Abstract
In June 2008, the Cedar River crested flooding more than 5,000 Cedar Rapids homes. Residents whose homes were flooded were invited to participate in this study. Household assessments and resident interviews were conducted between November 2008 and April 2009. We characterized exposures and symptoms experienced by individuals inhabiting 73 flood-damaged homes. Active air sampling and passive electrostatic dust collectors were used to assess exposures to: culturable mold, culturable bacteria, fungal spores, inhalable particulate matter (iPM), endotoxin, glucans, allergens, lead, asbestos, radon, carbon dioxide, and carbon monoxide. Wall moisture levels and relative humidity were also measured. Exposures and questionnaire-based health assessments were compared at two levels of remediation, in-progress and completed. Homes with remediation in-progress (n=24), as compared to the completed homes (n=49), had significantly higher airborne concentrations of mold, bacteria, iPM, endotoxin and glucan. Residents of in-progress homes had a significantly higher prevalence of doctor diagnosed allergies (adjusted OR=3.08; 95%CI: 1.05–9.02) and all residents had elevated prevalence of self-reported wheeze (adjusted OR=3.77; 95%CI: 2.06–6.92) and prescription medication use for breathing problems (adjusted OR=1.38; 95%CI: 1.01–1.88) after the flood as compared to before. Proper post-flood remediation led to improved air quality and lower exposures among residents living in flooded homes.
Introduction
Higher global mean temperatures over land induced by increased concentrations of greenhouse gases produce higher humidity and more frequent extreme precipitation events (Intergovernmental Panel on Climate Change, 2008). Data from the past 100 years suggest a rapidly changing hydrological cycle in the Northern Hemisphere with decreased extent of sea ice, lower glacial mass and increased incidents of flooding. Land use changes driven by increasing population and conversion of wetlands to agricultural use have increased the potential for and severity of flooding in the Midwest U.S. (Iowa Climate Change Impacts Committee, 2010).
The Cedar River flows from North Central Iowa through the city of Cedar Rapids and then joins the Iowa River near the confluence with the Mississippi River. On June 13, 2008, after a snowy winter and prolonged rainfall, the Cedar River crested flooding 10 square miles of the city and impacting 5,390 homes in what was considered a 500 year flood (Robinson, 2010) After the flood waters receded, public health authorities were inundated with concerns about possible contaminants in homes and were asked if it was safe for residents to return to their houses after remediation. Public health officials noted a paucity of research that could inform their decisions.
Studies of flood-ravaged homes prior to remediation reported that homes have elevated levels of fungi and bacteria (Chew et al., 2006; Rao et al., 2007). Chew et al. detected extremely high concentrations of several bioaerosols inside homes beginning three months after the homes were impacted by Hurricanes Katrina and Rita in New Orleans, Louisiana. That study analyzed bioaerosols in three houses before, during, and after intervention. The results showed the highest concentrations during intervention. Rao et al. entered 5 mildly damaged and 15 moderately to heavily water-damaged homes two months (October 2005) after the houses were impacted by Hurricane Katrina in New Orleans. Rao et al. detected high concentrations of endotoxin, glucan, spores, and culturable fungi in many of the homes.
The microorganisms that were sampled are important because they have previously been linked to adverse health effects in exposed humans. It is known that damp indoor environments promote the growth of mold and bacteria (Institute of Medicine, 2011). Occupational exposures to bioaerosols including mold, bacteria, fungal spores, allergens, endotoxin, and fungal glucan have been linked to lung inflammation, organic dust toxic syndrome, allergic hypersensitivities, asthma, and other respiratory problems(Douwes et al., 2003; Green et al., 2006; Kurup et al, 2000; Thorne et al., 2006; Thorne and Duchaine, 2007). Endotoxin is a lipopolysaccharide contained in the outer membrane of Gram-negative bacteria and fungal glucans are polysaccharide components of the fungal cell. Bioaerosols can act as pathogen-associated molecular patterns, or in some cases allergens, to trigger immune responses leading to upper and lower airway irritation and inflammation (Sigsgaard et al., 2008). Organic dust toxic syndrome occurs after exposure to high levels of organic dust and manifests as flu-like symptoms (malaise, cough, headache, nausea, etc.) (Schenker et al., 1998).
