Abstract
Short sleep duration has been associated with higher current body mass index (BMI) and subsequent weight gain. However, most prior longitudinal studies are limited by reliance on self-reported sleep duration, and none accounted for the potential confounding effect of sleep-disordered breathing. The associations of sleep duration with current BMI and BMI change were examined among 310 midlife women in the Study of Women’s Health Across the Nation (SWAN) Sleep Study (2003–2005). Sleep duration was assessed for approximately one month with concurrent wrist actigraphy and sleep diaries. The presence and severity of sleep-disordered breathing was quantified using the apnea-hypopnea index (AHI) based on in-home polysomnography. BMI was assessed annually through core SWAN visit 10 (2006 and 2008). Mean BMI increased from 29.6 (SD=7.8) kg/m2 to 30.0 (SD=8.0) kg/m2 over an average of 4.6 years (SD=1.0) of follow up. In cross-sectional analyses controlling for AHI, demographic variables, and several potential confounding variables, actigraphy (estimate=−1.22, 95%C.I.: −2.03, −.42) and diary (estimate=−.86, 95%C.I. −1.62, −.09) measures of sleep duration were inversely associated with BMI. Each hour of less sleep was associated with 1.22 kg/m2 greater BMI for actigraphy sleep duration, and a 0.86 kg/m2 greater BMI for diary sleep duration. Longitudinal associations between sleep duration and annual BMI change were non-significant in unadjusted and fully-adjusted models. In this cohort of midlife women, cross-sectional associations between sleep duration and current BMI were independent of sleep-disordered breathing, but sleep duration was not prospectively associated with weight change.
Identification of novel risk factors for weight gain may inform efforts to curb the ongoing obesity epidemic. One factor that has recently received increased attention is short sleep duration. Cross-sectional associations between short sleep duration and adiposity have been consistently found among children and adults, with short sleepers having 55%–89% higher odds of overweight or obesity (1,2). As much of the epidemiological literature consists of cross-sectional analyses, which cannot determine temporality, considerable debate remains as to whether short sleep duration may causally contribute to weight gain. Prospective studies in children have repeatedly found that short sleep duration predicts subsequent weight gain and risk of becoming overweight (3,4). Findings have been less consistent in prospective studies of adults (5,6). Of 14 published studies, 6 have reported associations between short sleep duration and subsequent weight gain in all subgroups examined (7–12), 4 found associations only in selected subgroups (13–16), and 4 found no association (17–20).
The inconsistency across prior prospective studies in adults may stem from several factors. All but one prior study (21) relied on self-reported sleep duration, usually measured with a single survey item, rather than objective measures such as sleep actigraphy (19). Additionally, very few studies have accounted for the presence of sleep-disordered breathing, and those that did relied on self-reported sleep-disordered breathing symptoms (e.g., snoring, “difficulty staying asleep”) rather than objective criteria such as the frequency of apneas and hypopneas observed during polysomnography. This is a critical limitation considering that obesity is a principal risk factor for sleep-disordered breathing (22), and the development and progression of sleep-disordered breathing represents a possible reverse causal path that must be ruled out to establish an influence of sleep duration on weight gain.
The Study of Women’s Health Across the Nation (SWAN) Sleep Study provides an opportunity to examine the prospective association between short sleep duration and subsequent weight change in midlife women, a group in which both sleep disturbance (23) and weight gain (24) have been documented. The present study overcame the major limitations of prior research by assessing sleep duration over approximately one month using both sleep actigraphy and sleep diaries. The presence of sleep-disordered breathing was also determined objectively through polysomnography. Analyses tested the hypotheses that shorter sleep duration would be cross-sectionally associated with higher BMI, and prospectively associated with a higher rate of weight gain, among midlife women, independent of sleep-disordered breathing.
