Abstract
Devising interventions to provide integrated treatment for addiction and medical problems is an urgent issue. This study piloted a structural intervention, Directly Administered Antiretroviral Therapy (DAART), to assist methadone-maintenance patients in HIV medication adherence. Twenty-four participants received: 1) antiretroviral medications at the methadone clinic daily before receiving their methadone; 2) take-home antiretroviral medication for days they were not scheduled to attend the methadone clinic, and 3) brief adherence counseling to address adherence barriers. DAART lasted 24 weeks, with a planned step-down to twice-weekly administration in weeks 25–36, followed by self-administration in weeks 37–48. Retention rates at weeks 24, 36, and 48 were 83%, 92%, and 75% respectively. DAART was associated with improvement in the proportion of participants achieving viral suppression as well as with high medication adherence rates (clinic-verified; 85% and self-reported 97%) during the active intervention phase. DAART was effective as an intervention but did not promote transition to self-administration. This study demonstrates that DAART is adaptable and simple enough to be implemented into methadone treatment programs interested in providing HIV adherence services.
Keywords: HIV/AIDS, Drug Abuse Treatment, Antiretroviral Therapy
1. Introduction
Adherence to HIV medications is crucial. It is well established that adherence to antiretroviral (ARV) medications is associated with relative improvements in immunologic and virologic markers (Low-Beer, Yip, O’Shaughnessy, Hogg, & Montaner, 2000; Paterson et al., 2001), increased body weight (Shikuma et al., 2004), and less rapid progression to Acquired Immunodeficiency Syndrome (AIDS) (Bangsberg et al., 2001). Substance abuse is consistently associated with low adherence to HIV medications (Gonzalez, Barinas, & O’Cleirigh, 2011; Lucas, 2011). Preventive interventions include patient-centered, behavioral skill approaches and environmental interventions that facilitate adherence, such as directly administered antiretroviral therapy (DAART). DAART’s main components include supervised medication administration, which involves structural clinical changes (Blankenship, Bray, & Merson, 2000), and individual patient support. Methadone maintenance treatment (MMT) programs are promising sites for using DAART. Staff are licensed to administer medications, and patients attend the clinics frequently, often daily. The efficacy of on-site dispensing of tuberculosis medications in MMT has been demonstrated (Batki, Gruber, Bradley, Bradley, & Delucchi, 2002), and positive effects of DAART in MMT have been shown (Lucas et al., 2006). A recent clinical trial found higher adherence and lower viral loads in the DAART group (Berg, Litwin, Li, Moonseong, & Arnsten, 2011) compared to treatment as usual.
Recent clinical guidelines for improving entry, retention, and antiretroviral adherence developed by an International Association of Physicians in AIDS Care Panel (Thompson et al., 2012) recommend DAART for individuals with substance use disorders. Specifically, integration of DAART into methadone maintenance treatment for opioid dependent patients is advised. Yet implementing DAART in methadone clinics confronts a number of barriers. The need for staff time to coordinate with the primary medical provider and the pharmacy; specific protocols for a wide range of procedures such as handling, storing, dispensing, and implementing changes in medications; and patient no-shows causing not only missed methadone doses but also missed ARV medications, are significant barriers we have observed.
The present study was conducted to assess the feasibility and effectiveness of providing DAART in a methadone clinic setting. The primary expectation was that DAART would be associated with an increase in the proportion of participants who achieved viral suppression. In a related secondary hypothesis we expected that adherence to ARV medication would improve.
2. Methods
2.1. Participants
Twenty-four adult opioid-dependent patients in MMT at San Francisco General Hospital (SFGH) were included, all receiving or starting ARV therapy. Inclusion criteria were: Detectable viral load, prescribed once-or-twice-daily ARV dosing, clinic methadone dosing schedule at least three times a week, and reported less than 95% adherence to ARVs during a two-week baseline period. Exclusion criteria were: Enrolled in another adherence program or residential treatment, cognitive impairment or psychosis interfering with ability to provide informed consent, or unavailable any time during the 48-week study period.