A recent report from the Institute of Medicine on Climate Change, the Indoor Environment, and Health found “sufficient evidence to conclude that there is an association between indoor- dampness-related agents and asthma exacerbation, upper respiratory tract symptoms, cough, and wheeze.” They estimate that about 20% of current asthma in the United States can be attributed to dampness in the home. In addition, aggravated and new onset symptoms may occur more frequently in individuals exposed to moldy conditions (Institute of Medicine, 2011). The high prevalence of asthma has prompted public health officials to seek causal linkages and initiate primary prevention.
Individuals entering flood-damaged structures expose themselves to potentially high levels of bioaerosols and frequently report respiratory ailments with names such as “Katrina Cough” and “Flood Crud” (Thorne, 2007). This malaise is accompanied by a persistent cough and often sinus congestion. A study of firefighters who had contact with floodwater in New Orleans after Hurricane Katrina had an ‘increased rate of developing new-onset upper respiratory symptoms’, such as sinus congestion, cough, and throat irritation (Tak et al., 2007).
We launched this project to address two aims: first, to determine the concentration of contaminants inside flood-damaged homes during and after remediation; and second, to determine if the residents of these homes were experiencing higher rates of adverse health effects. A home inspection, lab analysis, and health questionnaire were utilized. We anticipated that high exposures to airborne contaminants would give rise to adverse health effects. Individuals with pre-existing conditions and co-morbidities were expected to exhibit more symptoms than healthy individuals following exposure from post flood contamination
We hypothesized that 1) bioaerosol contaminants in homes with remediation completed would be significantly lower than in the in-progress homes; 2) prevalence of current adverse respiratory health effects would be lower among residents of completed homes; and 3) with proper construction techniques, remediated homes would have levels of indoor air pollutants within recommended indoor air quality (IAQ) guidelines.
Materials and Methods
Subject Recruitment
Residents were contacted via a mailer sent to the neighborhoods impacted by the flood of 2008. Individuals expressing interest in participation were scheduled for an assessment visit. Inclusion criteria were that the homes experienced water damage in the flood of 2008, were marked with a yellow or green placard by the Code Enforcement Division of Cedar Rapids (further detailed in Results), were either completed or in-progress of remediation, the residents of the homes had either moved back in or were planning to move back in upon completion, and the respondent was living in the house or was performing the remediation inside the home. Seventy-three residences were successfully enrolled on a first-response basis and participated in the study. The demographics of the enrolled subjects are shown in Table 1.
Table 1.
The remediation in-progress and completed home participants questionnaire responses.
| Characteristic | Remediation In-Progress | Remediation Completed |
|---|---|---|
| Air Assessment Total, n | 24 | 49 |
| Location | ||
| Cedar Rapids | 21 | 44 |
| Palo | 3 | 4 |
| Central City | 0 | 1 |
| House Conditions | ||
| Yellow Placard, % | 96% | 84% |
| Median Year Home Built(Range) | 1940(1890–2002) | 1962(1813–2008) |
| Re-occupied Home, n | 13 | 49 |
| Pets, % | ||
| Dog | 38 | 37 |
| Cat | 42 | 22 |
| Bird | 4 | 2 |
| Other | 0 | 6 |
| Total % with pets | 58 | 45 |
| Participants, n | 23 | 48 |
| Age, mean±SD | 49.8±9.1 | 53.6±19.0 |
| Sex, % women | 78 | 73 |
| Active/passive smoking: | ||
| Never, % | 48 | 44 |
| Passive smoking only, % | 26 | 6 |
| Active smoking, % | 17 | 25 |
| Former active smoking, % | 35 | 31 |
| Occupational status: | ||
| Manager/professional, % | 52 | 33 |
| Technicians, % | 4 | 2 |
| Other non-manual, % | 17 | 23 |
| Manual, % | 17 | 27 |
| Retired, % | 9 | 13 |
| Unknown,% | 0 | 2 |
Survey
Indoor air assessments, home inspections and administration of questionnaires were performed by one of the authors (KH). Enrollment procedures and questionnaires were approved by the Institutional Review Board and informed consent was obtained in writing from each participant. Questionnaires were used to assess demographics, pulmonary health history, current respiratory status, duration of time spent in the home, participation in remediation activities, the use of personal protective equipment (PPE), and other variables related to potential exposures. Questions were drawn from established instruments including the SF-36, the European Community Respiratory Health Survey II (ECRHS), the Louisa Environmental Intervention Project (LEIP), and several original questions related specifically to the flood (Siroux et al., 2008; Thorne et al., 2006; Ware et al., 1992). Questionnaire data were double entered into a database and verified.
