). In rats from both neonatal conditions that received a saline injection as adults, IL-1β protein was only increased after the learning experience (context + shock); *p<0.001, compared to context alone or shock alone. Neonatally-infected rats that received LPS 24 hours prior to behavioral testing exhibited an exaggerated IL-1β response, but only in response to learning (context + shock); **p<0.01 compared to control rats. These data indicate that normal learning induces the synthesis of IL-1β in the hippocampus, but neonatally-infected rats that receive an adult immune challenge have dysregulation of IL-1β at the time of learning. Subsequent experiments revealed that microglia were the sole source of IL-1β in these experiments [307]. (B) In a separate set of rats, fear memory for the context was assessed 72 h after conditioning. Rats from each neonatal condition that received saline 24 h prior to the learning experience show robust freezing behavior (fear) at the 72 h test, indicating that they remember the association between the shock and the context. Control rats that received LPS 24 h prior to conditioning also show robust freezing behavior (fear) at the 72 h test, indicating strong memory. In contrast, neonatally-infected rats that received LPS 24 h prior to conditioning exhibit significantly decreased freezing (fear) in the context (**p<0.05), indicating impaired memory only in this “2-hit” group (neonatal infection + adult immune challenge; see [307]). (C) Our working model is that IL-1β is produced by microglia within the hippocampus at the time of learning and is required for normal memory formation. In the absence of learning (shock alone or context alone), IL-1β is not produced in detectable levels. In neonatally-infected rats, long-term changes in neuroimmune function (microglial priming, see [307]) results in significantly exaggerated levels of IL-1β following a learning experience, which are “unmasked” by the adult LPS challenge. These exaggerated levels of IL-1β interfere with the consolidation of the learning experience and result in memory deficits. The cartoon illustrates microglia in a quiescent phenotype (left-hand tail of the inverted U), in a normal, active phenotype in which IL-1β is produced during normal learning to support memory (center), and in a sensitized/primed morphology in which exaggerated levels of IL-1β are observed in response to learning, but only in neonatally-infected rats that receive LPS as adults (right-hand tail of the inverted U). All data are represented from [307]).
These data indicate that LPS given 24 hours prior to learning and memory causes a significant shift in microglial function in neonatally-infected rats such that learning itself results in exaggerated IL-1β production, which impairs learning and memory. Consistent with this interpretation, inhibiting microglia with minocycline either at the time of LPS treatment or at the time of learning can reverse the memory impairment in neonatally-infected rats [307]. These collective data have led to three conclusions: (1) Microglia have a critical role in learning and memory via the production of IL-1β, (2) Microglial dysfunction (exaggerated IL-1β) results in cognitive dysfunction, and (3) Early-life events can significantly impact cognitive function later in life via long-term programming of microglial function.
BEYOND INFECTION: IMMUNE MOLECULES AND NEURAL DEVELOPMENT
A wide number of immune signaling molecules including cytokines, chemokines, MHC molecules, complement proteins, and TLRs have been identified for their critical roles in neural development [81] (Figure 5). Many of these molecules are glial-derived (microglia and astrocytes), or they critically interact with glial function to guide normal development. We and others [81, 288] have hypothesized that the colonization pattern of the developing brain by glia, and the large number of immune molecules important for brain development, has important implications for a wide number of insults or environmental stimuli that might activate the developing immune system either directly or indirectly, and in so doing exert enduring effects on neural function and behavior, and the evidence is mounting in support of this hypothesis.
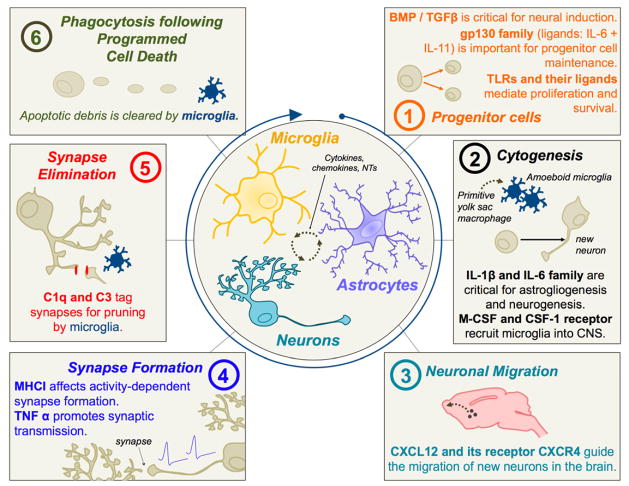
Figure 5. Immune molecules play a ubiquitous role in neural development.
Microglia, astrocytes, and neurons share a common molecular language within the CNS, and continually communicate via cytokines, chemokines, neurotransmitters and other factors (center circle). Many of the same molecules originally identified for their roles in immune function have now been implicated in neural development; representative examples are conceptualized here (see [48, 81] for comprehensive reviews). (1) Many cytokines are important for progenitor cell maintenance, proliferation, and differentiation. The bone-morphogenic protein (BMP)/transforming growth factor beta (TGFβ) family of cytokines is critical for neural induction [106]. The gp130 receptor and associated ligands are important for progenitor cell maintenance and proliferation [80, 117, 175]. TLR3 is important for maintaining progenitor cell populations and proliferation of dividing cells within the brain [157], and HMGB1, a known ligand for TLR4, impacts cell survival [319]. (2) Cytokines such as IL-1β and the IL-6 family of proteins have a demonstrated role in cytogenesis within the developing brain [50, 198]. IL-1β expression peaks during astrocytogenesis, which is dependent on the presence of amoeboid microglia [109]. Microglia begin to colonize the developing brain as primitive yolk sac macrophages beginning around E9–10 [107]; macrophage colony-stimulating factor (M-CSF) and the CSF-1 receptor are important for this recruitment [107, 250]. (3) Chemokines, in particular CXCL12 and its receptor CXCR4, guide the migration of new neurons in many brain regions, including the cerebellum [166, 321]. (4) MHC I is critical for the activity dependent formation of synapses within the visual cortex, and likely many other brain regions [60, 78, 261]. TNFα released by astrocytes promotes synaptic transmission and affects activity-dependent synaptic scaling [270]. (5) Complement proteins, C1q and C3, tag synapses for elimination [272]. Microglia recognize these proteins via the complement receptor 3 (CD11b), and phagocytose the labeled synapses as a mechanism of synaptic pruning [254]. (6) Microglia are the primary phagocytic cells of the CNS, and thus have an important role in phagocytosing apoptotic debris following programmed cell death, a process that occurs continuously and most abundantly within the developing brain [93]. Programmed cell death likely plays a major role in recruitment of microglia into the CNS [302].
Role of Microglia in Neurodevelopment
Microglia originate early in the life of the fetus and are potentially very long-lived, meaning they may have the capacity to reside in the brain for most of the life of the animal [168]. Microglial progenitor cells begin colonizing the rodent brain around embryonic day (E) 9–10 via the infiltration of primitive macrophage precursors from the yolk sac [58, 107, 168]. These primitive microglia enter the parenchyma via the blood stream and ventricles [66]. Microglia are detectable using immunohistochemical staining for the antigen Iba1 within the developing rodent brain around E13 or 14; they are initially located within the embryonic brain around subcortical regions such as the hippocampus and around the corpus callosum [301, 312]. From that point, microglia migrate to their final destination within the brain where they continue to proliferate. Similar to the rodent brain, colonization of microglia within the human brain is an orchestrated response that occurs early in fetal development alongside maturation of the nervous system [58]. In humans, the first cells with the characteristics of a macrophage appear within the yolk sac and mesenchyme (embryonic cells that will eventually develop into the circulatory and lymphatic system) around the 4th week of gestation. Vascularization of the neural plate commences at around 5 weeks of gestation and within the 5th and 6th week of gestation many of these yolk-sac derived cells appear within and just outside these newly formed blood vessels (see [168] for review).
To date, it is not well known what factors drive the infiltration and migration of immature microglia into the parenchyma. Some researchers have noted that the invasion of microglia within the developing brain coincides with naturally occurring cell death during early brain development [10, 11, 222]. Moreover, chemokines such as macrophage colony stimulating factor (M-CSF) and the CSF-1 receptor [107, 250], and intercellular cell adhesion molecule (ICAM)-2 [107, 236], may play a role. Many other chemokines (e.g., monocyte chemoattractant protein (MCP)-1) have an important role in microglial migration and neural development within the healthy brain [63–65, 235]. We have recently identified a significant number of chemokines that are up-regulated within the rat hippocampus and cortex at birth when compared to the adult brain, including CCL2, CCL3, CCL6, CCL7, CCL12, and Chemokine (C-X-C motif) ligand 6 (CXCL6) [259]. One might hypothesize these cytokines are important for attracting primitive macrophages and immature microglia into the brain from the periphery during early brain development.
Within the adult brain, microglia have a distinct ramified morphology represented by thin, long processes and small cell bodies. In contrast, microglia within the embryonic human and rodent brain have a larger, round, amoeboid morphology, similar to the morphology of microglia seen in the adult brain following activation or injury. From embryonic development to early postnatal development, and from adolescence to adulthood, microglia shift their morphology rapidly and dramatically in a brain region-dependent manner. This process has been explored predominantly within subcortical regions of the rodent brain and the retina [66, 258]. From birth to P4, cells change their morphology rapidly as they develop from a round amoeboid shape to a shape characterized by a smaller cell body with thinner, longer processes. However, even in the juvenile and adolescent rodent brain, microglia within certain brain regions continue to show a more activated morphology suggesting that these brain regions and the microglia within them are continuing to undergo maturational changes [66, 258].