2.2. Study Site
The SFGH Opiate Treatment Outpatient Program (OTOP) is a 600-patient clinic providing MMT and outpatient detoxification. OTOP has treated patients with HIV/AIDS since 1984 (Sorensen, Batki, Good, & Wilkinson, 1989) and provides HIV primary care to eighty patients. Adding DAART to the clinic’s services involved considerable preparation, including presentation to clinic staff, surveying staff to understand the barriers to conducting DAART, adapting an adherence counseling manual (Haug, Sorensen, Gruber, Lollo, & Roth, 2006) to include standardized DAART elements, educating clinic staff about HIV treatment, and developing procedures for administering HIV medications at the busy MMT dispensary.
2.3. Recruitment and Informed Consent
The UCSF IRB approved all study procedures. Recruitment methods were based on previous work at the clinic (Sorensen et al., 2007). Participants consented to the DAART intervention and to exchange of information with primary care providers concerning viral load tests and missed ARV doses. Participants were enrolled from September 12, 2007 through September 30, 2008.
2.4. Study Procedures
Participants completed a baseline assessment and provided blood and urine samples. They supplied detailed tracking information at baseline and every four weeks. Self-report measures were gathered through an audio computer assisted self-interview (A-CASI) at 14-day intervals.
2.5. The DAART Intervention
DAART was provided for 24 weeks. Following referral by their primary care provider and review by the methadone clinic nurse practitioner or physician to address possible interactions and needs for methadone does adjustments, participants took their ARV medications at the MMT clinic’s dispensing window before receiving methadone.
The procedures used were streamlined and adapted to fit the clinical setting. Although we had originally planned to conduct a study of “enhanced” DAART (see Altice et al., 2004), it was difficult to employ many of these components in the clinic. For example, it was not feasible to employ a DAART Specialist a position that coordinated care, outreach workers, or a HIV Clinical Pharmacist (Foisy & Akai, 2004) to perform adherence counseling; consequently these responsibilities were absorbed by the clinical and research staff. We also incorporated staff trainings on the project rationale, procedures, and outcomes to assist with organizational acceptability.
We adopted structural elements to address observed barriers: 1) take-home antiretroviral medication packets for days the participants were not scheduled to attend MMT and 2) extra medication if participants did not attend on scheduled days. By ensuring that participants always had access to their HIV medication, these procedures enabled the clinic to provide DAART to those whose clinic attendance was not ideal. Specifically, participants received packets containing HIV medications for evenings and days they were not scheduled to attend clinic, plus emergency packets for up to seven days of missed clinic visits. A backup prescription for an additional 7-day supply was kept on file with the participating pharmacy as an emergency back-up. Additional medications could be included in DAART and in emergency packets by primary provider request.
After 24 weeks of DAART, participants transitioned to twice-weekly direct administration, with self-administration on the other days (DAART step-down), and after 36 weeks they transitioned entirely to self-administration. However, as noted in the results section, the step-down process was not feasible for most participants, who continued to receive DAART with methadone. Participants, their primary care providers, methadone clinic staff, and the adherence counselor could suggest continuation in the regular DAART protocol instead of step-down transition to self-administration. Often participants asked to continue in DAART, or primary providers requested continued DAART for participants on salvage regimens for whom developing resistance entailed high medical risk.
In addition to DAART, brief adherence counseling was provided by a research project assistant with a bachelors’ degree in psychology, with supervision and backup by clinical psychologists (authors VG and JS). The approach was cognitive-behavioral, supportive, and psychoeducational with elements of motivational interviewing (see Cooperman, Parsons, Chabon, Berg, & Arnsten, 2007; Haug et al., 2006). The goal was to help participants take their at-home doses, and prepare for step-down from DAART. Adherence counseling sessions were scheduled in coordination with methadone dosing (i.e. the days and times participants came for dosing) and in conjunction with study visits.
Adherence counseling began in Study Week 1 and occurred every other week during DAART and every four weeks during DAART step-down phase. Each session lasted about 15 minutes and included assessment of adherence, addressing barriers to adherence, and a client adherence assignment.
2.6. Measures
2.6.1. Background and Follow-up Measures
Barriers to Adherence Checklist (Catz, Kelly, Bogart, Benotsch, & McAuliffe, 2000) includes 56 items scored on a 4-point scale ranging from 4 definitely true to 1 definitely false, with higher scores indicating higher barriers to adherence, resulting in a possible range from 4 to 224.
