Abstract
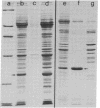
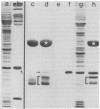
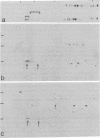
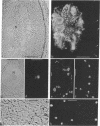
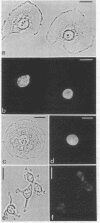
Nuclei of vitellogenic oocytes of the frog, Xenopus laevis, contain a prominent protein, representing about 10% of nuclear protein, which is characterized by a polypeptide of Mr 30,000. This protein is highly phosphorylated and acidic, displays several isoelectric variants with pI values ranging from 4.7 to 5.3, shows a high thermostability, is not stably complexed with other proteins, and is readily extracted in buffer solutions. Guinea pig antibodies against this protein have allowed its specific immunoprecipitation and localization by immunofluorescence microscopy, using both frozen tissue sections and cells grown in vitro. The protein is located almost exclusively in the nucleus where it appears to be spread throughout the nuclear interior. It is also a major nucleus-specific protein in vitellogenic and previtellogenic oocytes of other amphibian species as well as in other cell types, including hepatocytes, follicle epithelial cells, and cultured Xenopus cells, but is not detected in nuclei of transcriptionally inactive cells such as erythrocytes and spermatids. An immunologically related, if not identical, protein occurs in nuclei of higher vertebrate cells, including mammals. The properties of this abundant nuclear phosphoprotein and its possible relationship to the “nucleosome assembly factor” protein are discussed. It is suggested that this soluble protein serves a general nuclear function.
Keywords: phosphoproteins, nonhistones, nuclear sap, nucleocytoplasmic compartmentation, immunofluorescence microscopy
Full text
PDF

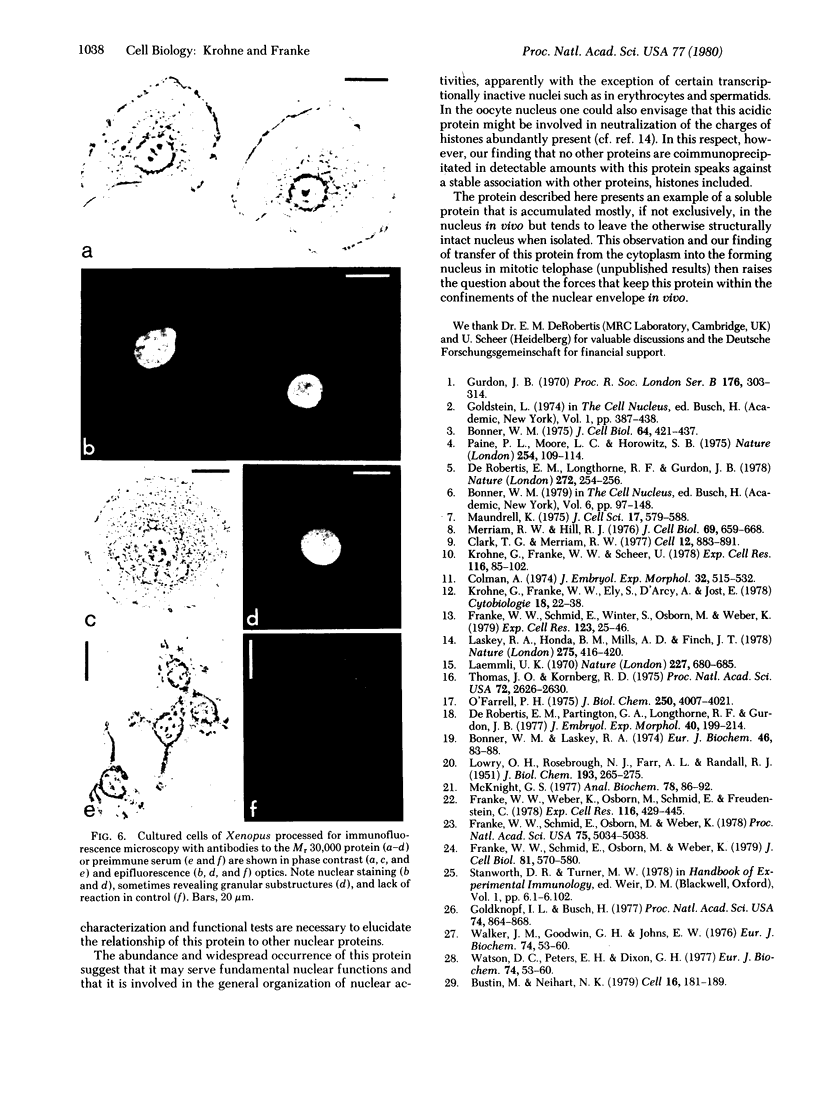
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975 Feb;64(2):431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Neihart N. K. Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells. Cell. 1979 Jan;16(1):181–189. doi: 10.1016/0092-8674(79)90199-5. [DOI] [PubMed] [Google Scholar]
- Clark T. G., Merriam R. W. Diffusible and bound actin nuclei of Xenopus laevis oocytes. Cell. 1977 Dec;12(4):883–891. doi: 10.1016/0092-8674(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Colman A. Synthesis of RNA in oocytes of Xenopus laevis during culture in vitro. J Embryol Exp Morphol. 1974 Oct;32(2):515–532. [PubMed] [Google Scholar]
- De Robertis E. M., Longthorne R. F., Gurdon J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978 Mar 16;272(5650):254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Partington G. A., Longthorne R. F., Gurdon J. B. Somatic nuclei in amphibian oocytes: evidence for selective gene expression. J Embryol Exp Morphol. 1977 Aug;40:199–214. [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Intermediate-sized filaments of human endothelial cells. J Cell Biol. 1979 Jun;81(3):570–580. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Ely S., D'Arcy A., Jost E. Localization of a nuclear envelope-associated protein by indirect immunofluorescence microscopy using antibodies against a major polypeptide from rat liver fractions enriched in nuclear envelope-associated material. Cytobiologie. 1978 Oct;18(1):22–38. [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Scheer U. The major polypeptides of the nuclear pore complex. Exp Cell Res. 1978 Oct 1;116(1):85–102. doi: 10.1016/0014-4827(78)90067-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Finch J. T. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature. 1978 Oct 5;275(5679):416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Proteins of the newt oocyte nucleus: analysis of the nonhistone proteins from lampbrush chromosomes, nucleoli and nuclear sap. J Cell Sci. 1975 May;17(3):579–588. doi: 10.1242/jcs.17.3.579. [DOI] [PubMed] [Google Scholar]
- McKnight G. S. A colorimetric method for the determination of submicrogram quantities of protein. Anal Biochem. 1977 Mar;78(1):86–92. doi: 10.1016/0003-2697(77)90011-2. [DOI] [PubMed] [Google Scholar]
- Merriam R. W., Hill R. J. The germinal vesicle nucleus of Xenopus laevis oocytes as a selective storage receptacle for proteins. J Cell Biol. 1976 Jun;69(3):659–668. doi: 10.1083/jcb.69.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. C., Peters E. H., Dixon G. H. The purification, characterization and partial sequence determination of a trout testis non-histone protein, HMG-T. Eur J Biochem. 1977 Mar 15;74(1):53–60. doi: 10.1111/j.1432-1033.1977.tb11365.x. [DOI] [PubMed] [Google Scholar]