Taking into consideration the morphology of immature microglia and the increased production of cytokines within the developing brain, one might assume that the primary role of microglia within the developing brain is related to their role as brain macrophages, specifically that they are actively engaged in the phagocytosis of cellular debris of apoptotic cells as well as the induction of apoptosis in other cells [30, 169, 301]. However, recent work suggests that microglia have a much more complex role in the developing brain. Microglia now have demonstrated roles in cellular differentiation and axon guidance [see [48, 81] for review]. Moreover, a critical role for microglia in developmental synapse elimination has recently been described [254]. C1q and C3, proteins within the classical complement cascade of the immune system, localize to synapses within the postnatal mouse brain intended for elimination [270]. Microglia that express the complement receptor (CD11b) for C3 are activated for phagocytosis of these “opsonized” or labeled, synapses [254, 272, 288]. Interestingly, a lack of CX3CR1 (fractalkine) receptor on microglia transiently reduces the number of microglia within the early postnatal brain (P8–P15), and impairs synapse elimination in mice [176]. Defects in synaptic function have been linked to a large number of mental health disorders, including depression, anxiety, and cognitive disruption, and pruning defects (either over or under pruning) are linked to autism and schizophrenia [149, 164, 251].
Role of Astrocytes in Neural Development
Astrocytes are derived from specific populations of progenitor cells [see [318] for review] toward the end of embryonic development. During mammalian nervous system development, neural progenitor cells (NPCs) generate neurons first and astrocytes second, though the switch that guides this determination is brain-region dependent [for review see [98]]. The differentiation of NPCs into neurons first and astrocytes second requires a discrete turning on and turning off of particular genes, such as gfap or s100b, using epigenetic mechanisms including DNA methylation [282]. Interestingly, a recent experiment determined that co-culture of neural progenitor cells with microglia can promote the differentiation of neural progenitors into astrocytes [119]. Protoplasmic astrocytes begin to sprout processes within the final weeks of embryonic development and the first weeks of postnatal development, and they develop long processes relatively rapidly. By P7, astrocytes within the hippocampus display thin processes similar to filopodia that often terminate with small bulbous structures, with usually one primary but sometimes more primary, thicker processes [54]. One week later, astrocytes within the hippocampus display much greater ramification of their processes, though many of these processes are still thin and filopodial in nature; and by P21, astrocytes display multiple primary processes, thinner processes are more ramified/mature, and astrocytes have established distinct boundaries from neighboring astrocytes. Astrocytes have a crucial function in synaptogenesis and synaptic scaling, through the release of diffusible factors and the production of extracellular matrix proteins (see [86] for review). Most synaptogenesis occurs after birth and depends significantly on astrocyte function. A single astrocyte can associate with nearly 2 million synapses [98], making up the “tripartite synapse” [8], and their role in synaptic plasticity mechanisms within the adult CNS is now well accepted [7, 226, 260]. Astrocytes express many neurotransmitter receptors, allowing them to rapidly perceive and respond to synaptic activity [273]. Astrocytes also produce the TNFα that mediates synaptic scaling following prolonged periods of inactivity within the HP [26, 270]. D-serine release from astrocytes is necessary for HP LTP [127, 314]. Importantly, a recent report showed that IL-1 type 1 receptor expression in astrocytes is also necessary for HP-dependent LTP and long-term memory [29]. Moreover, these cells are immunocompetent and respond to injury or infection in many ways that are analogous to microglia. In response to injury or immune stimulation, astrocytes exhibit hypertrophy and proliferation, a process known as reactive gliosis or astrocytosis, which has been implicated in a number of neurological disorders with discrete developmental origins, including seizures [266], ischemia [281], and cortical injury [53].
Cytokines
A large number of cytokines have been characterized for their importance in many neurodevelopmental processes including neurogenesis, neuronal and glial cell migration, proliferation, differentiation, and synaptic maturation and pruning (Figure 5). These include members of the gp130, bone morphogenetic protein (BMP), and transforming growth factor beta (TGFβ) super-families, “cytokine-like” hormones such as leptin, growth hormones, and prolactin, as well as many traditionally defined “pro-inflammatory” cytokines (e.g., IL-1β, TNFα) [49, 81, 104, 156, 176, 177, 219]. The basal expression of many cytokines early in development is significantly higher when compared to the adult brain. Coinciding with the appearance of amoeboid microglia during early brain development, researchers have reported a naturally occurring increase in cytokines. For example, IL-1β is produced at detectable levels within the cortex from approximately E14 to P7 [108], in contrast to the adult brain in which levels are difficult to detect basally. In contrast, the cerebellum, which develops significantly later, just prior to birth in rodents, has a peak in IL-1β levels that occurs from P2 to P14 [108]. Specifically within the hippocampus, IL-1β is increased nearly 6-fold at birth when compared to adult hippocampus [259]. Time dependence and regional specificity has also been demonstrated for the expression of other cytokines during brain development, suggesting a physiological role for these cytokines in the development of specific brain circuits. Both IL-1β and TNFα are present early in the developing sheep brain, declining by birth and peaking again around the time of synaptogenesis [82]. IL-6 is important for numerous developmental processes including prenatal CNS vascular development [92], and IL-6 increases markedly in striatum, hippocampus, and cortex throughout development, suggesting a neurotrophic role for this cytokine within these brain regions [101, 102]. IL-11 is increased 10-fold within the hippocampus at birth when compared to adults [259]. Not surprisingly, there is a corresponding increase in cytokine receptors early in development when compared to the adult brain. Specifically, IL-1 receptor 2, IL-2 receptor β, IL-2 receptor γ, and IL-6 receptor α are all significantly up-regulated at P0 within the hippocampus when compared to the adult [259]. These data suggest that elevated levels of particular cytokines may coincide with important processes of neurodevelopment in a brain region-dependent manner.
Chemokines
Chemokines are small 8–12 kD proteins important for T cell, B cell, and hematopoietic cell development [2, 129, 166, 196, 197, 321], and are critical mediators of chemotaxis in the context of disease; e.g., recruitment of leukocytes to sites of insult or injury [146, 153, 161, 202]. Increasing research has identified a ubiquitous role for chemokines in nervous system function as well. For instance, CXCR2 increases GluR1 affinity for glutamate following transfection into human embryonic kidney (HEK) cells [160]. Application of the CXCR2 ligand, CXCL2, onto cultured cerebellar Purkinje cells increases spontaneous AMPA-type glutamatergic excitation [160]. The chemokine CX3CL1 (fractalkine) reduces AMPA-mediated currents and alters EPSCs evoked by electrical stimulation of Schaffer collaterals in hippocampal neurons, via interactions with its receptor CX3CR1, which is notably highly expressed on microglia within the brain [159]. Numerous other chemokines, including CCL2 (MCP-1), CCL3 (macrophage inflammatory protein (MIP)-1α), and CXCL12, have been identified for their roles in neurotransmission within the CNS as well, particularly within the hippocampus [155, 203].
In addition to playing critical roles in neurotransmission, chemokines are important for neural stem cell migration within the brain [152, 284], including the developing brain. The chemokine CXCL12 (SDF-1) and its exclusive receptor CXCR4 have a critical role in the migration of neurons to their final destination. Mice lacking either CXCL12 or CXCL4 have deficits in cerebellar development, as the temporospatial migration of granule cells into the internal granule cell layer from the external layer is disrupted [166, 320, 321]. Since this discovery, others have discovered that CXCR4 and CXCL12 regulate the migration of cells within other developing brain regions including the proliferating cells of the dentate gyrus [163], GABAergic interneurons migrating into the cortex [280], and gonadotropin-releasing hormone (GnRH) neurons migrating from the vomeronasal organ to their destination within the hypothalamus [256]. CXCL12 also regulates axonal elongation and branching within hippocampal neurons via interactions with its receptor [229]. With more sophisticated tools aimed at targeting individual chemokines and their receptors, researchers will most likely identify many more neurodevelopmental processes that are regulated by these chemoattractant immune molecules. Notably, inflammatory stimuli increase the proliferation of neural stem cells (NSCs) in the dentate gyrus [28, 77], and NSCs express several chemokine receptors, including CXCR4, the receptor for CXCL12, and CXCR2, the receptor for CCL2/MCP-1 [139, 284, 285, 304], which has important implications for injury and repair, and long-term neurocircuitry organization and function.
MHC I
In addition to the members of the cytokine families, other immune molecules have a demonstrated role in neural development. MHC I proteins were described in the activity dependent formation of synapses within the visual cortex over a decade ago [60]. MHC molecules are cell-surface ligands that are best known for binding T cells within the periphery to regulate their activation. However, they are also present on neurons within the developing brain, where they are thought to be located postsynaptically near glutamate receptors and to interact with a number of putative presynaptic proteins expressed on axonal growth cones in the selection and guidance of activity-dependent synaptic formation and strengthening [261]. Since their initial identification within the visual pathway, MHC I family molecules have been identified in many neuronal types within the developing dorsal root ganglia, brainstem, hippocampus, and cerebellum, and most likely MHC I has an important role in modulating synapse formation within all of these brain regions. The MHC I family of genes is in general an attractive candidate for guiding synaptic specificity because of its enormous diversity [261].