Substance use was measured using the Time Line Follow Back assessment (Sobell & Sobell, 1992) for the previous 14 days (2 weeks) at baseline. For the 12 week, 24 week, and 48 week analyses number of days using out of the past 28 (4 weeks) was analyzed. We then dichotomized substance use into high (using on more than 1/3 of days) and low (using on less than 1/3 of the days) categories to address variable skewedness. This cut-off was chosen based on the distribution of the variables; many participants were either using heavily or not at all.
2.6.2. Outcome Measures
A timeline followback calendar procedure yielded self-reported adherence for the previous two weeks. MMT dispensing records were abstracted to document the proportions of days medications were taken as prescribed. Plasma HIV-1 RNA (viral load) was determined using Version 3.0 branched DNA assay with a lower limit of 75 copies/ml, and was measured at baseline and weeks 12, 24, 36, and 48. Undetectable viral load was defined as less than 75 copies per milliliter of plasma.
2.7. Monetary Compensation
Participants received cash compensation for study activities, including each assessment ($15), blood draw ($5), urine sample ($3), and a $25 bonus for completing all assessments on time.
2.8. Data Analysis
2.8.1. Data Quality and Data Reduction
The ARV medication doses received or missed were obtained from the methadone dispensary records and databases. These were checked against the research assistant’s daily log. With self-reported adherence we compared the number of doses missed during the 2-week baseline with the most recent 2 week (14 days) follow-ups at 24 and 48 weeks. We also compared the self-reported percentage of doses taken over the 2-week baseline with the percentage taken in the period covered by each follow-up. Listwise deletion was used to handle missing data.
2.8.2. Statistical Analyses
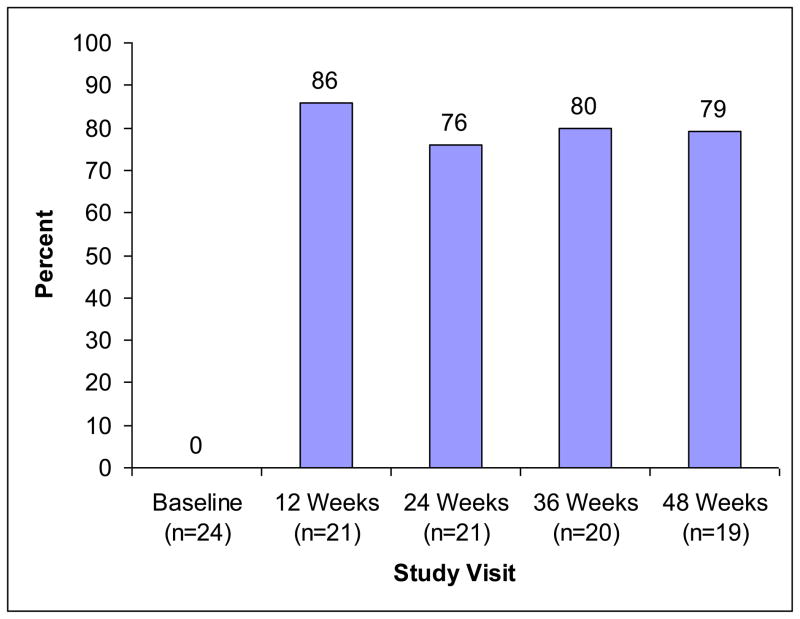
Comparisons between baseline and 24-week data examined initial effects of the intervention, and comparisons between baseline and 48-weeks explored the sustained impact of the intervention. Viral load data had a skewed distribution, so the distribution was dichotomized (detectable, undetectable), and McNemar’s chi square statistic was used to test for changes between baseline and 12-weeks. Similar analyses were used to examine differences between baseline and 24, 36, and 48-week viral load (Figure 1).
Figure 1.
Percent achieving viral suppression over time
3. Results
3.1. Cohort Description
3.1 1. Recruitment
Of 66 participants referred, 32 (48.5%) were ineligible (e.g. undetectable viral load, on methadone van, discharged from methadone maintenance since referral), and another 10 (15.2%) declined to participate.