Methods
Participants
The Study of Women’s Health Across the Nation (SWAN) is an ongoing multiracial/ethnic, multi-site cohort study focused on the identification of health determinants across the menopausal transition. Annual assessments have been conducted in a multiethnic cohort of 3,302 women since 1996. The SWAN Sleep Study is an ancillary cross-sectional study that occurred between 2003 and 2005 (coinciding with SWAN Core visits 5, 6 or 7) at four of the seven SWAN sites (Chicago, IL, Detroit area, MI, Oakland, CA, and Pittsburgh, PA). Women in the Core SWAN cohort were recruited for the SWAN Sleep Study if they were premenopausal or early perimenopausal, and had an intact uterus and at least one ovary. Eligibility criteria were later relaxed to include postmenopausal women. Women were excluded from the SWAN Sleep Study if they were using hormone replacement therapy or due to factors known to affect sleep, including shift/night work, active cancer treatment, oral corticosteroid use, or regular alcohol consumption exceeding 4 drinks per day. Additional eligibility criteria for the SWAN Core Study and SWAN Sleep Study have been described elsewhere (25). Institutional approval for the use of human subjects was obtained at each study site, and all participants provided written informed consent. Of the 370 African-American, Chinese, and Caucasian women between ages 48 and 59 who enrolled in the SWAN Sleep Study, data from 310 were available for analysis.
Measures
Body mass index (BMI)
BMI was calculated as weight(kg)/height2(m) from measurements taken at SWAN Core annual assessments. Analyses included BMI data from all assessments between the year of the SWAN Sleep Study (between 2003 and 2005) and Core SWAN visit 10, which was conducted between 2006 and 2008. BMI at the annual assessment that occurred closest in time to the SWAN Sleep Study (either before or after) was treated as the initial BMI observation.
Sleep measures
Participants completed a sleep assessment protocol that included three nights of in-home polysomnography to assess sleep-disordered breathing, followed by concurrent sleep actigraphy and sleep diary assessment for the shorter duration of either one menstrual cycle or 35 days. Analyses involving actigraphy were limited to days 4 through 30 of the sleep protocol because of the potential effect of the polysomnography sensors on movement during sleep, and because data were sparse after day 30. The sleep protocol began within 7 days of the onset of menstruation in menstruating women; non-menstruating women were scheduled at their convenience.
On the first night of the sleep protocol, study staff applied electrodes and calibrated polysomnography monitoring equipment (Vitaport-3 [TEMEC VP3]) in participants’ homes. Signals collected during polysomnography included central referential electroencephalography (channels C3 and C4 referenced to A1 + A2), bilateral electro-oculogram, submentalis electromyogram, electrocardiography, nasal pressure cannula, oral-nasal thermistor, inductance plethysmography of abdominal and thoracic respiratory effort, fingertip oximetry, and bilateral electromyogram of the anterior tibialis to assess periodic limb movement (26). Sleep stages were visually scored in 20-second epochs by trained polysomnography technicians according to standard criteria (27). The apnea-hypopnea index (AHI) was calculated as the number of complete (apneas) and partial (hypopneas) reductions in airflow from baseline, lasting at least 10 seconds, observed per hour (28). A detailed description of polysomnography scoring procedures in the SWAN Sleep Study can be found elsewhere (25,29).
Sleep diaries were completed each night immediately before going to bed and each morning upon waking throughout the sleep protocol. At bedtime, participants recorded the number of cigarettes and caffeinated and alcoholic beverages consumed that day. Morning sleep diaries assessed the times when participants got into bed, tried to fall asleep, fell asleep, woke up, and got out of bed, as well the frequency and duration of any nocturnal awakenings. Sleep duration was calculated as the difference between reported sleep onset and wake time, minus any time spent during nocturnal awakenings. Diary sleep duration was calculated using weighted averages from weekdays (weight=5/7 or .714) and weekend days (weight=2/7 or .286) to account for variability in the number of weekdays recorded during the sleep protocol.
Participants wore an actigraph (Actiwatch-64, MiniMitter, Respironics, Bend OR), a device similar to a wrist watch that contains a sensitive accelerometer that records wrist movements, on each night of the sleep protocol. Wrist actigraphy data was processed in one-minute epochs with Actiware version 5.04 software. An epoch was scored as sleep when the weighted sum of activity counts in that epoch (weight=1), the immediately adjacent epochs (weight=.20), and next two adjacent epochs (weight=.04) was ≤40. Bed times and wake times from the sleep diaries were used to define the suspected sleep period, and sleep time was calculated as the number of minutes scored as sleep within the suspected sleep period. Actigraphy sleep duration was calculated as the weighted average of sleep times on weekdays (weight=5/7, or .714) and weekend days (weight=2/7, or .286) throughout the sleep protocol.