Complement proteins
The classical complement cascade within the peripheral immune system consists of a large number of proteins, including the initiating molecule C1q and downstream effector C3, which organize interactions between cells of the innate and adaptive immune system in order to aid in pathogen detection and elimination [193]. As described above, a role for these same proteins in developmental synapse elimination, or pruning, within the CNS has recently been recognized. Complement proteins are widely expressed within neurons of the developing brain, particularly during periods of active synaptic remodeling [272]. C1q or C3 deficient mice exhibit impairments in synapse elimination, along with disordered layer segregation within the lateral geniculate nucleus of the developing visual system. The prediction is that these same molecules are important in a number of developmental disorders as well; e.g., psychiatric disorders in which defective synapse elimination has been implicated, including autism and schizophrenia [271], although this remains to be directly demonstrated.
PAMPs, DAMPs and Toll-Like Receptors
The innate immune system represents a complex set of tissues, cells, and molecules critical for the detection, containment, and elimination of a large number of pathogens, insults, and damage to cells. Pathogen-associated molecular patterns (PAMPs) are a diverse set of evolutionarily-conserved molecules present on micro-organisms such as bacteria and viruses, which are recognized by the innate immune system via the activation of the endogenous pattern recognition receptors (PRRs), most notably the TLRs. The pattern recognition receptor TLR3 is expressed by neural progenitor cells in the developing cortex and regulates the proliferation of these cells [157]. In vitro studies suggest TLR2 and 4 are important for stem cell fate determination, and mice lacking TLR2 exhibit impaired neurogenesis, whereas mice lacking TLR4 exhibit enhanced neurogenesis [240]. These data are especially intriguing given that the Toll family of receptors were initially characterized in Drosophila for their role in embryonic neural patterning, but are inseparable from the immune system in mammals [4]. Importantly, the function of some TLRs, including TLR2 and 4, extends beyond that of PAMP recognition to “danger” associated molecular patterns (DAMPs) recognition more broadly [172]. DAMPs include endogenous “alarmins” that are released in response to cellular or tissue distress [31]. There are many putative factors that have been identified as alarmins, including hyaluronan, high mobility group box (HMGB) 1, and heat shock proteins (see [31] for review).
A few of these alarmin molecules have an established role in brain development, neural function, and behavior. Most notably, HMGB1 is a ubiquitous component of chromatin which can be released by necrotic cells, is retained by apoptotic cells, and is actively secreted by cells undergoing an inflammatory challenge or biological stress [192]. However, HMGB1 is also highly expressed in the developing brain of vertebrate and non-vertebrate species (Figure 6), and is the only HMGB to be released even in the absence of immune activation [120, 137, 151, 233]. Years ago, it was determined that HMGB1 can induce neurite outgrowth from embryonic forebrain cultures derived from rats [233]. More recently, it has been determined that HMGB1 is necessary for the survival and differentiation of catecholaminergic neurons during the development of the forebrain in zebrafish [319]. Thus, similar to other immune molecules previously discussed, alarmin molecules appear to have a critical role in the development of functional circuits within the brain.
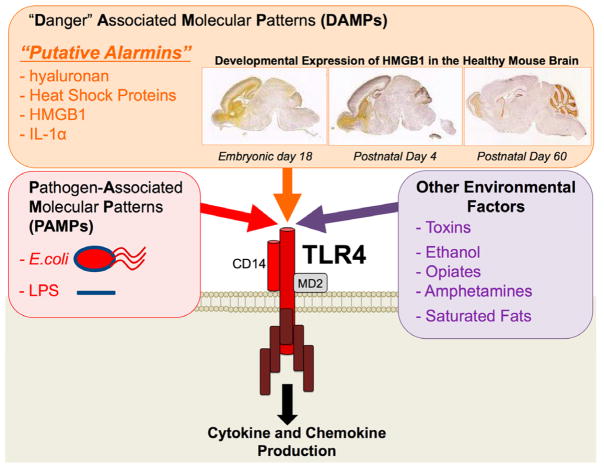
Figure 6. The list of factors hypothesized to activate immune cells via Toll-Like Receptors (TLRs), in particular TLR4.
Toll-like receptors are innate pattern recognition receptors which identify the pathogen-associated molecular patterns (PAMPs) of specific pathogens. For example, TLR4 recognizes the immunologically active cell wall pattern of gram-negative bacteria, lipopolysaccharide (LPS), which is also found on E. coli. The role of these receptors has recently expanded to include the broader recognition of “danger” associated molecular patterns (DAMPs). These include a novel subset of proteins that are produced and released by nearby cells undergoing cell death or distress, known as alarmins, which are putatively recognized by TLRs, including both TLR4 and TLR2. Proteins that have been identified as alarmins thus far include hyaluronan, Heat Shock Proteins, and high mobility group box (HMGB) 1. In addition, the list of exogenous or environmental factors that are hypothesized to activate TLR4 (directly or indirectly) is ever-expanding. These include drugs of abuse (e.g. opiates, amphetamines, and ethanol), saturated dietary fats, and other toxins (e.g. air pollution).
HMGB1’s role in neural function and behavior is exemplified in a recent experiment which determined that over-expression of HMGB1, caused by a functional knockout of Single-Ig-interleukin-1 related receptor (SIGIRR), a protein that constitutively down-regulates HMGB1 expression, causes significant impairments in hippocampal function in adults [62]. Specifically, these mice show deficits in novel object recognition, spatial reference memory, and LTP. Blocking TLR4, one of the innate immune receptors activated by HMGB1, completely blocks these deficits caused by exaggerated expression of HMGB1 signaling [62], suggesting that these effects of HMGB1 are exclusively driven by the activation of the innate immune system. These data support the hypothesis that dysfunction of the immune system – including in this case the dysregulation of alarmin proteins – can cause significant cognitive impairment even in the absence of an overt immune challenge.
Is there a sensitive period for the long-term consequences of early-life immune activation?
The development of the brain involves an exquisitely timed set of events that ultimately lead to the formation of functional circuits required for complex behaviors. Thus the consequences of an immune challenge early in life for potentially lifelong behavioral outcomes will undoubtedly vary depending on the ongoing developmental processes at the time of insult. We have recently reviewed this evidence [39]. As described in the previous section, the morphology, function, and synthesis of immune molecules from glial cells are markedly different during early postnatal development as these cells continue, themselves, to develop and direct the development of the cells around them. We have thus hypothesized that the distinct colonization pattern of microglia into the developing brain represents a sensitive period for long-term changes in their function as well as associated cognitive and behavioral outcomes; if true, then a challenge later in life should not have the same impact. Indeed, E. coli infection on P30, a time when the majority of microglia are in a mature ramified state in males [259], does not enduringly impact IL-1β expression, nor does it impact cognition in adulthood, either basally or following an LPS challenge [38]. Beyond changes in glia, and in immune molecules expression within the developing brain as described previously, there are numerous other factors that likely impact the long-term consequences of immune disruption or challenge early in life, including the maternal immune response, and the placental and blood-brain barriers.
The Maternal-Fetal Interface
The maternal immune system and placenta are two critical factors that can distinguish or differentially impact the neurodevelopmental outcomes of the offspring exposed to an immune challenge prenatally. Pregnancy is associated with many changes in the maternal immune response (see [239] for review). Over the course of pregnancy, there is a gradual shift from cell-mediated (Th1) to humoral (Th2)-dominant responses, a decrease in inflammatory macrophages and cytokines production, decreased febrile responses, and an increase in regulatory T cells, changes that are hypothesized to prevent fetal rejection [188, 239]. However, these changes also affect disease pathogenesis, including susceptibility to infectious diseases that are often increased [239]. Interestingly, maternal immune activation, while altered, nonetheless results in a robust increase in certain cytokines (e.g., IL-6) within the maternal blood stream [261]. Cytokines produced by the maternal immune system may in turn gain access to the fetal brain, via the placenta. The placenta is the first functional organ of the fetus [218], and research on “fetal programming” increasingly focuses on the placenta as a crucial source of variation in the development and physiology of the fetus in response to environmental change (reviewed in [111, 143, 195]). The majority of this research has focused on the impact of maternal stress or maternal nutrition on fetal outcomes, via changes in metabolic pathways (e.g., leptin, insulin) [9, 79, 143]. However, a significant literature on the role of placental endocrine-immune interactions is emerging. Maternal antibodies and immune cells contact the lining of the syncytiotrophoblast layer of the placenta, which in turn increases cytokine production. In addition, the placenta has its own macrophages, known as Hofbauer cells, which are capable of producing cytokines and chemokines [91]. While there is debate about whether maternal cytokines cross the placenta, cytokines that are produced directly by the placenta include IL-1, IL-2, IL-6, IL-8, and IL-10, interferons and TNFα, and likely many others [136, 218]. There is a current upsurge in interest into the mechanisms by which placental cytokine expression may impact fetal development; recent evidence suggests an impact on fetal growth via downstream modification of growth factors, such as IGF-1 [136], and leptin [47, 70, 158, 295, 309]. In sum, the placenta has the capacity to produce its own array of cytokines, which can enter the fetal circulation and thereby modulate or interfere with ongoing fetal growth and neurodevelopment [136, 218]. Notably, imbalanced cytokine production within the placenta has been observed during maternal infection, but also in response to preeclampsia, prenatal stress, and exposure to air pollution (47, 158, 307], suggesting the implications of placental neuroendocrine-immune interactions extend well beyond infection.