3.1.2. Participant Characteristics
Background characteristics of participants appear in Table 1. As noted, most participants were male, they came from varied racial/ethnic backgrounds with about 28% nonwhite, over 80% self-identified as heterosexual; and in age over two thirds were in their forties or fifties. Most were receiving long-term disability (SSI) and Medicaid, and the remaining participants were on General Assistance.
Table 1.
Participant Background Characteristics
| Baseline Demographic and Clinical Characteristics (N=24) | |
|---|---|
| Gender, n (%) | |
| Male | 14 (58) |
| Female | 8 (33) |
| Transgender | 2 (8) |
| Race/ethnicity, n (%) | |
| White | 10 (42) |
| Black | 6 (25) |
| Hispanic | 7 (29) |
| Other | 1 (4) |
| Sexual orientation, n (%) | |
| Heterosexual | 20 (83) |
| Homosexual | 2 (8) |
| Bisexual | 2 (8) |
| Age, mean (SD) | 44.1 (9.4) |
| Highest level of education | |
| Some high school or less | 13 (54) |
| High School Diploma or GED | 9 (38) |
| Some College | 2 (8) |
| Current employment status | |
| Employed (full time or part-time) | 0 (0) |
| Unemployed | 15 (63) |
| Retired | 9 (38) |
| Marital status | |
| Single | 12 (50) |
| Married | 0 (0) |
| Divorced | 5 (21) |
| Separated | 1 (4) |
| Widowed | 6 (25) |
| Living arrangements | |
| With significant other | 5 (21) |
| With family (not own children) | 3 (13) |
| With friends | 5 (21) |
| Alone | 8 (33) |
| Residential facility | 3 (13) |
| Homeless, n (%) | 10 (42) |
| Viral Load, n (%) | |
| 75–400 | 1 (4) |
| 401–10,000 | 6 (25) |
| 10,001–100,000 | 12 (50) |
| >100,000 | 5 (21) |
| Years since diagnosis, n (%) | |
| <1 year | 1 (4) |
| 1–9 years | 8 (33) |
| 10–19 years | 13 (54) |
| 20+ years | 2 (8) |
| Years in methadone maintenance, n (%) | |
| <1 year | 11 (46) |
| 1–9 years | 10 (42) |
| 10–19 years | 3 (13) |
| Substance use in past 14 days, mean (SD) | |
| Alcohol | 3.3 (5.2) |
| Marijuana | 4.3 (5.3) |
| Heroin | .6 (1.1) |
| Cocaine | 5.5 (5.8) |
| Opioids | 5.4 (5.9) |
| Benzodiazepines | 2.1 (4.3) |
| Methamphetamine | .3 (.6) |
| Proportion of high (>1/3) substance use days, n (%) | |
| Alcohol | 5 (21) |
| Marijuana | 4 (17) |
| Heroin | 0 |
| Cocaine | 7 (29) |
| Opioids | 7 (29) |
| Benzodiazepines | 2 (8) |
| Methamphetamine | 0 |
3.2. Retention
Regarding retention in the study, 21 (87.5%) of the 24 participants completed the 12-week assessment, 20 (83%) completed 24-week assessment, 22 (92%) completed 36-week assessment and 18 (75%) completed the 48-week assessment. Viral load data were obtained for more participants, including 21 participants (87.5%) at 12 weeks and 24 weeks, 20 participants (83.3%) at 36 weeks, and 19 participants (79.2%) at 48 weeks. Of those who dropped out of the study, the primary reasons were: Left MMT and could not be located (3 participants) and incarceration (2 participants). Number of participants completing weekly assessments ranged from 23 (96%) in Week 6 to 13 (54%) in Week 40 and averaged 18 participants (75%) through the study.
Regarding retention in the intervention, all 24 participants completed the 2-week baseline phase, and 17 (71%) completed the interventions (DAART through 24 weeks, followed by DAART step-down or transition to long-term clinic DAART). Of the participants who discontinued treatment, primary reasons were: discontinued MMT (3 participants) and transferred to another MMT program (2); one went to prison, and only one discontinued DAART. At the end of the study intervention phase, the MMT program voluntarily continued the DAART process with 9 participants, 2 were receiving DAART in hospice or residential care, and 6 participants had transitioned to self-administration.
