Abstract
Optimal therapy for post-transplant lymphoproliferative disease (PTLD) remains problematic. A phase II trial adding rituximab to a low-dose cyclophosphamide and prednisone regimen was conducted for pediatric patients with Epstein-Barr virus (EBV) (+), CD20 (+) PTLD. Fifty-five patients were enrolled. Toxicity was similar for cycles of therapy containing rituximab versus those without. Complete remission (CR) rate was 69% [95% confidence interval (CI); 57%-84%). Of 12 patients with radiographic evidence of persistent disease at the end of therapy, 8 were in CR 28 weeks later without further PTLD therapy. There were 10 deaths, 3 due to infections while receiving therapy and 7 from PTLD. The 2-year event free survival (alive with functioning original allograft and no PTLD) was 71% (95% CI: 57%-82%) and overall survival was 83% (95% CI: 69%-91%) with median follow-up of 4.8 years. Due to small numbers, we were unable to determine significance of tumor histology, stage of disease, allograft type or early response to treatment on outcome. These data suggest rituximab combined with low-dose chemotherapy is safe and effective in treating pediatric with EBV (+) PTLD following solid organ transplantation.
Keywords: PTLD, EBV, rituximab, chemotherapy
Introduction
Post-transplant lymphoproliferative disorder (PTLD) following solid organ transplant (SOT) is a heterogeneous disease. In the pediatric organ recipient population, the incidence of PTLD is higher than in the adult population and the vast majority of disease is Epstein-Barr virus (EBV) positive. (1, 2, 3) Additionally, two-thirds to over 90% of PTLD in the pediatric population is of B-cell origin. (4, 5) Reduction of immune suppression (RIS) is considered first-line therapy for EBV (+) PTLD, although the success rate in the literature varies widely and is dependent on several variables including stage and histology of the disease. (6, 7) A previous study for pediatric patients with EBV (+) PTLD, following a trial of RIS received a regimen consisting of 6 cycles of low-dose cyclophosphamide and prednisone resulting in a 2 year event free survival (alive with functioning original allograft and no PTLD) of 67% and an overall survival of 73%. (8) Anti-CD20 monoclonal antibody (rituximab) alone or with chemotherapy has been demonstrated to be effective in the treatment of adult patients with PTLD that is refractory to RIS. (9, 10, 11, 12, 13, 14) To assess the feasibility and efficacy of the addition of rituximab to the previous reported low-dose cyclophosphamide and prednisone regimen, the Children's Oncology Group (COG) conducted a phase II trial for refractory, CD20 (+), EBV (+) PTLD in pediatric patients following solid organ transplantation (SOT).
Methods
Eligibility criteria
Inclusion criteria were patients <30 years of age, with a histological diagnosis of PTLD defined by World Health Organization (WHO) criteria, (15) following a SOT. Patients were required to have measurable disease that was demonstrated to be CD20 and EBV positive. Radiographic evidence of PTLD and/or elevated EBV viral load without tissue biopsy was insufficient to meet eligibility criteria. Patients had to have had a least a trial of reduction of immunosuppression, defined as at least < 50% reduction of calcineurin inhibitor for > 1 week. Fulminant (F-PTLD) was defined clinically as presence of fever (>38° C), hypotension (for age), and evidence for multisystem organ failure in at least 2 of the following organ systems: bone marrow (pancytopenia), liver (coagulopathy, hyperbilirubinemia and/or transaminitis), pulmonary (interstitial pneumonitis, or effusion) or gastrointestinal hemorrhage. (8) F-PTLD patients were allowed to be enrolled without a trial of reduction of immunosuppression.
Exclusion criteria included: 1) previous rituximab therapy (within 30 days) and/or chemotherapy (within 4 weeks or without complete hematologic recovery from the chemotherapy) and/or 2) evidence of central nervous system (CNS) disease. Due to concern of hepatitis B virus reactivation, prior to giving the first dose of rituximab, hepatitis B serology was documented on all patients enrolled after 12/04. No patients had evidence of active or previous hepatitis B infection.