Blood-Brain Barrier (BBB)
The BBB is a collection of endothelial cells, astrocytes, pericytes, and microglia that separate the circulating blood and the extracellular fluid of the brain via the integrity of the tight junctions between these cells. The BBB must effectively bring nutrients and oxygen into the brain while effectively keeping out any foreign or invading substances that might be damaging to sensitive neural tissue. It is becoming more evident that the integrity of the BBB has a major role in the pathophysiology of many neuropsychiatric disorders [190, 315]; for instance, the passage of cytokines and chemokines through the BBB can not only affect neural function and behavior but also cause considerable damage to neural tissue. In general, dogma states that cytokines are large enough molecules that their passage across the BBB does not occur. However, it is now understood that certain cytokines can cross the BBB via saturable transport [17–19, 121]. Specifically, the BBB has selective cytokine binding sites that convey the cytokine across the barrier via transporters (see [16] for review). In addition, cytokines increase the permeability of the BBB [213–215], thereby increasing the likelihood of cytokine transport across. Thus, while a rare occurrence, in the event of trauma caused by hypoxia/ischemia or severe inflammation, metabolic changes such as diabetes, or even drugs of abuse, the composition of the BBB can change [125] and circulating cytokines from the periphery may enter the brain and affect its function.
The developing brain represents a new set of challenges to the dogma regarding BBB permeability. The BBB is significantly more permeable during early brain development, as it is relatively underdeveloped at this time. In general, tight junctions are present early in development such that the BBB is developed enough to keep out the majority of large proteins [see [252] for review]. However, the capacity for some lipid-insoluble molecules to enter the developing brain is greater than in the adult brain [21, 252, 253]. Neonatal infection with E. coli, for example, causes a significant increase in the gene expression of cytokines within the brain itself [35, 257], but also a robust increase in the serum protein levels of many cytokines (IL-1β, IL-6, and TNFα) [35]. It is likely that the invasion of cytokines and chemokines from the peripheral immune system into the brain is greater in the developing brain than in the adult brain. Moreover, E. coli enters the brain directly following peripheral infection of the newborn rat [35], but not in the adult. Such direct access of pathogens can in theory activate microglia directly via pathogen recognition receptors (e.g., TLRs), as opposed to indirectly via cytokines. As a result, the potential risk for damage from a peripheral immune challenge might be much greater in the neonate than in the adult.
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, we have reviewed the evidence that the immune system is critically involved in neural and endocrine function, in both sickness and in health. During brain development, a large number of immune molecules are implicated in various aspects of “building a brain”, including neural cell differentiation, migration, synapse formation and refinement, and programmed cell death. Microglia produce or express receptors for many of these molecules, and are especially active beginning in mid-gestation and throughout the perinatal period. For this reason, we have hypothesized that development represents a sensitive period for impacting microglial function, and subsequent brain and behavioral development. However, many outstanding questions remain.
For instance, many developmental disorders exhibit a striking sex difference in their etiology and prevalence. Males are more likely to be diagnosed with disorders that present at birth or early in childhood, such as autism and learning disabilities. In contrast, females are more often diagnosed with disorders that arise around the onset of adolescence or puberty [see [20] for review]. This epidemiology suggests that there are sex-based neurobiological differences, which are likely to arise during development, that either directly promote specific neuropsychiatric disorders or increase the susceptibility to environmental factors that lead to such disorders. At present, no sex-based differences have been described that can fully explain the sexual dimorphism of neuropsychiatric disorders. However, there are sex differences within the placenta in response to a wide number of environmental variables (reviewed in [135]). For instance, prenatal stress induces larger changes in genes important for fetal growth in males compared to females [189]. Moreover, we have recently reported a marked sex difference in the colonization of the developing brain by microglia [258, 259], wherein females have 5-fold fewer microglia in brain regions important for cognition during the first postnatal week compared to males. Notably, females also do not exhibit the same enduring impact of an E. coli infection on P4; i.e., exaggerated cytokine production and memory deficits are not observed in adult females infected on P4 [40], potentially due to a blunted microglial-derived inflammatory response to the initial infection. Interestingly, the sex difference in microglial colonization observed in the neonatal brain reverses entirely just prior to adolescence, in the juvenile brain, and this sex difference is maintained into adulthood. Microglia within the female brain also appear to have a more activated morphology than the microglia within the male brain just prior to adolescence and into adulthood, suggesting that microglia within the juvenile/adolescent female brain may have a very different functional output from the microglia in the male brain at this time. Whether and how sex steroids impact these sex differences in placental function and microglial development remain to be defined.
Another important question is a critical one for intervention, and that is whether longterm “programming” effects would be observed under less “impoverished” laboratory conditions. For instance, we have recently determined that 6 weeks of limited daily environmental enrichment in adult rats, independent of any early-life manipulation, increased microglial and astrocyte surface antigen expression within the dentate gyrus of the hippocampus (which may indicate an increase in their density), but notably decreased the proinflammatory cytokine response to a systemic LPS challenge compared to standard housing controls [306]. However, the implications for behavior, if any, remain to be determined. We have also demonstrated that a neonatal “handling” paradigm, which increases the quantity and quality of maternal care, reverses some but not all aspects of neonatal infection on adult function. Glial priming appears to be largely prevented; however, handling is unable to decrease anxiety or increase learning and memory in neonatally-infected rats, which is characteristic of this paradigm in normal control animals [37]. Nonetheless, these data have intriguing implications for the prevention or reversibility of immune activation early in life. Again, the answer likely depends on the timing of exposure, and the underlying neurodevelopmental processes at play, in combination with genetic factors.
Taken together, we propose that this field warrants further research into the role that sex and brain regional dependent mechanisms may play in hormone-immune interactions, glial colonization/differentiation, number, and function, and their potential contribution to neural and cognitive dysfunction. Enduring consequences for the brain may be due to cell-intrinsic changes within microglia, or to changes in a plethora of other immune molecules that we now know are critical for normal brain development. How and when immune molecules interact with hormones, such as sex steroids, metabolic factors such as leptin, insulin and growth hormones, which are all critical for proper neural and behavioral development, is clearly underexplored. In sum, because immune molecules are also critical for behavioral outcomes, including cognition, mood, and social interactions, an impact on their function by diverse challenges early in development is a candidate mechanism of risk or resilience for neuropsychiatric disorders, as well as a novel target for behavioral or pharmacological interventions.
Highlights.
The nervous, endocrine, and immune systems communicate during health and disease
Cytokine dysregulation is linked to several diseases and neuropsychiatric disorders
Immune molecules and microglia are critical for normal brain development
Perinatal stressors may cause neuroimmune dysregulation and behavioral disorders
Microglia may present a novel target for interventions
Acknowledgments
The authors acknowledge the funding sources for much of the work discussed in this review, including R01 DA025978 and R01 MH083698 to S.D.B., and F32-DA030136 to J.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Current opinion in infectious diseases. 2006;19:290–297. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- 2.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. The Journal of experimental medicine. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 5.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annual Review of Immunology. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anforth HR, Bluthe RM, Bristow A, Hopkins S, Lenczowski MJ, Luheshi G, Lundkvist J, Michaud B, Mistry Y, Van Dam AM, Zhen C, Dantzer R, Poole S, Rothwell NJ, Tilders FJ, Wollman EE. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. European cytokine network. 1998;9:279–288. [PubMed] [Google Scholar]
- 7.Araque A, Navarrete M. Glial cells in neuronal network function, Philosophical transactions of the Royal Society of London. Series B. Biological sciences. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 9.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? The Journal of physiology. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain research. Developmental brain research. 1990;55:219–230. doi: 10.1016/0165-3806(90)90203-b. [DOI] [PubMed] [Google Scholar]
- 11.Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Brain research. Developmental brain research. 1991;58:1–12. doi: 10.1016/0165-3806(91)90231-7. [DOI] [PubMed] [Google Scholar]
- 12.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain, behavior, and immunity. 2010 doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. Journal of neuroimmunology. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 15.Ban EM, Sarlieve LL, Haour FG. Interleukin-1 binding sites on astrocytes. Neuroscience. 1993;52:725–733. doi: 10.1016/0306-4522(93)90421-b. [DOI] [PubMed] [Google Scholar]
- 16.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Special Issue: Blood Brain Barrier. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA, Kastin AJ, Gutierrez EG. Interleukin-1 alpha in blood has direct access to cortical brain cells. Neuroscience letters. 1993;163:41–44. doi: 10.1016/0304-3940(93)90224-9. [DOI] [PubMed] [Google Scholar]
- 20.Bao AM, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders, The Neuroscientist: a review journal bringing neurobiology. neurology and psychiatry. 2010;16:550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- 21.Barichello T, Fagundes GD, Generoso JS, Paula Moreira A, Costa CS, Zanatta JR, Simoes LR, Petronilho F, Dal-Pizzol F, Carvalho Vilela M, Lucio Teixeira A. Brain-blood barrier breakdown and pro-inflammatory mediators in neonate rats submitted meningitis by Streptococcus pneumoniae. Brain research. 2012 doi: 10.1016/j.brainres.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behavioural brain research. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 24.Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1β administration. Journal of neuroimmunology. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Bauer ME, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R. Interplay between neuroimmunoendocrine systems during post-traumatic stress disorder: a minireview. Neuroimmunomodulation. 2010;17:192–195. doi: 10.1159/000258721. [DOI] [PubMed] [Google Scholar]