3.3. Participation in Adherence Counseling
Of the 13 adherence counseling sessions expected during the 24-week DAART phase, mean attendance was 11.1; 14 participants (58%) completed all 13 expected sessions. All 6 who continued to the step-down phase and 7 of the 9 who continued to long-term DAART provided by the methadone clinic completed all adherence counseling sessions.
Ten participants completed fewer sessions than intended. Of these, 6 had favorable or neutral outcomes, including 1 who chose to discontinue DAART, 2 who continued to long-term DAART, 2 who transferred to other methadone clinics, and 1 who transferred to HIV residential care with onsite DOT. Another 4 had unfavorable outcomes, including 3 who were discharged from the methadone clinic for nonattendance or behavior problems, and 1 who was incarcerated.
Between Weeks 24 and 36, an additional 3 adherence counseling sessions were scheduled for the 17 participants who went on to the step-down phase or to clinic DAART. Of the 6 in the step-down phase, 1 (16.7%) had only 2 of the 3 adherence counseling sessions, and of the 9 who transitioned to long-term clinic DAART, 2 (22.2%) had only 2 of the 3 sessions (decreased frequency while in residential treatment). All others completed all 3 intended sessions.
3.4. Adherence-Related Subjective Experiences
As Table 2 shows, DAART was associated with decreased barriers to adherence. Ratings improved from baseline to the 24-week interview and remained stable though the 48-week interview. Participants identified several barriers to adherence at baseline (principally “getting treatment reminds me that I am HIV+” and “I have trouble remembering the names of medicines and what they are for”). The number of barriers declined slightly by the 24-week interview and stayed at this level through the 48-week interview.
Table 2.
Participant Adherence Background and Outcomes
| Variable | Baseline n=24 mean (SD) | 24 Weeks n=20 mean (SD) | 48 Weeks n=18 mean (SD) | Absolute Mean Difference Baseline- 24 weeks | T-test statistic, (df) Baseline- 24 Weeks | Absolute Mean Difference Baseline- 48 weeks | T-test statistic, (df) Baseline- 48 Weeks |
|---|---|---|---|---|---|---|---|
| Adherence-related Subjective Experiences | |||||||
| Barriers to Adherence | 111.3 (27.7) | 89.1 (21.7) | 78.9 (20.2) | 22.2 | 4.4*, (17) | 32.4 | 4.7*, (15) |
| Adherence | |||||||
| Medication Dispensing Records---Percent of Doses Taken | n/a | 84 | 85 | n/a | n/a | n/a | n/a |
| Self-Reported Adherence---Percent of Days Adherent | 36 (43) | 94 (22) | 99 (2) | 58 | −5.2*, (19) | 63 | −6.0*, (17) |
p<.001
3.5 Medication Adherence
DAART was associated with improved adherence. Dispensing records (Table 2) indicate that during the 24-week DAART phase participants took 84% of their HAART doses. For the participants who transitioned to the DAART step-down phase (n=6) (Weeks 24 to 36) adherence increased to 96%.
Participants’ self-report of adherence also improved significantly from baseline to the 24-week follow-up and was maintained through the 48 week follow-up. Table 2 shows the percentage of days taking medications (days of adherence) in the 14 days before the assessment. Allowing time for stabilization over the first 12 weeks, in the second 12 weeks leading up to the 24-week assessment, participants reported an average of 94% adherence, compared to an average of 36% at baseline. There were no significant adherence differences between those individuals who transitioned into the step-down phase and those who did not.
3.6. Plasma HIV-1 RNA (viral load) (primary outcome)
Figure 1 displays the proportion of participants who achieved viral suppression from baseline through week 48. DAART was associated with viral load reduction. At baseline 0% of participants had undetectable viral loads. By 12 weeks 86% of the participants already had undetectable levels. McNemar’s test indicated significant change between baseline and each subsequent time point, including a sustained difference at 48 weeks (p<.001).
3.7. Substance Use Associations with Self-Reported HAART Adherence
The proportion of days of substance use was calculated, allowing comparisons between the 2-week baseline and the 24-week follow-up. For each substance, participants were then grouped into those with high use (used more than 1/3 of the days in the reporting period) and low use (used on 1/3 or fewer of the days in the reporting period).