Patient evaluation
All patients underwent computerized tomography or magnetic resonance imaging that included neck, chest, abdomen and pelvis; as well as bone marrow aspirate/biopsy and lumbar puncture at study entry. All patients were staged according to the Murphy staging system for pediatric non-Hodgkin lymphoma (NHL). (16) Response and toxicity were documented after the 2nd, 4th, and 6th cycle of chemotherapy and at 1 year (or 7, 13, 24, and 52 weeks from study entry). Toxicity was assessed and documented using the National Cancer Institute Clinical Toxicity Criteria and Adverse Event (NCI CTCAE version 3.0) grading system.
Study design and procedures
The study accrual goal was 50 non-fulminant (NF-PTLD) patients. Fifty-five patients were enrolled (51 with NF-PTLD and 4 with F-PTLD) were enrolled onto COG ANHL0221 (Clinicaltrials.gov NCT00066469). The study included a 24-week treatment phase and an observation period for at least 24 months after study enrollment. Centralized pathology review by the study pathologist (SP) was required. Central pathology review was performed using WHO classification criteria (15) for morphologic subtype of PTLD (polymorphic, monomorphic or Hodgkin-like PTLD), as well as confirmed CD20 expression by immunohistochemistry and EBV positivity by Epstein-Barr early RNA (EBER) in-situ hybridization. Institutional Review Board approval was obtained at all participating institutions prior to enrolling patients.
Treatment Regimen
Patients received a total of 6 cycles of therapy given every 3 weeks. The first 2 cycles included cyclophosphamide (600 mg/m2 intravenous) on day 1 of each cycle, prednisone (1 mg/kg orally twice a day) or methylprednisolone (0.8 mg/kg intravenous every 12 hours) on days 1 - 5 of each cycle and rituximab (375 mg/m2 intravenous) on days 1, 8 and 15 of each cycle for a total of 6 doses. The remaining 4 cycles of chemotherapy were given as above, but without rituximab. Supportive care was administered as per institutional guidelines, with recommendation for irradiation of blood products and intravenous gammaglobulin for immunoglobulin G levels < 25% of lower limit of normal for age.
Definitions
Evaluation of response was by imaging and marrow evaluation if positive at diagnosis. Response definitions included: complete response (CR) defined as no detectable residual disease, partial response (PR) as residual disease with > 50% reduction in the size of all measurable tumor areas and no evidence of marrow or CNS disease, stable disease (SD) as < 50% reduction in all sites of measurable disease or < 25% increase in tumor mass, and progressive disease (PD) as appearance of new tumor at any site or > 25% increase in tumor mass. Events were defined as death, failure to achieve complete remission, disease recurrence and/or loss of functioning allograft. Loss of functioning original allograft was defined as having undergone a subsequent allograft, resection of the original allograft, return to organ replacement therapy, or death from loss of allograft function. Patients were considered treatment failures and removed from the study for any event. Overall survival (OS) was defined as the time from enrollment to death or last contact, and patients alive at last contact were treated as censored.
Statistical Methods
An interim analysis for efficacy and futility was performed after 5 events were observed. The second interim analysis was performed about 1 year later. Patient characteristics, toxicity and response to treatment were summarized using frequencies and percentages. For event free survival (EFS) analysis, patients not experiencing an event as defined were treated as censored. Comparison of survival distributions by prognostic factors was performed with the log-rank test. To compare survival distributions by response at 13 weeks a landmark analysis was conducted. Landmark analysis defines EFS as time from completion of experimental intervention, i.e. rituximab, (the landmark) to relapse, progressive disease, allograft loss, death, or last contact whichever occurred first. Only patients alive, on study and evaluated as a CR, PR, or SD at 13 weeks were included in the landmark analysis. SAS™ software Version 9.2 was used for data analysis (SAS™ Institute Inc., Cary, NC).