- 26.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 27.Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D. Sympathetic modulation of immunity: relevance to disease. Cellular immunology. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben Menachem-Zidon O, Avital A, Ben-Menahem Y, Goshen I, Kreisel T, Shmueli EM, Segal M, Ben Hur T, Yirmiya R. Astrocytes support hippocampal-dependent memory and longterm potentiation via interleukin-1 signaling. Brain, behavior, and immunity. 2011;25:1008–1016. doi: 10.1016/j.bbi.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 32.Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiology of learning and memory. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilbo SD. How cytokines leave their mark: the role of the placenta in developmental programming of brain and behavior. Brain, behavior, and immunity. 2011;25:602–603. doi: 10.1016/j.bbi.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain, behavior, and immunity. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behavioral neuroscience. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- 37.Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain, behavior, and immunity. 2007;21:332–342. doi: 10.1016/j.bbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behavioural brain research. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Frontiers in behavioral neuroscience. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilbo SD, Smith SH, Schwarz JM. A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7:24–41. doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilbo SD, Wieseler JL, Barrientos RM, Tsang V, Watkins LR, Maier SF. Neonatal bacterial infection alters fever to live and simulated infections in adulthood. Psychoneuroendocrinology. 2010;35:369–381. doi: 10.1016/j.psyneuen.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Bilbo SD, Yirmiya R, Amat J, Paul ED, Watkins LR, Maier SF. Bacterial infection early in life protects against stressor-induced depressive-like symptoms in adult rats. Psychoneuroendocrinology. 2008;33:261–269. doi: 10.1016/j.psyneuen.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bland ST, Beckley JT, Watkins LR, Maier SF, Bilbo SD. Neonatal Escherichia coli infection alters glial, cytokine, and neuronal gene expression in response to acute amphetamine in adolescent rats. Neuroscience letters. 2010;474:52–57. doi: 10.1016/j.neulet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain, behavior, and immunity. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blatteis CM. The onset of fever: new insights into its mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- 47.Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, Bilbo SD. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012 doi: 10.1096/fj.12-210989. in press. [DOI] [PubMed] [Google Scholar]
- 48.Boulanger LM. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Bouret SG, Bates SH, Chen S, Myers MG, Jr, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:1244–1252. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowen KK, Dempsey RJ, Vemuganti R. Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport. 2011;22:126–130. doi: 10.1097/WNR.0b013e3283430a44. [DOI] [PubMed] [Google Scholar]
- 51.Brask J, Kristensson K, Hill RH. Exposure to interferon-gamma during synaptogenesis increases inhibitory activity after a latent period in cultured rat hippocampal neurons. The European journal of neuroscience. 2004;19:3193–3201. doi: 10.1111/j.0953-816X.2004.03445.x. [DOI] [PubMed] [Google Scholar]
- 52.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of General Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 53.Burns KA, Murphy B, Danzer SC, Kuan CY. Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57:1115–1129. doi: 10.1002/glia.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. International Journal of Developmental Neuroscience. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cellular immunology. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatric research. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Cardona AE, Li M, Liu L, Savarin C, Ransohoff RM. Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. Journal of leukocyte biology. 2008;84:587–594. doi: 10.1189/jlb.1107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Research Reviews. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Choy KH, de Visser YP, van den Buuse M. The effect of ‘two hit’ neonatal and young-adult stress on dopaminergic modulation of prepulse inhibition and dopamine receptor density. British journal of pharmacology. 2009;156:388–396. doi: 10.1111/j.1476-5381.2008.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 61.Costa-Pinto FA, Palermo-Neto J. Neuroimmune interactions in stress. Neuroimmunomodulation. 2010;17:196–199. doi: 10.1159/000258722. [DOI] [PubMed] [Google Scholar]
- 62.Costello DA, Lyons A, Browne T, Denieffe S, Cox FF, Lynch MA. Long-term potentiation is impaired in CD200-deficient mice: a role for Toll-like receptor activation. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.280826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowell RM, Silverstein FS. Developmental changes in the expression of chemokine receptor CCR1 in the rat cerebellum. The Journal of comparative neurology. 2003;457:7–23. doi: 10.1002/cne.10554. [DOI] [PubMed] [Google Scholar]
- 64.Cowell RM, Xu H, Parent JM, Silverstein FS. Microglial expression of chemokine receptor CCR5 during rat forebrain development and after perinatal hypoxia-ischemia. Journal of neuroimmunology. 2006;173:155–165. doi: 10.1016/j.jneuroim.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Cross AK, Woodroofe MN. Chemokines induce migration and changes in actin polymerization in adult rat brain microglia and a human fetal microglial cell line in vitro. Journal of neuroscience research. 1999;55:17–23. doi: 10.1002/(SICI)1097-4547(19990101)55:1<17::AID-JNR3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 66.Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Progress in neurobiology. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 67.Cunningham AJ, Murray CA, O’Neill LAJ, Lynch MA, O’Connor JJ. Interleukin-1β (IL-1β) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neuroscience letters. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 68.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunningham ET, Jr, De Souza EB. Interleukin 1 receptors in the brain and endocrine tissues. Immunology today. 1993;14:171–176. doi: 10.1016/0167-5699(93)90281-o. [DOI] [PubMed] [Google Scholar]
- 70.Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A, Bjorntorp P, Albertsson Wikland K, Holmang A. Prenatal cytokine exposure results in obesity and gender-specific programming. American journal of physiology. Endocrinology and metabolism. 2001;281:E326–334. doi: 10.1152/ajpendo.2001.281.2.E326. [DOI] [PubMed] [Google Scholar]
- 71.Dantzer R. Cytokine, sickness behavior, and depression. Neurologic clinics. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, Kelley KW. Molecular basis of sickness behavior. Annals of the New York Academy of Sciences. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 73.Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Annals of the New York Academy of Sciences. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 74.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, behavior, and immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dantzer R, Konsman JP, Bluthe RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Autonomic Neuroscience: Basic & Clinical. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- 76.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 78.Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. The Australian & New Zealand Journal of Obstetrics & Gynaecology. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 80.Derouet D, Rousseau F, Alfonsi F, Froger J, Hermann J, Barbier F, Perret D, Diveu C, Guillet C, Preisser L, Dumont A, Barbado M, Morel A, deLapeyriere O, Gascan H, Chevalier S. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4827–4832. doi: 10.1073/pnas.0306178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Dziegielewska KM, Moller JE, Potter AM, Ek J, Lane MA, Saunders NR. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell and tissue research. 2000;299:335–345. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- 83.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacological reviews. 2000;52:595–638. [PubMed] [Google Scholar]
- 84.Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:1519–1521. doi: 10.1096/fj.04-3569fje. [DOI] [PubMed] [Google Scholar]
- 85.Ellis S, Mouihate A, Pittman QJ. Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. The Journal of physiology. 2006;571:695–701. doi: 10.1113/jphysiol.2005.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 88.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural brain research. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 89.Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. Journal of immunology (Baltimore, Md: 1950) 1987;139:459–463. [PubMed] [Google Scholar]
- 90.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophrenia bulletin. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faulk WP, Hunt JS. Human placentae: view from an immunological bias. American journal of reproductive immunology (New York, NY: 1989) 1989;21:108–113. doi: 10.1111/j.1600-0897.1989.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 92.Fee D, Grzybicki D, Dobbs M, Ihyer S, Clotfelter J, Macvilay S, Hart MN, Sandor M, Fabry Z. Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine. 2000;12:655–665. doi: 10.1006/cyto.1999.0599. [DOI] [PubMed] [Google Scholar]
- 93.Ferrer I, Serrano T, Soriano E. Naturally occurring cell death in the subicular complex and hippocampus in the rat during development. Neuroscience research. 1990;8:60–66. doi: 10.1016/0168-0102(90)90058-m. [DOI] [PubMed] [Google Scholar]
- 94.Fidel PL, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. American Journal of Obstetrics and Gynecology. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 95.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. American journal of physiology. Regulatory, integrative and comparative physiology. 2004;287:R759–766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 96.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiology of aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 97.Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: Immunophenotypic and functional characteristics. Journal of neuroscience methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 98.Freeman MR. Specification and morphogenesis of astrocytes. Science (New York, NY) 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fruntes V, Limosin F. Schizophrenia and viral infection during neurodevelopment: a pathogenesis model? Medical science monitor: international medical journal of experimental and clinical research. 2008;14:RA71–77. [PubMed] [Google Scholar]
- 100.Fujioka M, Nishio K, Miyamoto S, Hiramatsu KI, Sakaki T, Okuchi K, Taoka T, Fujioka S. Hippocampal damage in the human brain after cardiac arrest. Cerebrovascular diseases (Basel, Switzerland) 2000;10:2–7. doi: 10.1159/000016018. [DOI] [PubMed] [Google Scholar]