Home Inspection
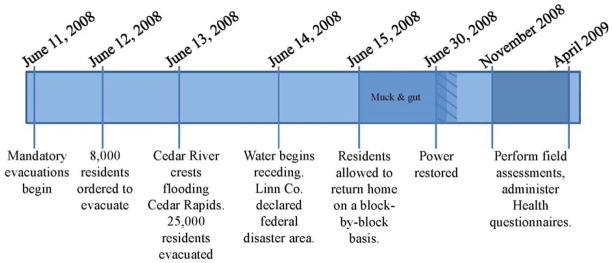
Homes were assessed at two levels of remediation during the field sampling, beginning four months after the flood (Figure 1): those homes where remediation was completed and those where remediation was in-progress. Remediation followed “mucking and gutting” and generally entailed removal and replacement of cabinetry, drywall, flooring and insulation with a drying out period between removal and replacement, as categorized in Table 2. An established home inspection survey served to collect information to assess variables that could contribute to adverse health effects or impact the IAQ. Carbon monoxide (CO), carbon dioxide (CO2), temperature, and relative humidity were measured using a Q-Trak sampler™ (Model 7545, TSI Inc., Shoreview, MN). Wall moisture readings were collected using a Moisture Encounter (Tramex Ltd., Littleton, CO) on the main support beams, drywall, and on the basement walls of the homes.
Figure 1.
Timeline of this study and of the flood in Cedar Rapids, Iowa.
Table 2.
Questionnaire data for remediation methods and exposure risk factors.
| Remediation In-Progress | Remediation Completed | |
|---|---|---|
| Participants, n | 23 | 48 |
| Removal, % | ||
| Drywall | 100 | 73 |
| Appliances | 100 | 88 |
| Furniture | 91 | 83 |
| Flooring | 96 | 81 |
| Other | 61 | 42 |
| Drying, % | 96 | 96 |
| Fans | 83 | 83 |
| Dehumidifiers | 74 | 90 |
| Opened Windows | 91 | 4 |
| Nothing | 0 | 19 |
| Other | 9 | 19 |
| How soon after the flood did you re-enter home to begin the muck & gut, % | ||
| less than 24 hrs | 17 | 10 |
| 1day - 1 week | 57 | 60 |
| 1–3 weeks | 26 | 23 |
| Over a month | 0 | 6 |
| Precautionary methods during mucking & gutting, % | ||
| Wear mask | 74 | 67 |
| Wear gloves | 74 | 73 |
| Disposed of clothes | 87 | 83 |
| Boots/hipwaders | 83 | 54 |
| No protective gear | 9 | 10 |
| Did not participate in cleanup | 4 | 0 |
Air Sampling
An IAQ assessment was conducted to quantify asbestos, lead, culturable bacteria, culturable mold, fungal spores, iPM, endotoxin, glucan, and eight common household allergens. Active samplers included closed-face cassettes for asbestos and lead, Andersen Microbial Samplers (AMS) for culturable bacteria and fungi, Air-O-Cell cassettes for fungal spores, and Button inhalable fraction samplers for iPM, endotoxin, and glucans (Douwes et al. 2003). Passive sampling was performed over a 14-day period using electrostatic dust collectors (EDC) for endotoxin, glucans, and allergens (Noss et al., 2008). Radon kits (AirChek, Inc.) were also deployed during the assessment. The State Hygienic Laboratory at The University of Iowa conducted the analysis of the asbestos and lead samples using NIOSH method 7400 for the asbestos and NIOSH Method 7300 for the lead (NIOSH, 1994; NIOSH, 2003).