Results
Patient and Disease Characteristics
Fifty-five patients were enrolled (51 with NF-PTLD and 4 with F-PTLD). One patient was ineligible for not meeting inclusion criteria, due to less than 1 week of reduction of immunosuppression before enrollment. The study opened at 75 centers, but patients were enrolled at only 23 centers from 4/2004 and 7/2008. Characteristics of patients and disease are summarized in Table 1.
Table 1.
Patient and Disease Characteristics
| Total | ||
|---|---|---|
| N | 54 | |
| Gender, N (%) | Female | 21 (39%) |
| Male | 33 (61%) | |
| Median Age (years) (Range) | 9.7 (0.8 – 19.4) | |
| Age (years), N (%) | < 4 years | 12 (22%) |
| 4 – 15 years | 28 (52%) | |
| > 15 years | 14 (26%) | |
| Race | Caucasian | 37 (69%) |
| African American | 7 (13%) | |
| Native American | 1 (2%) | |
| Unknown | 9 (16%) | |
| Ethnicity | Latino/Hispanic | 7 (13%) |
| Not Latino/Hispanic | 45 (83%) | |
| Unknown | 2 (4%) | |
| Type of organ transplanted, N (%) | Heart | 11 (20%) |
| Kidney/renal | 17 (31%) | |
| Liver | 17 (31%) | |
| Lung | 5 (9%) | |
| Intestine | 2 (4%) | |
| Multiple organs | 2 (4%) | |
| Histology, N (%) | Monomorphic | 29 (73%) |
| Polymorphic | 8 (20%) | |
| Monomorphic & Polymorphic | 3 (7.5%) | |
| Not reviewed | 14 | |
| Clonality, N (%) | Monoclonal | 12 (55%) |
| Polyclonal | 9 (41%) | |
| Monoclonal & Polyclonal | 1 (4%) | |
| Not reviewed/Not Performed | 32 | |
| St. Jude's Stage, N (%) | I | 4 (7%) |
| II | 11 (20%) | |
| III | 37 (69%) | |
| IV | 2 (4%) |
NF-PTLD - non-fulminant PTLD, F-PTLD – fulminant PTLD
Disease characteristics varied widely amongst the patients in this study. Seventy-three percent had disseminated disease (stage III/IV) and 2 patients had marrow involvement. Pathology reports were reviewed on all patients and sufficient material for central pathology review was available for 40 patients (74%). The majority of patients had monomorphic disease (73%); while three cases had discordant morphology at different biopsied sites of disease, i.e. monomorphic disease found at one diagnostic biopsy site and polymorphic disease at another. Clonality determination was available in 22/54 cases and determined to be monoclonal in 12 cases.
Toxicity and Allograft Status
Toxicity on all patients was graded and reported after cycle 2 (chemotherapy and all 6 doses of rituximab) and after cycle 4 and 6 (chemotherapy only). One patient experienced supraventicular tachycardia with rituximab administration and received no further doses. In all other patients, rituximab did not appear to add significant overall toxicity. The only Grade III/IV occurring in > 10% of patients was neutropenia (absolute neutrophil count <1000/mm3), which occurred in 14% of patients. Fever or documented infection was reported in 7% of patients with neutropenia (7%), while infection with normal neutrophil counts occurred in an additional 7% of patients. There was no difference in the incidence of and grade III/IV toxicities after cycles 2 (with rituximab) and cycles 4, 6 (without rituximab). There was no delay in therapy or dose reductions due to complications, except for one patient who was in complete remission after 4 cycles of treatment but did not receive the last two cycles of chemotherapy due prolonged neutropenia; this patient remained in complete remission long-term. Ten patients died, three (6%) due to infection while receiving therapy, and 7 due to PTLD. While 5 patients (7%) lost their allografts, 2 kidney recipients had a nephrectomy performed to remove PTLD that was stable disease; one following 2 cycles of chemotherapy and rituximab and one at completion of therapy. The other 3 patients had loss of allograft function at 2, 2.5 and 4 years from study enrollment. Two secondary malignancies were observed, an EBV (+) leiomyosarcoma and a basal cell carcinoma, occurring 2.5 and 4 years after therapy, respectively.