Covariates
Covariates were selected based on their potential to influence sleep, BMI, or their longitudinal associations. Person-level covariates documented at SWAN baseline were age at the time of the sleep study (derived from date of birth), study site, education level, and self-reported African-American, Chinese, or Caucasian race/ethnicity. Time-varying covariates, which were assessed during Core SWAN assessments, included current smoking status (yes/no), menopausal status, and physical activity. Menopausal status was determined by menstrual bleeding patterns (pre-/ early peri-menopausal, late peri-menopausal, post-menopausal). Women taking hormone therapy (n=17), which affects menstruation, were categorized as having undetermined menopausal status. Engagement in non-occupational physical activity was assessed in Core SWAN visits 6 and 9 only using a composite index of 5-point Likert and ordinal quantitative scales (30), with higher values indicating more frequent engagement in physical activity. Mean daily use of cigarettes and consumption of alcoholic and caffeinated drinks during the sleep protocol were also included as covariates.
Statistical analysis
Descriptive statistics were used to characterize the sample and evaluate variable distributions for outliers. The mean values for the two sleep duration variables differed from one another, so it was not possible to create meaningful cutpoints for sleep duration that would have been comparable across measures. Therefore, sleep duration was treated continuously in all analyses. As fewer than 5% of women had sleep durations exceeding 8 hours on both sleep measures, we did not explore nonlinear relations in which both short and long sleep duration might predict increased weight change. The variable distribution of AHI was skewed, so participants were categorized into groups reflecting absent (<5), mild (≥5 to <15), and moderate-to-severe (≥15) sleep-disordered breathing (31). Race/ethnicity, study site, menopausal status, and smoking status were treated as categorical variables. Education was treated as ordinal data with five levels.
Cross-sectional associations between sleep duration and initial BMI were tested using linear regression. Linear mixed models were used to test longitudinal associations between sleep duration at the time of the SWAN Sleep Study and change in BMI across subsequent annual Core SWAN assessments. The dependent variable in these models was each woman’s observed BMI at each annual Core SWAN assessment occurring after baseline. Longitudinal change in BMI was modeled with the main effect of time, which was measured in years since the initial BMI assessment. The sleep by time interaction term captured the degree to which longitudinal change in BMI varies according to sleep duration. Models featured random intercepts and slopes to account for between-person variability in both baseline BMI and BMI change over time. Three sets of longitudinal models were calculated. Unadjusted models tested the association between sleep duration and subsequent BMI change trajectories. A second set of linear mixed models controlled for initial BMI to test whether sleep duration explained variability in BMI change trajectories beyond that already explained by women’s initial BMI. A third set of fully-adjusted models controlled for initial BMI and all other covariates. Effect modification of the cross-sectional and longitudinal associations between sleep duration and BMI by AHI category was tested in exploratory models. The studentized residuals in all models were approximately normally distributed. Analyses were peformed in SAS 9.2 (SAS Institute, Cary, NC).
Results
The analyzed sample was composed of 116 African-American, 55 Chinese, and 139 Caucasian women with a mean age of 49.7 (SD=2.0) years (Table 1). The average length of follow up for BMI change was 4.6 years (SD=1.0). Ninety percent of women completed the initial BMI assessment within ±60 days of the sleep protocol (M=6.7 days prior, SD=75.2 days). The percentage of participants providing 5, 4, and 3 or fewer longitudinal BMI observations were 84.1%, 11.0%, and 4.9%, respectively. Across the sample, mean BMI increased from 29.6 (SD=7.8) kg/m2 at the time of SWAN Sleep Study to 30.0 (SD=8.0) kg/m2 at the most recent follow up, which corresponds to a mean increase of approximately 0.08 (SD=0.53) kg/m2 per year. The 25th, 50th, and 75th percentiles of BMI change across the entire follow up period were −.55, .56, and 1.54 kg/m2, respectively.
Table 1.