AMS used tryptic soy agar with 0.1 g/L of cyclohexamide to culture bacteria and malt extract agar with 0.1 g/L of chloramphenicol was used to culture mold. The AMS were attached to a tripod set at 155 cm to approximate the breathing height and were operated at a calibrated flow rate of 28.3 L/min. AMS sampling was performed at 4 intervals: 0 (blank control), 1, 3, and 5 min in duplicate in the family room (FR) and a bedroom (BR). The plates were incubated for up to eight days at 28°C for bacteria and at 22°C for mold and a positive hole correction was applied to the colony forming units (CFU) (Macher, 1989). Air-O-Cell sampling cassettes were used to quantify fungal spores and were sampled for 5 min at 15.0 L/min. Air-O-Cells were stained using lactophenol blue for enumeration via microscopy using the transverse-traverse method (Yang and Heinsohn, 2007).
Inhalable particulate matter (iPM) was sampled using Button samplers cleaned with 1% E-toxa Clean (Sigma-Aldrich, St. Louis, MO) and rinsed five times with nanopure water. The samplers were loaded with endotoxin-free 25 mm glass fiber filters (SKC Inc., Eighty Four, PA) that were pre-weighed using a microbalance (reproducibility, 0.8 μg ; readability 1.0 μg) (MT-5, Mettler-Toledo Inc., Columbus, OH). Samplers were placed on the main level of the home away from vents and doors and set to run for 1440 min at 4.0 L/min. After post-weighing, the filters were stored at −20°C to await endotoxin and glucan extractions.
Air filter’s aqueous extracts were evaluated for endotoxin using the kinetic chromogenic Limulus Amebocyte Lysate (LAL) assay as previously described (Thorne, 2000). Linear (1→3)-β-D-glucan and branched (1→3)(1→6)-β-D-glucan were analyzed using a custom ELISA with polyclonal rabbit-antilaminarin IgG (U1) (1:2000 for capture) and biotinylated monoclonal mouse anti-laminarin IgG (B2) (1:1000 for labeling). The standards were curdlan for the linear (1→3)-β-D-glucan and baker’s yeast for branched (1→3)(1→6)-β-D-glucan (further described in the supplement).
EDC were assembled as described previously by Noss et. al. and were deployed nominally for 14-days on a flat surface to allow the folder to lay open with the cloths facing the ceiling. EDCs were placed at least 130 cm from the floor and 45 cm from the ceiling. The EDCs contained four electrostatic cloths, each with a surface area of 0.0205 m2, that were used for the analysis of endotoxin, glucans, and indoor allergens. One cloth was archived frozen. The extraction of endotoxin and glucan from the EDC cloths was performed as previously described in Noss et al. (2008). Allergens were analyzed using the Multiplex Array for Indoor Allergens protocol (Indoor Biotechnologies, Charlottesville, VA). Assessed allergens were: Der p1, Der f 1, mite group 2, Fel d 1, Can f 1, Mus m 1, Rat n 1, and Bla g 2. A time-weighted average was applied to the results to normalize deployment to 14-days.
Statistics
The bioaerosol concentrations and iPM data were log-normally distributed; therefore geometric mean (GM) and geometric standard deviation (GSD) were used to summarize the data. Other parameters were assessed using arithmetic means (AM) and SD. Values below the limit of detection (LOD) were assigned the value of the LOD/√2 (Hornung and Reed, 1990). Statistical analyses used SAS Version 9.2 (SAS Inc., Cary, NC) and included two-sample t-tests with test of equal variance to compare exposure measurements between the in-progress and completed homes. For health data, logistic regression was used to calculate odds ratios using a 95% confidence interval (CI) comparing in-progress and completed homes adjusting for age, sex, and smoking status (ever/never) and to determine whether there was a difference in the odds of experiencing the symptom during the past six months as compared to the odds of experiencing it 12 months ago, adjusting for remediation stage (in-progress/completed), age, sex, and smoking status (ever/never).