Response and Outcome
Early treatment response rate (CR + PR) following the first two cycles of chemotherapy and 6 doses of rituximab was 72% (95% CI: 57%-84%). All 4 F-PTLD patients achieved a CR by the end of cycle 4 of therapy. Twenty-nine patients achieved CR by the end of therapy. Twelve patients had residual disease by radiographic imaging at the end of therapy, but 8 of these 12 patients were reported to be in CR one year from study enrollment without any other therapy for PTLD. Therefore, 37 patients (69%, 95% CI: 56%-81%) ultimately achieved CR with this therapy. Nineteen patients experienced an event [progressive disease before achieving CR (n=8), relapse after achieving CR (n=3) allograft loss (n=5), death due to infection while on treatment (n=3)]. One relapse occurred at 2.7 years after study enrollment, the other two occurred within the first year.
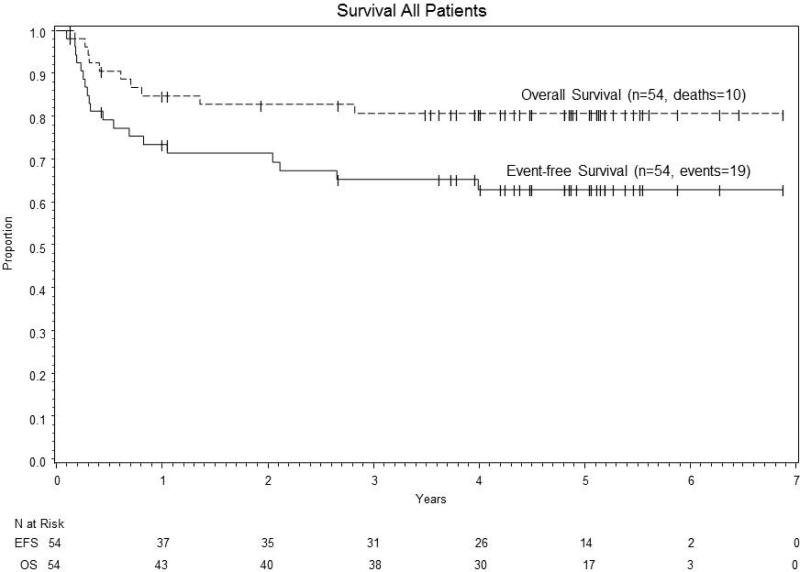
The 2 year event-free survival was 71% (95% CI: 57%-82%). (Figure 1A) All five patients with loss of function of allograft remained alive and disease-free with either organ replacement therapy or re-transplant. Five of the 11 patients with progressive disease or relapse achieved a CR with further therapy, though one patient died of unknown causes 2 years after achieving CR. Therefore, the 2 year overall survival (OS) was 83% (95% CI: 69%-91%). (Figure 1) The median follow-up for patients is 4.8 years (0.1 – 6.9 years).
Figure 1.
Event –free survival and overall survival using low-dose chemotherapy and rituximab for PTLD
Prognostic factors
Though the study was not designed to have sufficient power to assess risk factors; tumor morphology (monomorphic vs. polymorphic), clinical stage (localized stage I/II vs. disseminated stage III/V), allograft type (heart, kidney, liver and other) and response after cycle 4 of therapy were analyzed for predicting outcome. (Table 2) The 3 cases with both monomorphic and polymorphic morphology in different biopsies at diagnosis were excluded. Though patients with monomorphic disease appeared to have had a better outcome compared to polymorphic disease this was not statistical significant. Outcome for patients with disseminated disease (stage III/IV) appeared to be superior to patients with localized disease (stage I/II) but again this difference was not significantly different. There was no significant difference in outcome by allograft type or patients who had achieved complete response, partial response or had stable disease after 4 cycles of therapy.
Table 2.