Sample characteristics for SWAN Sleep Study participants included in analyses
| Overall (N=310) | |
|---|---|
| Mean (SD) | |
| BMI at SWAN Sleep Study (kg/m2) | 29.6 (7.8) |
| BMI at last SWAN follow up (kg/m2) | 30.0 (8.0) |
| Age at SWAN Sleep Study (y) | 49.7 (2.0) |
| AHI (events/h) | 8.1 (9.3) |
| Total sleep duration (h/day) | |
| Actigraphy | 5.9 (1.0) |
| Sleep diary | 6.7 (0.9) |
| Physical activity levela | 2.8 (1.1) |
| Cigarette use during sleep protocol (per day) | 0.7 (2.7) |
| Alcohol use during sleep protocol (alcoholic drinks/day) | 0.3 (0.5) |
| Caffeine use during sleep protocol (caffeinated drinks/day) | 1.5 (1.4) |
| n (%) | |
| Study site | |
| Pittsburgh, PA | 79 (25.5) |
| Detroit area, MI | 64 (20.6) |
| Chicago, IL | 67 (21.6) |
| Oakland, CA | 100 (32.3) |
| Race/ethnicity | |
| African-American | 116 (37.4) |
| Chinese | 55 (17.7) |
| Caucasian | 139 (44.8) |
| Menopausal status at SWAN Sleep Study | |
| Pre-/early peri-menopausal | 196 (63.2) |
| Late peri-menopausal | 64 (20.7) |
| Post-menopausal | 33 (10.7) |
| Undetermined | 17 (5.5) |
| Current smoker at SWAN Sleep Study | 27 (8.7) |
Abbreviations: AHI, apnea-hypopnea index; BMI, body mass index; SD, standard deviation.
Averaged across available years of follow up.
Diary sleep duration (M=6.7 h/day, SD=0.9 h/day) was calculated for 310 participants based on an average of 26.7 (SD=4.9) nights of diary data. Actigraphy sleep duration (M=5.9 h/day, SD=1.0 h/day) was calculated for 310 participants based on an average of 23.8 (SD=4.8) nights of actigraphy data. The correlation between the sleep duration measures was r=.52 (p<.0001). Of 294 participants with available polysomnography data, 142 (48.3%) did not have sleep-disordered breathing, 112 (38.1%) had mild sleep-disordered breathing, and 40 (13.6%) had moderate-to-severe sleep-disordered breathing based on AHI score. AHI was positively correlated with BMI (r(292)=.32, p<.0001) and inversely related to actigraphy sleep duration (r(292)= −.17, p=.003), but was not associated with diary sleep duration (r(292)= −.10, p=.09).
Cross-sectional analyses
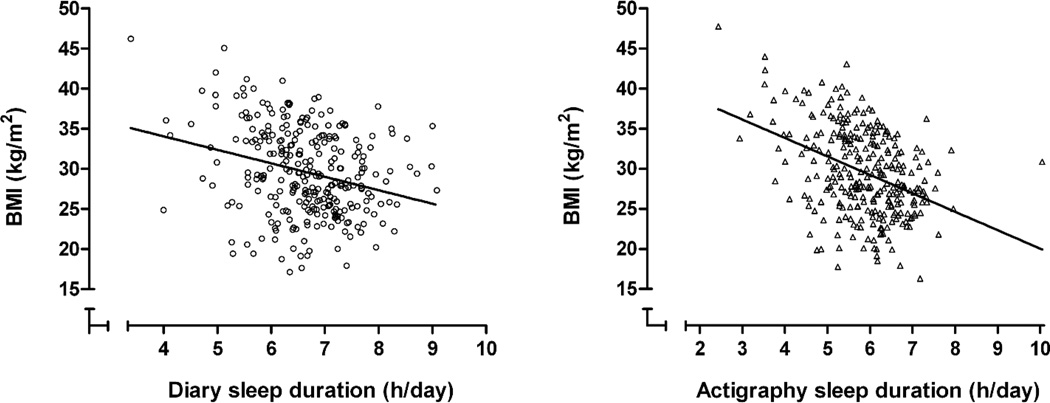
Higher BMI was associated with shorter sleep duration as measured by both actigraphy and diary in unadjusted cross-sectional models (Table 2, Figure 1). These associations were somewhat attenuated in fully adjusted models, but remained significant at the p<.05 level. After adjustment for covariates, each hour of less sleep was associated with 1.22 kg/m2 greater BMI for actigraphy sleep duration, and a 0.86 kg/m2 greater BMI for diary sleep duration.
Table 2.