- 101.Gadient RA, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain research. 1994;637:10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- 102.Gadient RA, Otten U. Identification of interleukin-6 (IL-6)-expressing neurons in the cerebellum and hippocampus of normal adult rats. Neuroscience letters. 1994;182:243–246. doi: 10.1016/0304-3940(94)90807-9. [DOI] [PubMed] [Google Scholar]
- 103.Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. Journal of neuroimmunology. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- 104.Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Frontiers in synaptic neuroscience. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 106.Gaulden J, Reiter JF. Neur-ons and neur-offs: regulators of neural induction in vertebrate embryos and embryonic stem cells. Hum Mol Genet. 2008;17:R60–66. doi: 10.1093/hmg/ddn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunology and allergy clinics of North America. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 111.Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002;23(Suppl A):S20–27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 112.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Autonomic Neuroscience: Basic & Clinical. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 113.Goines P, Van de Water J. The immune system’s role in the biology of autism. Current opinion in neurology. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 115.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 116.Goudochnikov VI. The role of glucocorticoids in aging and age-related pharmacotherapy, Advances in gerontology = Uspekhi gerontologii/Rossiiskaia akademiia nauk. Gerontologicheskoe obshchestvo. 2011;24:48–53. [PubMed] [Google Scholar]
- 117.Gregg C, Weiss S. CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain. Development. 2005;132:565–578. doi: 10.1242/dev.01592. [DOI] [PubMed] [Google Scholar]
- 118.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gu F, Wang J, Fu L, Ma YJ. Co-culture with microglia promotes neural stem cells differentiation into astrocytes. Chinese medical journal. 2011;124:3394–3398. [PubMed] [Google Scholar]
- 120.Guerin A, d’Aubenton-Carafa Y, Marrakchi E, Da Silva C, Wincker P, Mazan S, Retaux S. Neurodevelopment genes in lampreys reveal trends for forebrain evolution in craniates. PloS one. 2009;4:e5374. doi: 10.1371/journal.pone.0005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. Journal of neuroimmunology. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 122.Han X, Li N, Meng Q, Shao F, Wang W. Maternal immune activation impairs reversal learning and increases serum tumor necrosis factor-alpha in offspring. Neuropsychobiology. 2011;64:9–14. doi: 10.1159/000322455. [DOI] [PubMed] [Google Scholar]
- 123.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 124.Hart BL. Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 125.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 126.Henn A, Kirner S, Leist M. TLR2 hypersensitivity of astrocytes as functional consequence of previous inflammatory episodes. Journal of immunology. 2011;186:3237–3247. doi: 10.4049/jimmunol.1002787. [DOI] [PubMed] [Google Scholar]
- 127.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. Journal of neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hernandez-Lopez C, Varas A, Sacedon R, Jimenez E, Munoz JJ, Zapata AG, Vicente A. Stromal cell-derived factor 1/CXCR4 signaling is critical for early human T-cell development. Blood. 2002;99:546–554. doi: 10.1182/blood.v99.2.546. [DOI] [PubMed] [Google Scholar]
- 130.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstetrics and gynecology. 1993;81:941–948. [PubMed] [Google Scholar]
- 131.Hodyl NA, Krivanek KM, Clifton VL, Hodgson DM. Innate immune dysfunction in the neonatal rat following prenatal endotoxin exposure. Journal of neuroimmunology. 2008;204:126–130. doi: 10.1016/j.jneuroim.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 132.Hodyl NA, Walker FR, Krivanek KM, Clifton VL, Hodgson DM. Prenatal endotoxin exposure alters behavioural pain responses to lipopolysaccharide in adult offspring. Physiology & Behavior. 2010;100:143–147. doi: 10.1016/j.physbeh.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 133.Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends in neurosciences. 1995;18:83–88. [PubMed] [Google Scholar]
- 134.Hornig M, Weissenbock H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Howerton CL, Bale TL. Prenatal programing: At the intersection of maternal stress and immune activation. Hormones and behavior. 2012 doi: 10.1016/j.yhbeh.2012.03.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain, behavior, and immunity. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang X, Wang L, Zhang H. Developmental expression of the high mobility group B gene in the amphioxus, Branchiostoma belcheri tsingtauense. The International journal of developmental biology. 2005;49:49–52. doi: 10.1387/ijdb.041915xh. [DOI] [PubMed] [Google Scholar]
- 138.Husband AJ. The immune system and integrated homeostasis. Immunol Cell Biol. 1995;73:377–382. doi: 10.1038/icb.1995.58. [DOI] [PubMed] [Google Scholar]
- 139.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain, behavior, and immunity. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.WMJI, Glaser R. Stress hormones and immune function. Cell Immunology. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 142.Jankowsky JL, Derrick BE, Patterson PH. Cytokine responses to LTP induction in the rat hippocampus: a comparison of in vitro and in vivo techniques. Learning & memory. 2000;7:400–412. doi: 10.1101/lm.32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clinical science. 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 144.Johnson WJ, Marino PA, Schreiber RD, Adams DO. Sequential activation of murine mononuclear phagocytes for tumor cytolysis: differential expression of markers by macrophages in the several stages of development. Journal of immunology (Baltimore, Md: 1950) 1983;131:1038–1043. [PubMed] [Google Scholar]
- 145.Johnston GR, Webster NR. Cytokines and the immunomodulatory function of the vagus nerve. British journal of anaesthesia. 2009;102:453–462. doi: 10.1093/bja/aep037. [DOI] [PubMed] [Google Scholar]
- 146.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Molecular interventions. 2010;10:263–270. doi: 10.1124/mi.10.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits longterm potentiation in the CA3 region of mouse hippocampal slices. European journal of pharmacology. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- 148.Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. Journal of psychiatric research. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 149.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of psychiatric research. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 150.Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Development and psychopathology. 1999;11:525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- 151.Kinoshita M, Hatada S, Asashima M, Noda M. HMG-X, a Xenopus gene encoding an HMG1 homolog, is abundantly expressed in the developing nervous system. FEBS letters. 1994;352:191–196. doi: 10.1016/0014-5793(94)00909-0. [DOI] [PubMed] [Google Scholar]
- 152.Kolodziej A, Schulz S, Guyon A, Wu DF, Pfeiffer M, Odemis V, Hollt V, Stumm R. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koziolek MJ, Vasko R, Bramlage C, Muller GA, Strutz F. The CX(3)C-chemokine fractalkine in kidney diseases. Mini reviews in medicinal chemistry. 2009;9:1215–1228. doi: 10.2174/138955709789055252. [DOI] [PubMed] [Google Scholar]
- 154.Kubota T, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. American journal of physiology. Regulatory, integrative and comparative physiology. 2000;279:R404–413. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 155.Kuijpers M, van Gassen KL, de Graan PN, Gruol D. Chronic exposure to the chemokine CCL3 enhances neuronal network activity in rat hippocampal cultures. Journal of neuroimmunology. 2010;229:73–80. doi: 10.1016/j.jneuroim.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain, behavior, and immunity. 2012;26:201–209. doi: 10.1016/j.bbi.2011.07.233. [DOI] [PubMed] [Google Scholar]
- 157.Lathia JD, Okun E, Tang SC, Griffioen K, Cheng A, Mughal MR, Laryea G, Selvaraj PK, ffrench-Constant C, Magnus T, Arumugam TV, Mattson MP. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:13978–13984. doi: 10.1523/JNEUROSCI.2140-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Roosli M, Schaub B. Exposure to Moderate Air Pollution during Late Pregnancy and Cord Blood Cytokine Secretion in Healthy Neonates. PloS one. 2011;6:e23130. doi: 10.1371/journal.pone.0023130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. Journal of immunology. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- 160.Lax P, Limatola C, Fucile S, Trettel F, Di Bartolomeo S, Renzi M, Ragozzino D, Eusebi F. Chemokine receptor CXCR2 regulates the functional properties of AMPA-type glutamate receptor GluR1 in HEK cells. Journal of neuroimmunology. 2002;129:66–73. doi: 10.1016/s0165-5728(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 161.Liu H, Jiang D. Fractalkine/CX3CR1 and atherosclerosis. Clinica chimica acta; international journal of clinical chemistry. 2011;412:1180–1186. doi: 10.1016/j.cca.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 162.Lord G. Role of leptin in immunology. Nutrition reviews. 2002;60:S35–38. doi: 10.1301/002966402320634913. discussion S68–84, 85–37. [DOI] [PubMed] [Google Scholar]
- 163.Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luscher C, Isaac JT. The synapse: center stage for many brain diseases. The Journal of physiology. 2009;587:727–729. doi: 10.1113/jphysiol.2008.167742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Frontiers in aging neuroscience. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 168.Male D, Rezaie P. Colonisation of the human central nervous system by microglia: the roles of chemokines and vascular adhesion molecules. Progress in brain research. 2001;132:81–93. doi: 10.1016/S0079-6123(01)32067-8. [DOI] [PubMed] [Google Scholar]
- 169.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 170.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in bioscience: a journal and virtual library. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 171.Mattson MP. Awareness of hormesis will enhance future research in basic and applied neuroscience. Critical reviews in toxicology. 2008;38:633–639. doi: 10.1080/10408440802026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Matzinger P. An innate sense of danger. Annals of the New York Academy of Sciences. 2002;961:341–342. doi: 10.1111/j.1749-6632.2002.tb03118.x. [DOI] [PubMed] [Google Scholar]
- 173.Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophrenia bulletin. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- 174.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neuroscience and biobehavioral reviews. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 175.Mehler MF, Marmur R, Gross R, Mabie PC, Zang Z, Papavasiliou A, Kessler JA. Cytokines regulate the cellular phenotype of developing neural lineage species. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 1995;13:213–240. doi: 10.1016/0736-5748(94)00060-g. [DOI] [PubMed] [Google Scholar]
- 176.Merrill JE. Effects of interleukin-1 and tumor necrosis factor-alpha on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Developmental neuroscience. 1991;13:130–137. doi: 10.1159/000112150. [DOI] [PubMed] [Google Scholar]
- 177.Merrill JE. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Developmental neuroscience. 1992;14:1–10. doi: 10.1159/000111642. [DOI] [PubMed] [Google Scholar]