Factors in patients and outcome
| Event-free survival | Overall Survival | |||
|---|---|---|---|---|
| 2-year rate | P-value | 2-year rate | P-value | |
| All PTLD | 71% (57%-82%) | 83% (69%-91%) | ||
| Stage | ||||
| I/II | 53% (26%-74%) | 0.072 | 67% (38%-85%) | 0.068 |
| III/IV | 79% (61%-89%) | 89% (73%-96%) | ||
| Allograft type | ||||
| Heart | 70% (33%-89%) | 0.34 | 90% (47%-99%) | 0.46 |
| Kidney | 64% (37%-82%) | 76% (49%-90%) | ||
| Liver | 82% (54%-94%) | 94% (65%-99%) | ||
| Other | 67% (28%-88%) | 65% (25%-87%) | ||
| Morphologic appearance | ||||
| Monomorphic | 76% (56%-88%) | 0.59 | 90% (71%-97%) | ** |
| Polymorphic | 58% (18%-84%) | 71% (26%-92%) | ||
| Landmark analysis (event-free survival from 13 weeks) | ||||
| 13 Week Response | ||||
| Complete remission | 80% (50%-93%) | 0.67 | 87% (56%-96%) | ** |
| Partial remission | 78% (52%-91%) | 100% | ||
| Stable disease | 71% (26%-92%) | 100% | ||
NF-PTLD - non-fulminant PTLD, CR – complete remission
too few events for significant estimation
Discussion
This study represents the largest cohort of pediatric PTLD patients treated on a prospective trial using a uniform treatment regimen. This study demonstrates that combining low-dose cyclophosphamide, prednisone and 6 weekly doses of rituximab is effective in treating pediatric patients with PTLD. The efficacy of this regimen has been reported previously in small series. (17, 18) It is difficult to determine the effect of the addition of rituximab to this chemotherapy backbone by comparing this cohort to the historical cohort. (8) Even with eligibility criteria and endpoint definitions being the same for both studies, definitive conclusions cannot be made due to the heterogeneity of PTLD and the small numbers in both studies. However, the addition of rituximab does not appear to add significant toxicity to this chemotherapy regimen. There was no higher incidence of grade III/IV toxicity with cycles that included rituximab compared to cycles with chemotherapy only. Though both studies required a trial of reduction of immunosuppression (RIS), the duration of RIS was not captured in either study, and the number of previous therapies or time from first diagnosis of PTLD until study enrollment was not collected for either study. The current study had a lower complete remission (CR) rate of 69% compared to 84% in the previous study without rituximab. This might suggest that patients in this current study had more aggressive or resistant disease upon study entry. The numbers of patients with fulminant (F-PTLD) in both studies were small. However, such patients appear to benefit from the addition of rituximab, as 4/4 patients were event-free survivors with rituximab as compared to no survivors utilizing this low-dose chemotherapy regimen alone. (8)
An interesting finding involved the outcome of 12 patients reported to have persistent disease at end of therapy. One of these patients was lost to follow-up, one had removal of allograft with stable disease at end of therapy, but 8 (67%) were reported to be in complete remission 28 weeks later without further therapy for their PTLD. There are several possible explanations for this observation. Since no biopsy reports of residual masses were available, a likely explanation is there was no viable residual tumor present despite a residual mass. However, there may have been a delayed effect of rituximab, as delayed remissions were not seen in the previous study without rituximab and delayed responses have been reported in the treatment of non-Hodgkin lymphoma (NHL) with rituximab. (19) A study using rituximab combined with chemotherapy to treat pediatric B-cell NHL demonstrated that the half-life of rituximab to be 28 days with detectable levels of rituximab in blood up to 9 months after last infusion. (20) Another possible explanation is that the recovering immune system eradicated residual tumor. Following chemotherapy, the decision of when and how to restart immune suppression was left to the discretion of the treating physician, although physicians were encouraged to maintain as low of level of immunosuppression as possible without compromising the allograft. Additionally, there are animal data that suggests low-dose cyclophosphamide is an effective adjuvant for tumor vaccines, perhaps by inhibiting regulatory T-cells. (20) Of note, two patients were rendered tumor free by removal of their allograft when it was felt the tumor was non-responsive. The results of this study suggest that patients, even with stable disease, may continue to respond at a very slow pace; therefore, an expectant approach in situations of persistent disease might allow patients to retain their allograft while having the PTLD eradicated.