Cross-sectional linear regression models of BMI (kg/m2).
| Actigraphy | Diary | |||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Unadjusted model | ||||
| Sleep duration (h/day) | −1.961 (−2.833,−1.089) | <.0001 | −1.298 (−2.280, −.316) | .001 |
| Adjusted model | ||||
| Sleep duration (h/day) | −1.223 (−2.030,−.416) | .003 | −.856 (−1.620,−.092) | .03 |
| AHI category (per level) | 1.971 (.935,3.007) | .0002 | 2.251 (1.215,3.287) | <.0001 |
| Age (years) | .196 (−.177,.570) | .30 | .165 (−.215,.544) | .39 |
| Study site | ||||
| Pittsburgh, PA | 0 | 0 | ||
| Detroit area, MI | 2.282 (.192,4.371) | .03 | 2.934 (.849,5.018) | .006 |
| Chicago, IL | −1.369 (−3.472,.733) | .20 | −1.541 (−3.656,.575) | .15 |
| Oakland, CA | −.574 (−3.056,1.908) | .65 | −.184 (−2.699,2.332) | .89 |
| Race/ethnicity | ||||
| Caucasian | 0 | 0 | ||
| African-American | 2.561 (.578,4.544) | .01 | 3.474 (1.550,5.398) | .0004 |
| Chinese | −6.969 (−9.531,−4.407) | <.0001 | −7.195 (−9.796, −4.594) | <.0001 |
| Menopausal status | ||||
| Pre-/early peri-menopausal | 0 | 0 | ||
| Late peri-menopausal | .018 (−1.857, 1.894) | .99 | .159 (−1.742, 2.061) | .87 |
| Post-menopausal | .829 (−1.539, 3.196) | .49 | .058 (−2.281,2.397) | .96 |
| Undetermined | .775 (−3.975,2.425) | .63 | .845 (−2.079,.3.769) | .57 |
| Education (per level) | .189 (−.514,.893) | .60 | .185 (−.529,.899) | .61 |
| Cigarette use during sleep protocol (per day) | −.198 (−.548,.153) | .27 | −.225 (−.578,.127) | .21 |
| Alcohol use during sleep protocol (drinks/day) | −2.233 (−3.919,−.548) | .01 | −2.401 (−4.105,−.696) | .006 |
| Caffeine intake during sleep protocol (drinks/day) | .363 (−.241,.967) | .24 | .455 (−.154,1.063) | .14 |
| Current smoker at SWAN Sleep Study | −2.203(−5.496,1.091) | .19 | −2.738 (−5.955,0.480) | .10 |
| Physical activity levela | −1.911(−2.562,−1.262) | <.0001 | −1.836 (−2.488,−1.183) | <.0001 |
Abbreviations: CI=confidence interval.
Measured at core SWAN assessment 6.
Figure.
Cross-sectional associations between sleep duration and BMI.
Exploratory models tested for effect modification of the association between sleep duration and BMI by AHI category. The sleep duration X AHI interaction term, in units of kg/m2 per h/day of sleep per AHI category, was not significantly related to BMI in adjusted cross-sectional models with either sleep measure (actigraphy, estimate=−.14, 95%C.I.: −1.16,.89; diary, estimate=.30, 95%C.I.: −.82,1.42).
Longitudinal analyses
Annual BMI change was found to be negatively related to initial BMI such that each additional kg/m2 at the time of the SWAN Sleep Study was associated with a −.46 kg/m2 (95%C.I.: −.87, −.06) lower rate of annual BMI change. Therefore, random slopes for the effect of time were incorporated to model each woman’s annual BMI change independent of initial BMI.
The association between sleep duration and longitudinal BMI change, represented as the interactions of actigraphy and diary sleep duration with time, were not significant in unadjusted (actigraphy, estimate=.02, 95%C.I.: −.04,.09; diary, estimate=−.03, 95%C.I.: −.11,.04), partially-adjusted (actigraphy, estimate=.03, 95%C.I.:−.05,.11; diary, estimate=−.06, 95%C.I.: −.14,.03), or fully-adjusted models (actigraphy, estimate=.06, 95%C.I.: −.03,.14; diary, estimate=−.04, 95%C.I.: −.13,.04) (Table 3). Study site was the only covariate that remained significantly associated with annual BMI change in the fully-adjusted model. Given the observed variability in annual BMI change and α=.05, the sample size of N=310 women provided .80 power to detect associations between sleep duration and BMI change as small as 0.078 kg/m2 per year for every additional hour of actigraphy sleep, and as small as 0.080 kg/m2 per year for every additional hour of diary sleep.
Table 3.