- 178.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Festschrift in Honour of Jeffrey Gray - Issue 2: Schizophrenia and Consciousness. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 179.Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: A link between neurodevelopment and adult psychopathology. Stress, Genetics, and Immunity. 2006;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 180.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain, behavior, and immunity. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 182.Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Williston Park, NY) 2000;14:75–79. discussion 79, 81–72, 85. [PubMed] [Google Scholar]
- 183.Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Autonomic & autacoid pharmacology. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 184.Miller LC, Isa S, LoPreste G, Schaller JG, Dinarello CA. Neonatal interleukin-1β, interleukin-6, and tumor necrosis factor: Cord blood levels and cellular production. The Journal of pediatrics. 1990;117:961–965. doi: 10.1016/s0022-3476(05)80145-3. [DOI] [PubMed] [Google Scholar]
- 185.Milton NG, Hillhouse EW, Milton AS. Activation of the hypothalamo-pituitary-adrenocortical axis in the conscious rabbit by the pyrogen polyinosinic:polycytidylic acid is dependent on corticotrophin-releasing factor-41. The Journal of endocrinology. 1992;135:69–75. doi: 10.1677/joe.0.1350069. [DOI] [PubMed] [Google Scholar]
- 186.Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, Andersen PK, Melbye M. Effects of family history and place and season of birth on the risk of schizophrenia. The New England journal of medicine. 1999;340:603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- 187.Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, Pittman QJ. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:7975–7983. doi: 10.1523/JNEUROSCI.6078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mouihate A, Harre EM, Martin S, Pittman QJ. Suppression of the febrile response in late gestation: evidence, mechanisms and outcomes. Journal of neuroendocrinology. 2008;20:508–514. doi: 10.1111/j.1365-2826.2008.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Muller N, Ackenheil M. Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Progress in neuro-psychopharmacology & biological psychiatry. 1998;22:1–33. doi: 10.1016/s0278-5846(97)00179-6. [DOI] [PubMed] [Google Scholar]
- 191.Muller N, Schwarz MJ. Immune System and Schizophrenia. Current immunology reviews. 2010;6:213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. Journal of internal medicine. 2004;255:332–343. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 193.Murphy K, Travers P, Walport M. Janeway’s Immunobiology. 7. Garland Science; New York: 2008. [Google Scholar]
- 194.Mustafa MM, Lebel MH, Ramilo O, Olsen KD, Reisch JS, Beutler B, McCracken GH., Jr Correlation of interleukin-1 beta and cachectin concentrations in cerebrospinal fluid and outcome from bacterial meningitis. The Journal of pediatrics. 1989;115:208–213. doi: 10.1016/s0022-3476(89)80067-8. [DOI] [PubMed] [Google Scholar]
- 195.Myatt L. Placental adaptive responses and fetal programming. The Journal of physiology. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 197.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. The European journal of neuroscience. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- 199.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, behavior, and immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nawa H, Takei N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neuroscience research. 2006;56:2–13. doi: 10.1016/j.neures.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 201.Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Current opinion in neurology. 2000;13:133–139. doi: 10.1097/00019052-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 202.Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, Hunter KD, Graham GJ. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. The Journal of clinical investigation. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nicolai J, Burbassi S, Rubin J, Meucci O. CXCL12 inhibits expression of the NMDA receptor’s NR2B subunit through a histone deacetylase-dependent pathway contributing to neuronal survival. Cell death & disease. 2010;1:e33. doi: 10.1038/cddis.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 205.Oh-Nishi A, Obayashi S, Sugihara I, Minamimoto T, Suhara T. Maternal immune activation by polyriboinosinic-polyribocytidilic acid injection produces synaptic dysfunction but not neuronal loss in the hippocampus of juvenile rat offspring. Brain research. 2010;1363:170–179. doi: 10.1016/j.brainres.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 206.Ortega A, Jadeja V, Zhou H. Postnatal development of lipopolysaccharide-induced inflammatory response in the brain. Inflammation research: official journal of the European Histamine Research Society …[et al.] 2010 doi: 10.1007/s00011-010-0252-y. [DOI] [PubMed] [Google Scholar]
- 207.Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour, Prenatal Programming of Behavior. Physiology and Cognition. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 208.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biological psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 209.Pace JL, Russell SW, Torres BA, Johnson HM, Gray PW. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. Journal of immunology (Baltimore, Md: 1950) 1983;130:2011–2013. [PubMed] [Google Scholar]
- 210.Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain, behavior, and immunity. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 211.Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Annals of the New York Academy of Sciences. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Palin K, McCusker RH, Strle K, Moos F, Dantzer R, Kelley KW. Tumor necrosis factor-alpha-induced sickness behavior is impaired by central administration of an inhibitor of c-jun N-terminal kinase. Psychopharmacology. 2008;197:629–635. doi: 10.1007/s00213-008-1086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pan W, Kastin AJ. Upregulation of the transport system for TNFalpha at the blood-brain barrier. Archives of Physiology and Biochemistry. 2001;109:350–353. doi: 10.1076/apab.109.4.350.4238. [DOI] [PubMed] [Google Scholar]
- 214.Pan W, Kastin AJ. Cytokine transport across the injured blood-spinal cord barrier. Current pharmaceutical design. 2008;14:1620–1624. doi: 10.2174/138161208784705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pan W, Zhang L, Liao J, Csernus B, Kastin AJ. Selective increase in TNF alpha permeation across the blood-spinal cord barrier after SCI. Journal of neuroimmunology. 2003;134:111–117. doi: 10.1016/s0165-5728(02)00426-5. [DOI] [PubMed] [Google Scholar]
- 216.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Developmental Brain Research. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 217.Pantelis C, Yucel M, Wood SJ, McGorry PD, Velakoulis D. Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. The Australian and New Zealand Journal of Psychiatry. 2003;37:399–406. doi: 10.1046/j.1440-1614.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- 218.Pasca AMPAA. The Placenta: The Lost Neuroendocrine Organ. Neonatology. 2010;11:64–75. [Google Scholar]
- 219.Pathipati P, Gorba T, Scheepens A, Goffin V, Sun Y, Fraser M. Growth hormone and prolactin regulate human neural stem cell regenerative activity. Neuroscience. 2011;190:409–427. doi: 10.1016/j.neuroscience.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 220.Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Molecular psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- 221.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain, behavior, and immunity. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 222.Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 223.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nature reviews. Neuroscience. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 224.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature reviews. Neurology. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 225.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 226.Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Research Reviews. 2010;63:39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 227.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 228.Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behavioural brain research. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 229.Pujol F, Kitabgi P, Boudin H. The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. Journal of cell science. 2005;118:1071–1080. doi: 10.1242/jcs.01694. [DOI] [PubMed] [Google Scholar]
- 230.Quan N, Banks WA. Brain-immune communication pathways. Brain, behavior, and immunity. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 231.Rabin BS, Ganguli R, Lysle DT, Cunnick JE. Interaction between the brain and the immune system. Immunology series. 1990;52:125–154. [PubMed] [Google Scholar]
- 232.Rantakallio P, Jones P, Moring J, Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. International journal of epidemiology. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- 233.Rauvala H, Pihlaskari R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. The Journal of biological chemistry. 1987;262:16625–16635. [PubMed] [Google Scholar]
- 234.Redelman D, Welniak LA, Taub D, Murphy WJ. Neuroendocrine hormones such as growth hormone and prolactin are integral members of the immunological cytokine network. Cellular immunology. 2008;252:111–121. doi: 10.1016/j.cellimm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rezaie P, Male D. Mesoglia & microglia--a historical review of the concept of mononuclear phagocytes within the central nervous system. Journal of the history of the neurosciences. 2002;11:325–374. doi: 10.1076/jhin.11.4.325.8531. [DOI] [PubMed] [Google Scholar]
- 236.Rezaie P, Patel K, Male DK. Microglia in the human fetal spinal cord--patterns of distribution, morphology and phenotype. Brain research. Developmental brain research. 1999;115:71–81. doi: 10.1016/s0165-3806(99)00043-7. [DOI] [PubMed] [Google Scholar]
- 237.Rivest S. Molecular insights on the cerebral innate immune system. Brain, behavior, and immunity. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 238.Rivest S. Interactions between the immune and neuroendocrine systems. Progress in brain research. 2010;181:43–53. doi: 10.1016/S0079-6123(08)81004-7. [DOI] [PubMed] [Google Scholar]
- 239.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Hormones and behavior. 2012 doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nature cell biology. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 241.Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. Journal of neuroimmunology. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 242.Rostene W, Dansereau MA, Godefroy D, Van Steenwinckel J, Reaux-Le Goazigo A, Melik-Parsadaniantz S, Apartis E, Hunot S, Beaudet N, Sarret P. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. Journal of neurochemistry. 2011;118:680–694. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- 243.Rostene W, Guyon A, Kular L, Godefroy D, Barbieri F, Bajetto A, Banisadr G, Callewaere C, Conductier G, Rovere C, Melik-Parsadaniantz S, Florio T. Chemokines and chemokine receptors: new actors in neuroendocrine regulations. Frontiers in neuroendocrinology. 2011;32:10–24. doi: 10.1016/j.yfrne.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 244.Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends in neurosciences. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 245.Rotondo D, Abul HT, Milton AS, Davidson J. Pyrogenic immunomodulators increase the level of prostaglandin E2 in the blood simultaneously with the onset of fever. European journal of pharmacology. 1988;154:145–152. doi: 10.1016/0014-2999(88)90091-x. [DOI] [PubMed] [Google Scholar]