We employed geographic information system (GIS) mapping to assess whether locations and flooding impacted the in-progress and completed homes differently (ArcGIS version 9.3.1 ESRI, Redlands, CA, USA). Homes were geocoded using E911 address locations obtained from the Iowa GIS Data Repository. Figure S-1 (on-line supplement) shows the geocoded locations of the homes referenced to the Cedar River. A t-test revealed no significant difference in proximity to the Cedar River between the in-progress or completed homes. We used Moran’s I spatial statistics tool to test if spatial autocorrelation existed between the home locations and the concentrations or air contaminants (further discussed in online supplement).
Results
Demographics
Study homes required extensive clean out, demolition and reconstruction (Table 2). All of the remediation-completed participants were living in their homes as were the majority of the in-progress participants; however, 10 of the in-progress participants were working on their homes but sleeping elsewhere. On average, the remediation-completed participants took 83 days to reoccupy their homes. The remediation in-progress homes reported more extensive remediation compared with the completed homes; but the same percent (96%) of homeowners reported using an active house drying method post-flood (e.g., fans and dehumidifiers). Of the residents who participated in the remediation, 91% (in-progress) and 90% (completed) reported using some form of PPE. Seventy-four percent of the in-progress residents and 67% of the completed residents reported wearing a dust mask during mucking and gutting and/or remediation, although no subjects used sufficient respiratory protective measures such as full-face cartridge respirators or powered air purifying respirators.
Smoking history and occupational status for the cohort are presented in Table 1. The U.S. National Health Interview Survey reported that 21% of adults were current cigarette smokers and 21% were ex-smokers (Pleis, 2010). In our cohort, 17% of the in-progress participants and 25% of the completed participants were current smokers. However, the percent of former smokers was 52% and 56% for in-progress and completed homes, respectively. The occupations were classified as in the ECRHS study into five categories: manager/professional, technician, other non-manual, manual, and unknown/unclassified (Siroux et al., 2008). Participants engaged in manual labor averaged 17% and 27% with the higher representation in the remediation-completed group.
Environmental Assessment
Most enrolled households (88%) had been marked with a yellow placard by the Code Enforcement Division of the City of Cedar Rapids indicating that the structure had received some damage and limited entry was allowed. The remaining households were green placarded indicating that the homes had no structural damage. No homes that had been marked with a red placard (severe structural damage) were assessed since they were ultimately demolished. As shown in Table 1, environmental assessments were completed in 24 homes where remediation was in-progress and 49 homes where it was completed. Homes ranged widely in year of construction from 1813 to 2008. Households with pets included 58% of the in-progress homes and 45% of the completed homes, distributed as shown in Table 1.
Asbestos, lead, and radon sampling were conducted as a service to the residents. These results are displayed in Table 3 (grouped by the remediation stage), along with the gas-phase pollutants and moisture measurements obtained inside the homes. Asbestos and lead were a concern due to the remediation process and the advanced age of many of the homes. Renovation can mobilize asbestos and lead dust allowing for respiratory exposure and producing a potential health hazard. Fortunately, low levels of asbestos and unquantifiable lead concentrations were detected inside the homes. All of the asbestos concentrations were less than the workplace permissible exposure limit of 0.1 fibers/cc (OSHA 2002)
Table 3.
Arithmetic Mean (SD)(range) or Geometric mean (GSD)(range).of concentrations in the family room of study homes.
| Exposures | Remediation In-Progress | n/N | Remediation Completed | n/Nb |
|---|---|---|---|---|
| Asbestos (Fibers/cc) | 0.02(0.01) (0.01–0.06) | 13/21 | 0.03(0.02) (0.01–0.08) | 27/47 |
| Lead (mg/m3) | <Quantitation Limit | 0/24 | <Quantitation Limit | 0/49 |
| Radon (pCi/L) a | 2.4(3.0) (<0.3–18.9) | 14/24 | 2.3(2.2) (<0.3–11.2) | 37/45 |
| CO (ppm) | 0.03(0.16) (0–0.80) | 1/24 | 0.14(0.42) (0–2.3) | 6/49 |
| CO2 (ppm) | 560(210) (300–1240) | 24/24 | 860(470) (380–2900) | 49/49 |
| Relative Humidity (RH%) | 42(8.8) (28–65) | 24/24 | 37(10) (20–67) | 49/49 |
| Temperature (°C) | 17(4.5) (8–25) | 24/24 | 20(2.7) (9–25) | 49/49 |
| Wall Moisture (%) | 15.1(2.3) (7–20) | 24/24 | 15.6(3.3) (14–22) | 47/49 |
GSD: Geometric Standard Deviation
Geometric mean (GSD)(range).