Linear mixed models testing longitudinal associations between sleep duration and annual change in BMI (kg/m2).
| Actigraphy | Diary | |||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Unadjusted modela | ||||
| Sleep duration (h/day) | −1.976 (−2.843,−1.109) | <.0001 | −1.234 (−2.211,−.257) | .01 |
| Time (y) | −.034 (−.437,.368) | .87 | .329 (−.171,.828) | .20 |
| Sleep duration (h/day) X Time (y) | .024 (−.043,.091) | .48 | −.033 (−.107,.041) | .38 |
| Partially-adjusted modelb | ||||
| Sleep duration (h/day) | −.081 (−.282,.119) | .43 | .106 (−.110,.323) | .34 |
| Time (y) | −.045 (−.503,.413) | .85 | .495 (−.072,1.063) | .09 |
| Initial BMI (kg/m2) | .975 (.953,.997) | <.0001 | .977 (.956,.999) | <.0001 |
| Sleep duration (h/day) X Time (y) | .029 (−.048,.106) | .46 | −.055 (−.140,.029) | .20 |
| Fully-adjusted modelb | ||||
| Sleep duration (h/day) | −.088 (−.319,.143) | .46 | .127 (−.105,.359) | .28 |
| Time (y) | −.226 (−.723,.271) | .37 | .399 (−.197,.995) | .19 |
| Initial BMI (kg/m2) | .963 (.934,.993) | <.0001 | .965 (.936,.994) | <.0001 |
| Sleep duration (h/day) X Time (y) | .057 (−.025,.139) | .17 | −.044 (−.132,.043) | .32 |
| AHI category (per level) | −.197 (−.466,.073) | .15 | −.182 (−.450,.085) | .18 |
| Age (years) | .025 (−.065,.115) | .58 | .029 (−.061,.119) | .53 |
| Study site | ||||
| Pittsburgh, PA | 0 | 0 | ||
| Detroit area, MI | −.845 (−1.374,−.316) | .002 | −.844 (−1.374,−.315) | .002 |
| Chicago, IL | −.138 (−.674,.399) | .62 | −.134 (−.671,.402) | .62 |
| Oakland, CA | −.173 (−.791,.446) | .58 | −.190 (−.810,.431) | .55 |
| Race/ethnicity | ||||
| Caucasian | 0 | 0 | ||
| African-American | .388 (−.122,.898) | .14 | .412 (−.084,.907) | .10 |
| Chinese | −.514 (−1.174,.146) | .13 | −.476 (−1.137,.186) | .16 |
| Education (per level) | −.111 (−.290,.068) | .22 | −.117 (−.295,.062) | .20 |
| Current smoker at annual assessments | −.194 (−.830,.443) | .55 | −.191 (−.828,.445) | .56 |
| Cigarette use during sleep protocol (per day) | −.016 (−.105,.074) | .73 | −.016 (−.105,.074) | .73 |
| Alcohol use during sleep protocol (drinks/day) | −.196 (−.624,.232) | .37 | −.196 (−.624,.233) | .37 |
| Caffeine intake during sleep protocol (drinks/day) Menopausal status | .086 (−.069,.241) | .28 | .084 (−.070,.239) | .28 |
| Pre-/early peri-menopausal | 0 | 0 | ||
| Late peri-menopausal | .377 (−.303,1.057) | .28 | .364 (−.309,1.037) | .29 |
| Post-menopausal | −.302 (−1.115,.511) | .47 | −.287 (−1.101,.527) | .49 |
| Undetermined | −.210 (−.905,.485) | .55 | −.185 (−.879,.509) | .60 |
| Physical activity levelc | −.084 (−.231,.064) | .27 | −.082 (−.229,.066) | .28 |
Abbreviations: CI=confidence interval; AHI, apnea-hypopnea index.
Modeled annual change in BMI, treating BMI within the year of the SWAN Sleep Study as the first longitudinal observation.
Modeled annual change in BMI at core SWAN assessments, controlling for initial BMI in the year of the SWAN Sleep Study.
Measured at core SWAN assessments 6 and 10.
In exploratory models, the interaction term testing whether AHI category moderated the effect of sleep duration on BMI change, in units of kg/m2 per year for each h/day of sleep per AHI category, was not statistically significant for either sleep measure (actigraphy, estimate=.07, 95%C.I.: −.05,.18; diary, estimate=−.02, 95%C.I.: −.15,.10).