Less than half of the patients with progressive or relapsed disease were salvaged with more aggressive chemotherapy. This illustrates the need to predict which patients may benefit from more aggressive therapy upfront. The literature has conflicting data on prognostic parameters in PTLD, which is likely a reflection of low number of patients, heterogeneous disease and/or treatments in a particular study. In this study, since uniform therapy was given and all PTLD was EBV and CD20 positive, we attempted to identify prognostic factors. However, the small numbers provided limited power to detect differences.
In this study, morphology was not found to be a predictor of outcome. The finding of 3 patients with different morphology at two different affected sites at diagnosis illustrates that PTLD may be heterogeneous even in the same patient and a single biopsied site may not be representative of all disease. (15) The majority (73%) of patients in this study had a biopsy demonstrating monomorphic disease, which is higher than other large series of pediatric PTLD (5, 21). Polymorphic PTLD in pediatrics appears to respond more frequently to RIS and therefore fewer patients with polymorphic disease may have been eligible for this study. (6) The results of this trial suggest that after a trial of RIS that morphology is a poor predictor of outcome. It has been reported that Burkitt lymphoma histology in PTLD predicts worse outcome. (22, 23) There were only 5 cases with Burkitt histology in this cohort but 4 were long-term disease-free survivors. However, cytogenetics and/or c-myc rearrangement status is not known in all of these cases. Others have shown that marrow or CNS involvement, i.e. stage IV disease, predict poor outcome. (4, 23, 24) Patients with CNS involvement were excluded from this study, due to the concern that these low doses of cyclophosphamide and rituximab would not penetrate the blood-brain barrier effectively. There were only 2 patients with marrow involvement in this cohort with one being a long-term disease-free survivor.
In summary, this regimen appears to be effective and safe for pediatric patients with PTLD even in patients with an aggressive fulminant form of the disease. It is difficult to extrapolate these results to all PTLD. This study was restricted to CD20 (+) PTLD. There are reports that CD20 (-) disease has inferior outcome, but others studies have not been able to confirm this. (25, 26, 27) It is also unclear if this approach is effective for adult patients with EBV (+), CD20 (+) PTLD. These results also suggest that parameters that usually predict prognosis in non-Hodgkin lymphoma, i.e. histology, stage or initial response to therapy, may not be as relevant for PTLD. The most pertinent question that remains is how to predict which patients will respond to reduction of immunosuppression only, which patients will respond to rituximab alone, which patients will require a low-dose chemotherapy regimen +/- rituximab and which patients might benefit from more aggressive chemotherapy upfront. Future prospective large multi-center, prospective trials will hopefully increase our insight into this disease.
Acknowledgements
Research is supported by the Chair's Grant U10 CA98543 and Statistics and Data Center Grant U10 CA98413 of the Children's Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH.
Abbreviations
- PTLD
Post-transplant lymphoproliferative disease
- EBV
Epstein-Barr virus
- RIS
reduced immunosuppression
- CR
complete remission
- WHO
World Health Organization
- SOT
solid organ transplant
- F-PTLD
Fulminant post-transplant lymphoproliferative disease
- CNS
central nervous system
- NCI CTCAE
National Cancer Institute Clinical Toxicity and Adverse Event
- COG
Children's Oncology Group
- EBER
Epstein-Barr early RNA
- PR
partial remission
- SD
stable disease
- PD
progressive disease
- EFS
event free survival
- NF-PTLD
non-fulminant post-traansplant lymphoproliferative disease
- OS
overall survival
- NHL
non-Hodgkin lymphoma
- CI
confidence interval
Footnotes
Preliminary data of this work was presented at 5th World Congress of the International Pediatric Transplant Association in 2009 and 3rd International Symposium on Childhood, Adolescent and Young Adult Non-Hodgkin's Lymphoma in 2009.