Number detected (n)/Total number analyzed (N)
Due to the geological makeup of the State of Iowa, a large proportion of homes across Iowa exceed the federal standard for indoor radon of 4.0 pCi/L (Field et al., 2000). Radon testing was provided to the participants because radon is a priority environmental health concern and reconstruction provides an economical opportunity to install a radon mitigation system. Homes that were still undergoing significant remediation, and had an open building envelop, were given a radon test kit and directions to sample after closed conditions could be achieved. Radon measurements were obtained in 51 homes, 13 of which had a radon level above 4.0 pCi/L (Table 3). These participants were advised to install mitigation systems.
No homes had elevated CO levels, thus demonstrating proper exhaust of combustion products from appliances. CO2 measurements were performed to give insight into the occupancy and adequacy of ventilation of the home. The ASHRAE 62-1999 standard suggests levels should be maintained below 1000 ppm for an occupied home (ASHRAE, 1989). Eleven of the 73 homes assessed had CO2 concentrations above 1000 ppm and three of these homes registered over 2000 ppm. CO2 levels in the completed homes were significantly higher than the in-progress homes (arithmetic means: 860 ppm vs. 560 ppm; p<0.001) (Table 3). The homes in the study had acceptable relative humidity levels around 40%, the EPA recommends indoor air have 30–60% relative humidity, and wall moisture levels were low with means of 15.1% and 15.6% for in-progress and completed homes (U.S. Environmental Protection Agency, 2012). A study by Baxter et al. using the Tramex Moisture Encounter, classified homes have <15% wall moisture levels to be “clean” (Baxter et al., 2005). This was an important finding that suggested home owners and contractors were well informed regarding drying of wooden framed homes at the initial stages of remediation.
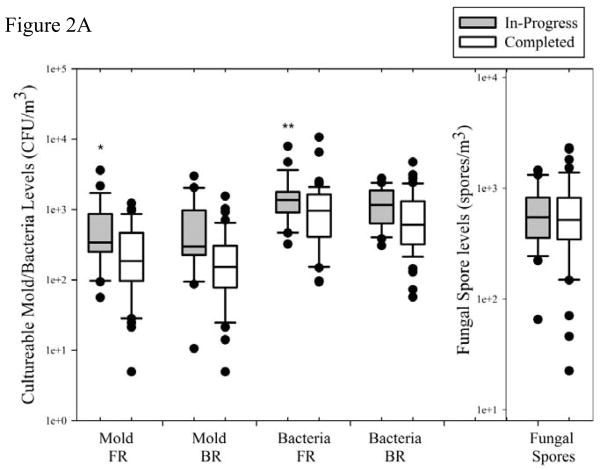
The IAQ assessment results in Supplement Table 1 show the GM, GSD, and ranges for all parameters assessed as well as the number of detectable samples (n) over the total number sampled (N). The fold change was calculated as the GM concentration for in-progress homes compared to the completed homes. Figures 2–3 are box plots that illustrate bioaerosol concentrations. There are no established standards for acceptable concentrations of culturable bacteria and mold. However, a common benchmark indicating an elevated level is more than 1000 CFU/m3.In the in-progress homes, 18/24 (75%) homes had airborne culturable bacteria concentrations above 1000 CFU/m3 in the FR and 10/20 (50%) homes had BR concentrations exceeding this benchmark (Figure 2A). In comparison, the completed homes had 23/48 (48%) in the FR and 17/49 (35%) in the BR with concentrations that exceeded 1000 CFU/m3. There was no significant difference in culturable bacteria and mold concentrations between the FR and BR of each home; therefore geometric means of the two locations were used as the measurement for culturable bacteria or mold in each home for subsequent statistical analysis. As shown in Supplement Table 1, the culturable bacteria concentrations were 1.6-fold higher (p=0.026) in the in-progress homes and the culturable mold concentrations were 2.6-fold higher (p=0.002).