Discussion
In this multi-ethnic cohort of midlife women, actigraphy and diary measures of sleep duration demonstrated strong cross-sectional associations with BMI that were found to be independent of objectively-assessed sleep-disordered breathing. However, sleep duration was not prospectively associated with BMI change over approximately 4.6 years of follow up in unadjusted and adjusted models.
This study extends prior research in at least two important ways. First, the use of two methods of sleep assessment, daily actigraphy and sleep diaries assessed concurrently over approximately one month, represents a significant strength as most prior longitudinal studies have relied solely on brief self-report measures of typical sleep duration. The use of objective sleep measures such as actigraphy is particularly valuable because subjective measures of sleep duration are prone to systematic overreporting (21). Second, this is also the first study to use objectively-assessed apnea and hypopnea events to address the potentially confounding effect of sleep-disordered breathing. Obesity is a strong risk factor for sleep-disordered breathing (22), and one prior study suggested that cross-sectional associations between sleep duration and BMI may be confined to individuals with self-reported sleep-disordered breathing symptoms (19). No effect modification by AHI category was observed in this study, and sleep duration and AHI category were independently associated with BMI.
The only prior analysis of the associations between objectively-measured sleep duration (actigraphy) and future weight change was conducted among 612 men and women in the CARDIA Sleep Study (19). Similar to the current findings, shorter sleep duration was associated with higher BMI in cross-sectional analyses, but was not predictive of weight change over 5 years of follow up. Initial BMI at the time of the SWAN Sleep Study captured a very large portion of the variability in annual BMI change across subsequent years, and all variables other than study site (including physical activity level) became non-significant in the fully adjusted models. The findings observed both in SWAN and CARDIA indicate that short sleep duration does not contribute to weight gain in midlife.
Associations between sleep and weight gain have been more consistent among children and young adults than middle-aged and older adults, suggesting that the effect of sleep on weight change may weaken with age (1,6,18). One possibility is that the effect of short sleep on weight gain reaches a plateau prior to midlife with the accumulation of additional body mass, as the increased energy requirements of a larger body will eventually offset the obesogenic effect of short sleep (32). Further research using experimental designs should explore whether short sleep duration might continue to affect energy balance in midlife without causing further increases in weight. Additionally, future studies of sleep and weight gain in children are needed to definitively rule out reverse causation and potential confounding of this association by other factors (e.g., physical activity).
This study had several noteworthy limitations. The SWAN Sleep Study cohort consists of midlife women of three racial backgrounds, contains disproportionately few post-menopausal women, and excludes individuals with characteristics known to influence sleep. Therefore, the observed associations may not generalize to other populations. While actigraphy and sleep diaries are superior measures to brief survey measures, polysomnography is considered the “gold standard” for sleep assessment. Polysomnography was collected for up to three nights in the SWAN Sleep Study, but polysomnography is relatively disruptive due to the placement of numerous sensors and leads on the body, and is known to affect the quantity and quality of sleep on the first night of observation (33). Therefore, it was not considered a suitable measure of typical sleep duration. As the menopausal transition is characterized by increased sleep disturbance (23,34), potential changes in sleep duration during follow-up may not have been captured in our single sleep assessment period. Additionally, our sleep measures did not capture daytime napping, which is inversely related to both sleep duration and BMI in midlife adults (35). Though sample sizes were reasonably large, our longitudinal analyses were underpowered to detect small effects of sleep duration on annual BMI change that could potentially accumulate over many years to produce clinically significant adiposity. Our sample size also provided insufficient power to explore interactions between sleep and factors such as race/ethnicity, physical activity level, or menopausal status. The observed change in BMI during follow up was relatively modest, and prospective associations between sleep duration and weight change might be more apparent in samples which demonstrate greater variability in BMI change or provide a longer follow-up period. Also, very few women had sleep durations exceeding 8 hours, which precluded the possibility of testing for non-linear associations in which both short and long sleep duration were associated with BMI change, as reported in a few prior studies (6). Finally, though we also accounted for a number of potential confounds, it is possible that residual confounding or unmeasured factors influenced the current results.
While associated with current BMI among midlife women, sleep duration was not associated with weight gain. Additional investigation is needed to better understand how sleep potentially contributes to weight gain across the lifespan, and test the possibility that sleep duration affects energy balance without resulting in continued weight increases at midlife.
Acknowledgements
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). Sleep data were processed with the support of RR024153. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994 – present; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
| Steering Committee: | Susan Johnson, Current Chair |
| Chris Gallagher, Former Chair |
We thank the study staff at each site and all the women who participated in SWAN.