At baseline, those with high opioid use reported missing fewer days of their HIV medications (mean days of missed medications =3.78) than those with low opioid use (mean days of missed medications =12.07), (F1,23 =18.71, p<.001). No adherence differences were found in high vs. low alcohol (F1,23 = .63, ns) or cocaine users (F 1,23 =.09, ns). Baseline data showed that of the 24 participants 5 (21%) were categorized as high alcohol users, 7 (29%) were high cocaine users, and 7 (29%) were high opiate users (Table 1). At 24 weeks no adherence differences were found between high and low opioid (F 1, 14 = .62, ns), alcohol (F 1,14 = 3.34, ns), or cocaine users (F 1,14 = .07, ns). At 24 weeks we had substance use data for 15 participants. Of these, 3 participants (20%) had high alcohol use, 4 (27%) reported high cocaine use, 5 (33%) reported high opiate use, and 2 reported high marijuana use (13%). No participants reported high methamphetamine, high heroin, or high benzodiazepine use at 24 weeks.
3.8. Participants who Transitioned to Step-Down and Self-Administration
Of the 6 participants who transitioned to step-down and then self-administration, almost all were housed (n=5, 83.3%, vs. only 58.3% housed in the overall sample) and ethnic minority (n=5, 83.3%, vs. only 28% minority in the overall sample). There were an equal number of men and women. There were no significant substance use or adherence differences between those who transitioned and those who did not.
3.9. Clinic Continuation on DAART after End of Study Intervention
The methadone clinic transitioned 9 participants to long-term DAART at the methadone dispensary window. At six months past the end of the 48-week study, these 9 participants were still receiving their ARVs at the dispensary window.
Due to clinic budget limitations and the less certain benefits of adherence counseling, the clinic did not to continue the regular adherence counseling. Instead, a nurse practitioner provided pharmacy coordination (prescription changes and problem resolution), and as-needed adherence counseling.
4. Discussion
The present study contributes to existing DAART literature by providing programmatic data that shows DAART is adaptable and simple enough to be a highly generalizable model for other MMT programs interested in providing HIV adherence services. This work piloted DAART, an intervention that included directly dispensing ARVs in MMT and individual adherence counseling. DAART, with convenience packets for at-home ARV doses and missed visits, supplemented by adherence counseling and pharmacy coordination, increased ARV adherence despite substance use and homelessness. DAART had acceptable retention (75% continued through 24 weeks); was associated with greatly improved adherence to HIV medications; and the proportion of participants with undetectable viral loads rose from 0 to 86%. Attempts to step participants down from DAART were mixed. At 24 weeks, when the step-down phase began, there were no differences observed between those who began step-down and those who did not. Only 6 of the 24 participants succeeded in transitioning to self-administration. Almost all of those had stable housing, and a majority were ethnic minorities. The MMT program voluntarily continued the DAART process with 9 participants after the intervention ended, reflecting positive perceptions by both participants and clinic staff. These results mirror other demonstrations (Berg et al., 2011; Conway et al., 2004; Lucas et al. 2006) that MMT can be a useful site for DAART interventions.
In this study, the modal participant continued DAART beyond the planned 24-week intervention period. The planned step-down from DAART to self-administration was not feasible for most participants. DAART served as a long-term rather than a short-term intervention, despite adherence counseling designed to help participants achieve self-administration. These results are congruent with a trial of DAART versus self-administered therapy among injection drug users (Altice, Maru, Bruce, Springer, & Friedland, 2007), which demonstrated the effectiveness of DAART at improving 24-week outcomes, yet virological benefits did not persist after the intervention ended (Maru, Bruce, Walton, Springer, & Altice, 2009). These results are consistent with the idea that DAART should be considered a long-term intervention because the benefits for adherence and viral load cease once DAART is discontinued (Berg et al., 2011). A positive aspect of this study is that the structural aspects of the intervention, including staff training and identifying DAART barriers at the clinic, coupled with significant improvements in patients on DAART, helped the clinic to adopt this intervention beyond the planned research study.