Author contributions- significant participation in study design, data collection and interpretation and manuscript preparation (TGG, MAO, SLP, JRP,MSC, RJH) study design and data collection (JCL), data collection, analysis and interpretation and manuscript preparation (LMS).
Disclosure
Genentech USA Inc/Roche Advisory Board (TGG). None of the other authors have conflicts of interests to disclose as described by the American Journal of Transplantation.
References
- 1.Dharnidharka VR, Tejani AH, Ho Pl, Harmon WE. Post-transplant lymphoproliferative disorder in the United States: young Caucasian males at highest risk. Am J Transplant. 2002;2(10):993–998. doi: 10.1034/j.1600-6143.2002.21019.x. [DOI] [PubMed] [Google Scholar]
- 2.Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, et al. Posttransplant lymphoproliferative disease in pediatric liver transplantation. Interplay between primary Epstein-Barr virus infection and immunosuppression. Transplantation. 1996;62(3):370–375. doi: 10.1097/00007890-199608150-00012. [DOI] [PubMed] [Google Scholar]
- 3.Allen UD, Farkas G, Hébert D, Weitzman S, Stephens D, Petric M, et al. Risk factors for post-transplant lymphoproliferative disorder in pediatric patients: a case-control study. Pediatr Transplant. 2005;9(4):450–455. doi: 10.1111/j.1399-3046.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 4.Maecker B, Jack T, Zimmermann M, Abdul-Khaliq H, Burdelski M, Fuchs A, et al. CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation. J Clin Oncol. 2007;25(31):4902–8. doi: 10.1200/JCO.2006.10.2392. [DOI] [PubMed] [Google Scholar]
- 5.Webber SA, Naftel DC, Fricker FJ, Olesnevich P, Blume ED, Addonizio L, et al. Lymphoproliferative disorders after paediatric heart transplantation: multi-institutional study. Lancet. 2006;367:233–239. doi: 10.1016/S0140-6736(06)67933-6. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi RJ, Kraus MD, Patel AL, Canter C, Cohen AH, Hmiel P, et al. Posttransplant lymphoproliferative disease in children: correlation of histology to clinical behavior. J Pediatr Hematol Oncol. 2001;23(1):14–8. doi: 10.1097/00043426-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am J Transplant. 2011;11(2):336–347. doi: 10.1111/j.1600-6143.2010.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross TG, Bucuvalas JC, Park JR, Greiner TC, Hinrich SH, Kaufman SS, et al. Low-dose chemotherapy for Epstein-Barr virus-positive post-transplantation lymphoproliferative disease in children after solid organ transplantation. J Clin Oncol. 2005;23(27):6481–8. doi: 10.1200/JCO.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 9.Oertel SH, Verschuuren E, Reinke P, Zeidler K, Papp-Vary M, Babel N, et al. Effect of anti-CD20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transplant. 2005;5(12):2901–2906. doi: 10.1111/j.1600-6143.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 10.Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006;107(8):3053–3057. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 11.Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, et al. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6(3):569–576. doi: 10.1111/j.1600-6143.2005.01211.x. [DOI] [PubMed] [Google Scholar]
- 12.Trappe RU, Choquet S, Reinke P, Dreyling M, Mergenthaler HG, Jäger U, et al. Salvage therapy for relapsed posttransplant lymphoproliferative disorders (PTLD) with a second progression of PTLD after upfront chemotherapy: the role of single-agent rituximab. Transplantation. 2007;84(12):1708–1712. doi: 10.1097/01.tp.0000295987.12996.19. [DOI] [PubMed] [Google Scholar]
- 13.Choquet S, Trappe R, Leblond V, Jäger U, Davi F, Oertel S. CHOP-21 for the treatment of post-transplant lymphoproliferative disorders (PTLD) following solid organ transplantation. Haematologica. 2007;92(2):273–274. doi: 10.3324/haematol.10595. [DOI] [PubMed] [Google Scholar]