Institutions where the study was performed: Rush University Medical Center, Chicago, IL; University of California-Davis, Davis, CA; University of Michigan, Ann Arbor, MI; University of Pittsburgh, Pittsburgh, PA.
Footnotes
Disclosure
The authors have no relevant conflicts of interest to disclose.
References
- 1.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16(2):265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 3.Landhuis CE, Poulton R, Welch D, Hancox RJ. Childhood sleep time and long-term risk for obesity: A 32-year prospective birth cohort study. Pediatrics. 2008;122(5):955–960. doi: 10.1542/peds.2007-3521. [DOI] [PubMed] [Google Scholar]
- 4.Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Arch Pediatr Adolesc Med. 2010;164(9):840–845. doi: 10.1001/archpediatrics.2010.143. [DOI] [PubMed] [Google Scholar]
- 5.Knutson KL. Sleep duration and cardiometabolic risk: A review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity (Silver Spring) 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: A 6-year prospective study from the Quebec Family Study. Sleep. 2008;31(4):517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunderson EP, Rifas-Shiman SL, Oken E, et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167(2):178–187. doi: 10.1093/aje/kwm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: A 13-year prospective study. Sleep. 2004;27(4):661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 10.Nishiura C, Hashimoto H. A 4-year study of the association between short sleep duration and change in body mass index in Japanese male workers. J Epidemiol. 2010;20(5):385–390. doi: 10.2188/jea.JE20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Gunderson EP, Stuebe AM, Mantzoros CS. Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity (Silver Spring) 2011;19(1):171–178. doi: 10.1038/oby.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itani O, Kaneita Y, Murata A, Yokoyama E, Ohida T. Association of onset of obesity with sleep duration and shift work among Japanese adults. Sleep Med. 2011;12(4):341–345. doi: 10.1016/j.sleep.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87(2):310–316. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 15.Lyytikainen P, Rahkonen O, Lahelma E, Lallukka T. Association of sleep duration with weight and weight gain: A prospective follow-up study. J Sleep Res. 2011;20(2):298–302. doi: 10.1111/j.1365-2869.2010.00903.x. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: A large-scale prospective study. Sleep. 2010;33(2):161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorkelund C, Bondyr-Carlsson D, Lapidus L, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: The prospective population study of women in Gothenburg. Diabetes Care. 2005;28(11):2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 18.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: Analyses of the NHANES I. Sleep. 2005;28(10):1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 19.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: The CARDIA Sleep Study. Am J Epidemiol. 2009;170(7):805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stranges S, Cappuccio FP, Kandala NB, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: The Whitehall II Study. Am J Epidemiol. 2008;167(3):321–329. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Ford ES, Zhao G, Croft JB, Balluz LS, Mokdad AH. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005–2006. Prev Med. 2010;51(1):18–23. doi: 10.1016/j.ypmed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 24.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: The SWAN Sleep Study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 26.The Atlas Task Force. Recording and scoring leg movements. Sleep. 1993;16(8):748–759. [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, Md: U.S. Dept. of Health, Education, and Welfare, Public Health Services-National Institutes of Health, National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. [Google Scholar]
- 28.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 29.Kravitz HM, Avery E, Sowers MR, et al. Relationships between menopausal and mood symptoms and EEG sleep measures in a multi-ethnic sample of middle-aged women: The SWAN Sleep Study. Sleep. 2011;34(9):1221–1232. doi: 10.5665/SLEEP.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 31.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 32.Magee LL, Hale LE. Re: "cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: The CARDIA Sleep Study". Am J Epidemiol. 2010;171(6):745. doi: 10.1093/aje/kwq018. [DOI] [PubMed] [Google Scholar]
- 33.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14(1):13–17. [PubMed] [Google Scholar]
- 34.Hall MH, Gold EB, Kravitz HM, Matthews KA, Sowers M. Acute and persistent sleep disturbances in mid-life women: Race matters. [Abstract 0908] Sleep. 2011;34:A311. (Abstract Supplement) [Google Scholar]
- 35.Owens JF, Buysse DJ, Hall M, et al. Napping, nighttime sleep, and cardiovascular risk factors in midlife adults. J Clin Sleep Med. 2010;6(4):330–335. [PMC free article] [PubMed] [Google Scholar]