Study limitations included the small sample size, which prevented examining independent contributions of intervention components and predictors of response to DAART. So we are uncertain about the independent or combined contribution of supervised dosing, and adherence counseling. Generalizability was limited by the single-clinic setting, and we note that only 24 of 68 people referred to the study participated. Longer-term efficacy is unknown at this time. The measure of adherence to take-home medications depended on self-report, which is a limited measure of medication adherence. Surprisingly, at baseline the high users of opioids self-reported better adherence than the low-users of opioids, a counterintuitive finding that should be investigated using a more objectively observable measure of medication adherence. Also there was no comparison group. We also note that transition from DAART to self-administration was not achieved and may have been an unreasonable expectation.
Despite the study’s restrictions, this work adds to a growing literature demonstrating the value of integrating HIV treatment into MMT programs and substance abuse treatment in general. Recently Volkow & Montaner (2011) point out the vital importance of providing comprehensive care for drug users with HIV, integrating treatments of addiction and medical problems. The present study supports the conclusion that adherence to HIV medications can be improved with the use of DAART. By implementing DAART, methadone clinics can help patients to take their ARVs as scheduled, despite adherence challenges such as substance use and homelessness. This allows patients who may otherwise be deemed too unstable to receive ARVs, decreasing their viral load and HIV disease progression.
Implementing DAART services in a MMT program requires clinical leadership, care coordination, and ongoing medical staff participation. The clinic needs a “champion”—a clinical leader who is committed to the project and can educate and inspire the team in the early days of the intervention, before the life-saving nature of the work is apparent. DAART is protocol intensive and requires significant up front planning to accommodate the flexibility needed to meet patients’ changing needs. A few examples of common situations that should be included in protocols are: medication side effects; not showing up for scheduled dosing; refusing DAART medications; and requesting to take medications later in the day. Oversight and coordination between the prescribing clinician, pharmacy, and methadone dispensary is essential to avoid errors and limit delays at the busy dispensary windows. The methadone clinic needs to have a medical provider available in case same-day order changes are needed or a serious side effect needs evaluation. A single pharmacy capable of delivering medications in easy-to-administer packaging should be used for all DAART patients, and all medications received from the pharmacy need to be reviewed by nursing staff to ensure accuracy. While the DAART program has been managed without increased staff, many staff members have had an increase in workload and responsibilities which a smaller, non-hospital based program, may not be able to accommodate. Finally, the issue of compensation for DAART services was not explored in this pilot but will be important in dissemination to other sites.
As demonstrated in this study, DAART can be implemented in methadone clinics if nursing and medical staff can be made available for pharmacy coordination. In this study the adherence counseling was conducted by a staff member provided by the research grant. If time allows, nursing, pharmacy or medical staff may be able to provide, or supervise an HIV-savvy counselor or health educator to provide adherence counseling as well. With limited staff, clinics may want to serve a small number of patients, prioritizing the most unstable ones who might otherwise not be started on ARVs.
Acknowledgments
5.1. Financial Support
The research was supported by grants from the National Institutes of Health (R21DA020369, P50DA09253, U10DA015815, and T32DA07250), and a Pilot Award from the UCSF AIDS Research Institute. These agencies had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
We are grateful for the support of the staff and patients of the Division of Substance Abuse and Addiction Medicine at San Francisco General Hospital and its Opiate Treatment Outpatient Program
We appreciate the support of these research and clinical colleagues: Kevin Delucchi, PhD, Stephen Dominy, MD;, CNS; Levis Owens, RN, MS, RNP; Susan Flores, MS, RN, RNP; Nikitia Hardwick, RN; James Murray, RN; Mel Bettancourt, LVN; Glories Heft, LVN; Christine Gruta, PharmD; Joshua Alexander, MPH, DO; Nicholas Hengl, and Yong S. Song, PhD.
Footnotes
5.2. Contributors
Authors Nancy A. Haug, James L. Sorensen, and Valerie A. Gruber designed the study. Authors Nancy A. Haug, Valerie A. Gruber, Elisabeth Powelson, Sandra Larios, Bradley Shapiro, and James L. Sorensen wrote the protocol. Authors Elisabeth Powelson, Sandra Larios, and Valerie Gruber screened participants, collected data, and delivered the adherence counseling. Authors Jacqueline Tulsky, Bradley Shapiro, and Deborah P. Logan delivered and supervised medical and substance abuse treatment issues related to the study activities. Authors Sandra Larios and Valerie A. Gruber oversaw the data quality and data analysis activities. Author James Sorensen wrote the first draft of the manuscript with assistance from authors Nancy A. Haug, Sandra Larios, and Valerie A. Gruber. All authors have approved the final manuscript.