- 14.Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196–206. doi: 10.1016/S1470-2045(11)70300-X. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow SH, Webber SA, Chadburn A. Post-transplant lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. International Agency for Research on Cancer; Lyon, France: 2008. pp. 343–9. [Google Scholar]
- 16.Murphy SB, Fairclough DL, Hutchison RE, Berard CW. Non-Hodgkin's lymphomas of childhood: an analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol. 1909;7(2):186–193. doi: 10.1200/JCO.1989.7.2.186. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Fricker FJ, González-Peralta RP, Slayton WB, Schuler PM, Dharnidharka VR. Post-transplant lymphoproliferative disorder in children: recent outcomes and response to dual rituximab/low-dose chemotherapy combination. Pediatr Transplant. 2010;14(7):896–902. doi: 10.1111/j.1399-3046.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 18.Orjuela M, Gross TG, Cheung YK, Alobeid B, Morris E, Cairo MS. A pilot study of chemoimmunotherapy (cyclophosphamide, prednisone, and rituximab) in patients with post-transplant lymphoproliferative disorder following solid organ transplantation. Clin Cancer Res. 2003;9(10 Pt 2):3945S–3952S. [PubMed] [Google Scholar]
- 19.Coiffer B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multi-center phase II study. Blood. 1998;92(6):1927–1932. [PubMed] [Google Scholar]
- 20.Cairo MS, Lynch JC, Harrison L, Perkins SL, Shiramizu B, Gross TG, et al. Safety, kinetics, and outcome following rituximab (R) in combination with FAB chemotherapy in children and adolescents (C+A) with stage III/IV (Group B) and BM+/CNS+ (Group C) mature B-NHL: A Children's Oncology Group report. J Clin Oncol. 2010;28(15S):687s. [Google Scholar]
- 21.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, et al. Cyclophosphamide induces type I interferon and augments the number of CD44hi T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95(6):2024–2030. [PubMed] [Google Scholar]
- 22.Picarsic J, Jaffe R, Mazariegos G, Webber SA, Ellis D, Green MD, et al. Post-transplant Burkitt lymphoma is a more aggressive and distinct form of post-transplant lymphoproliferative disorder. Cancer. 2011;117(19):4540–50. doi: 10.1002/cncr.26001. [DOI] [PubMed] [Google Scholar]
- 23.Windebank K, Walwyn T, Kirk R, Parry G, Hasan A, Bown N, et al. Post cardiac transplantation lymphoproliferative disorder presenting as t(8;14)Burkitt leukaemia/lymphoma treated with low intensity chemotherapy and rituximab. Pediatr Blood Cancer. 2009;53(3):392–3966. doi: 10.1002/pbc.22070. [DOI] [PubMed] [Google Scholar]
- 24.Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P, First MR, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc. 2005;37(2):954–955. doi: 10.1016/j.transproceed.2004.12.130. [DOI] [PubMed] [Google Scholar]
- 25.Orjuela MA, Alobeid B, Liu X, Siebert AL, Kott ER, Addonizio LJ, et al. CD20 expression predicts survival in paediatric post-transplant lymphoproliferative disease (PTLD) following solid organ transplantation. Br J Haematol. 2011;152(6):733–42. doi: 10.1111/j.1365-2141.2010.08448.x. [DOI] [PubMed] [Google Scholar]
- 26.Ghobrial IM, Habermann TM, Ristow KM, Ansell SM, Macon W, Geyer SM, McGregor CG. Prognostic factors in patients with post-transplant lymphoproliferative disorders (PTLD) in the rituximab era. Leuk Lymphoma. 2005;46(2):191–6. doi: 10.1080/10428190400012011. [DOI] [PubMed] [Google Scholar]
- 27.Dharnidharka VR, Martz KL, Stablein DM, Benfield MR. Improved survival with recent Post-Transplant Lymphoproliferative Disorder (PTLD) in children with kidney transplants. Am J Transplant. 2011;11(4):751–8. doi: 10.1111/j.1600-6143.2011.03470.x. [DOI] [PubMed] [Google Scholar]