- 246.Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurobiology of Cognition in Laboratory Animals: Challenges and Opportunites. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 247.Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, affective & behavioral neuroscience. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- 248.Salmenpera T, Kalviainen R, Partanen K, Pitkanen A. Hippocampal damage caused by seizures in temporal lobe epilepsy. Lancet. 1998;351:35. doi: 10.1016/S0140-6736(05)78092-2. [DOI] [PubMed] [Google Scholar]
- 249.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 250.Sasaki A, Yokoo H, Naito M, Kaizu C, Shultz LD, Nakazato Y. Effects of macrophage-colony-stimulating factor deficiency on the maturation of microglia and brain macrophages and on their expression of scavenger receptor. Neuropathology: official journal of the Japanese Society of Neuropathology. 2000;20:134–142. doi: 10.1046/j.1440-1789.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 251.Saugstad LF. Infantile autism: a chronic psychosis since infancy due to synaptic pruning of the supplementary motor area. Nutrition and health. 2011;20:171–182. doi: 10.1177/026010601102000402. [DOI] [PubMed] [Google Scholar]
- 252.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clinical and experimental pharmacology & physiology. 1999;26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- 253.Saunders NR, Knott GW, Dziegielewska KM. Barriers in the immature brain. Cellular and molecular neurobiology. 2000;20:29–40. doi: 10.1023/a:1006991809927. [DOI] [PubMed] [Google Scholar]
- 254.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schwarting GA, Henion TR, Nugent JD, Caplan B, Tobet S. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:6834–6840. doi: 10.1523/JNEUROSCI.1728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schwarz JM, Bilbo SD. LPS elicits a much larger and broader inflammatory response than Escherichia coli infection within the hippocampus of neonatal rats. Neuroscience letters. 2011;497:110–115. doi: 10.1016/j.neulet.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Schwarz JM, Bilbo SD. Sex, glia, and development: Interactions in health and disease. Hormones and behavior. 2012 doi: 10.1016/j.yhbeh.2012.02.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. Journal of neurochemistry. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sharif A, Prevot V. ErbB receptor signaling in astrocytes: a mediator of neuron-glia communication in the mature central nervous system. Neurochemistry international. 2010;57:344–358. doi: 10.1016/j.neuint.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 261.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain, behavior, and immunity. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012 doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- 265.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder, American journal of medical genetics. Part B. Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2011 doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Somera-Molina KC, Robin B, Somera CA, Anderson C, Stine C, Koh S, Behanna HA, Van Eldik LJ, Watterson DM, Wainwright MS. Glial activation links early-life seizures and long-term neurologic dysfunction: evidence using a small molecule inhibitor of proinflammatory cytokine upregulation. Epilepsia. 2007;48:1785–1800. doi: 10.1111/j.1528-1167.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- 267.Sorce S, Myburgh R, Krause KH. The chemokine receptor CCR5 in the central nervous system. Progress in neurobiology. 2011;93:297–311. doi: 10.1016/j.pneurobio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 268.Spulber S, Bartfai T, Schultzberg M. IL-1/IL-1ra balance in the brain revisited - evidence from transgenic mouse models. Brain, behavior, and immunity. 2009;23:573–579. doi: 10.1016/j.bbi.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 269.Spulber S, Mateos L, Oprica M, Cedazo-Minguez A, Bartfai T, Winblad B, Schultzberg M. Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. Journal of neuroimmunology. 2009;208:46–53. doi: 10.1016/j.jneuroim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 270.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 271.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annual Review of Neuroscience. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 272.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 273.Stipursky J, Romao L, Tortelli V, Neto VM, Gomes FC. Neuron-glia signaling: Implications for astrocyte differentiation and synapse formation. Life Sciences. 2011;89:524–531. doi: 10.1016/j.lfs.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 274.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD National Institute of Child H Human Development Neonatal Research N. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA: the journal of the American Medical Association. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 275.Streit WJ. Microglia and the response to brain injury. Ernst Schering Research Foundation workshop. 2002:11–24. doi: 10.1007/978-3-662-05073-6_2. [DOI] [PubMed] [Google Scholar]
- 276.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 277.Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurological research. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- 279.Streit WJ, Xue QS. Life and death of microglia. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 280.Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sullivan SM, Sullivan RK, Miller SM, Ireland Z, Bjorkman ST, Pow DV, Colditz PB. Phosphorylation of GFAP is Associated with Injury in the Neonatal Pig Hypoxic-Ischemic Brain. Neurochemical research. 2012 doi: 10.1007/s11064-012-0774-5. in press. [DOI] [PubMed] [Google Scholar]
- 282.Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Developmental cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 283.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. Journal of neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. The Journal of comparative neurology. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- 286.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Communicative & integrative biology. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The Role of Microglia in the Healthy Brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiological Reviews. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 290.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophrenia research. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 291.Veenstra M, Ransohoff RM. Chemokine receptor CXCR2: physiology regulator and neuroinflammation controller? Journal of neuroimmunology. 2012;246:1–9. doi: 10.1016/j.jneuroim.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. The Journal of biological chemistry. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- 293.Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain, behavior, and immunity. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 294.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain, behavior, and immunity. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 295.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 296.Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? Journal of neurochemistry. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- 297.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Walker FR, Hodyl NA, Krivanek KM, Hodgson DM. Early life host-bacteria relations and development: long-term individual differences in neuroimmune function following neonatal endotoxin challenge. Physiology & Behavior. 2006;87:126–134. doi: 10.1016/j.physbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 299.Walker FR, Knott B, Hodgson DM. Neonatal endotoxin exposure modifies the acoustic startle response and circulating levels of corticosterone in the adult rat but only following acute stress. Journal of psychiatric research. 2008;42:1094–1103. doi: 10.1016/j.jpsychires.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 300.Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behavioural brain research. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 301.Wang C-C, Wu C-H, Shieh J-Y, Wen C-Y. Microglial distribution and apoptosis in fetal rat brain. Developmental Brain Research. 2002;139:337–342. doi: 10.1016/s0165-3806(02)00584-9. [DOI] [PubMed] [Google Scholar]
- 302.Wang CC, Wu CH, Shieh JY, Wen CY. Microglial distribution and apoptosis in fetal rat brain. Brain research. Developmental brain research. 2002;139:337–342. doi: 10.1016/s0165-3806(02)00584-9. [DOI] [PubMed] [Google Scholar]
- 303.Westbrook RF, Good AJ, Kiernan MJ. Effects of the interval between exposure to a novel environment and the occurrence of shock on the freezing responses of rats, The Quarterly journal of experimental psychology.B. Comparative and physiological psychology. 1994;47:427–446. [PubMed] [Google Scholar]
- 304.Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- 305.Wiese S, Karus M, Faissner A. Astrocytes as a Source for Extracellular Matrix Molecules and Cytokines. Frontiers in pharmacology. 2012;3:120. doi: 10.3389/fphar.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Williamson LL, Chao A, Bilbo SD. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain, behavior, and immunity. 2012;26:500–510. doi: 10.1016/j.bbi.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Williamson LL, Sholar PW, Mistry RM, Smith SH, Bilbo SD. Microglia and Memory: Modulation by Early-Life Infection. Journal of Neuroscience. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2009;12:513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- 309.Wright RJ. Perinatal stress and early life programming of lung structure and function. Biol Psychol. 2010;84:46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 311.Wuchert F, Ott D, Murgott J, Rafalzik S, Hitzel N, Roth J, Gerstberger R. Rat area postrema microglial cells act as sensors for the toll-like receptor-4 agonist lipopolysaccharide. Journal of neuroimmunology. 2008;204:66–74. doi: 10.1016/j.jneuroim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 312.Xu J, Kaur C, Ling EA. Variation with age in the labelling of amoeboid microglial cells in rats following intraperitoneal or intravenous injection of a fluorescent dye. Journal of anatomy. 1993;182(Pt 1):55–63. [PMC free article] [PubMed] [Google Scholar]
- 313.Yang SN, Yang JM, Wu JN, Kao YH, Hsieh WY, Chao CC, Tao PL. Prenatal exposure to morphine alters kinetic properties of NMDA receptor-mediated synaptic currents in the hippocampus of rat offspring. Hippocampus. 2000;10:654–662. doi: 10.1002/1098-1063(2000)10:6<654::AID-HIPO1003>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 314.Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont (Pa: Township)) 2009;6:18–22. [PMC free article] [PubMed] [Google Scholar]
- 316.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 317.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. American Journal of Obstetrics and Gynecology. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 318.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Neuronal and glial cell biology: New technologies. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 319.Zhao X, Kuja-Panula J, Rouhiainen A, Chen YC, Panula P, Rauvala H. High mobility group box-1 (HMGB1; amphoterin) is required for zebrafish brain development. The Journal of biological chemistry. 2011;286:23200–23213. doi: 10.1074/jbc.M111.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nature neuroscience. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 322.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 323.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. Journal of psychiatric research. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]