5.3. Conflict of Interest
No conflicts declared.
References
- Altice FL, Metger J, Hodges J, Bruce RD, Marinovich A, Walton M, Springer SA, Friedland GH. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: Implications for program replication. Clinical Infectious Diseases. 2004;38:S376–S387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: A prospective, randomized, controlled trial. Clinical Infectious Diseases. 2007;45(8):770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, Moss A. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug and Alcohol Dependence. 2002;66(3):283–293. doi: 10.1016/s0376-8716(01)00208-3. [DOI] [PubMed] [Google Scholar]
- Berg KM, Litwin A, Li X, Moonseong H, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: A randomized controlled trial. Drug and Alcohol Dependence. 2011;113:192–199. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship KM, Bray SJ, Merson MH. Structural interventions in public health. AIDS. 2000;14:S11–S21. doi: 10.1097/00002030-200006001-00003. [DOI] [PubMed] [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19(2):124–133. [PubMed] [Google Scholar]
- Cooperman NA, Parsons JP, Chabon B, Berg KM, Arnsten JH. The development and feasibility of an intervention to improve antiretroviral adherence among HIV-positive patients receiving primary-care in methadone clinics. Journal of HIV/AIDS and Social Services. 2007;6(1/2):101–120. [Google Scholar]
- Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, Smith N, Mead A, DeVlaming S. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clinical Infectious Diseases. 2004;38(Suppl 5):S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- Foisy MM, Akai PS. Pharmaceutical care for HIV patients on directly observed therapy. The Annals of Pharmacotherapy. 2004;38:550–556. doi: 10.1345/aph.1D444. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Barinas J, O’Cleirigh C. Substance use: impact on adherence and HIV medical treatment. Current HIV/AIDS Reports. 2011;8(4):223–234. doi: 10.1007/s11904-011-0093-5. [DOI] [PubMed] [Google Scholar]
- Haug NA, Sorensen JL, Gruber VA, Lollo N, Roth G. HAART adherence strategies for methadone clients who are HIV-positive: A treatment manual for implementing contingency management and medication coaching. Behavioral Modification. 2006;20(6):752–781. doi: 10.1177/0145445506288229. [DOI] [PubMed] [Google Scholar]
- Low-Beer S, Yip B, O’Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. Journal of Acquired Immunodeficiency Syndrome. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- Lucas G. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sciences. 2011;88(21–22):948–952. doi: 10.1016/j.lfs.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clinical Infectious Diseases. 2006;42(11):1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- Maru DS, Bruce RD, Walton M, Springer SA, Altice FL. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: Results from a randomized controlled trial. Journal of Acquired Immune Deficiency Syndrome. 2009;30(2):176–181. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagner MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2001;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Shikuma CM, Zackin R, Sattler F, Mildvan D, Nyangweso P, Alston B, Evans S, Mulligan K The AIDS Clinical Trial Group 892 Team. Changes in weight and lean body mass during highly active antiretroviral therapy. Clinical and Infectious Diseases. 2004;39(8):1223–1230. doi: 10.1086/424665. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sorensen JL, Batki SL, Good P, Wilkinson K. Methadone maintenance program for AIDS-affected opiate addicts. Journal of Substance Abuse Treatment. 1989;6:87–94. doi: 10.1016/0740-5472(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, Tulsky JP, Barnett PG, Hall S. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: A randomized trial. Drug and Alcohol Dependence. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Orrell C, Altice FL, Bangsberg DR, Bartlett JG, Beckwith CG, Dowshen N, Gordon CM, Horn T, Kumar P, Scott JD, Stirratt MJ, Remien RH, Simoni JM, Nachega JB. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: Evidence-based recommendations from an International Association of Physicians in AIDS Care Panel. Annals of Internal Medicine. 2012;156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Montaner J. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Affairs. 2011;30(8):1411–1419. doi: 10.1377/hlthaff.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]