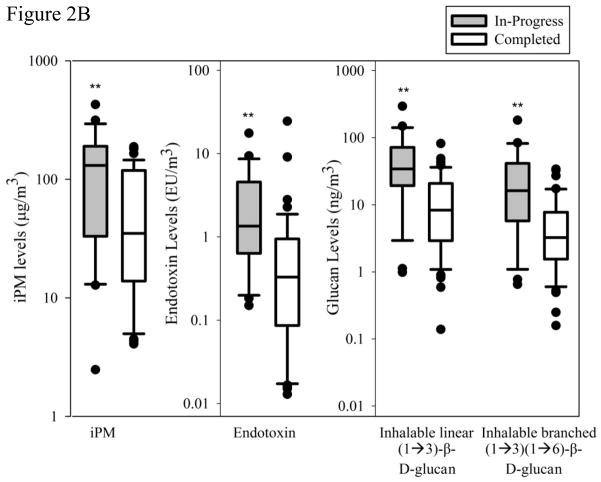
Figure 2.
Contaminant concentrations from active air sampling. The middle line on the Box plots represents the median value, the boundaries of the box represent the 25th and 75th percentile, the whisker caps represent the 10th and 90th percentile, and the dots represent the outliers. In-progress significantly higher than completed homes; *p < 0.05, **p < 0.01
Figure 2A. Culturable mold and bacteria concentrations in Family Room (FR), Bedroom (BR) for in-progress and completed homes. Fungal spore samples were collected in the FR.
Figure 2B. Inhalable particulate matter (iPM), endotoxin, linear (1→3)-β-D-glucan, and branched (1→3)(1→6)-β-D-glucan for the in-progress and completed homes.
Figure 3.
Contaminant concentrations from passive sampling.
Figure 3A. Endotoxin, linear (1→3)-β-D-glucan, and branched (1→3)(1→6)-β-D-glucan collected on the EDC for the in-progress and completed homes
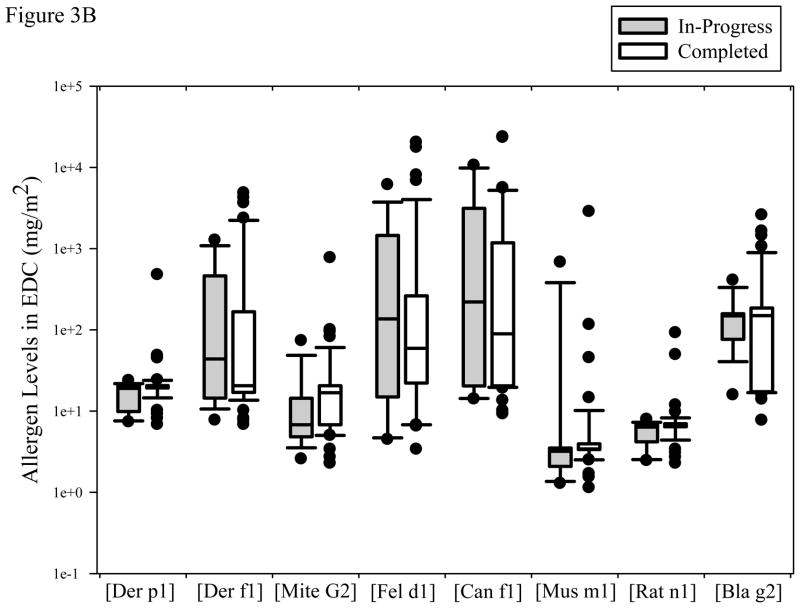
Figure 3B. Concentrations of 8 common household allergens for the in-progress and completed homes. Box plots were drawn as described in Figure 2.
None of the homes sampled exceeded >10,000 spores/m3 and there was no significant difference (p=0.59) in the fungal spore concentrations between the two remediation stages (Supplement Table 1 and Figure 2A). We conducted a previous study of bioaerosols in Iowa homes and found the mean fungal spore concentrations in the noncompliant (comparison group) in the basement to be about 3.8×103 spores/m3 (DeKoster and Thorne, 1995). Our samples were collected on the main level of homes and are substantially lower than previously found in our earlier study of Iowa homes. This could be attributed to seasonal differences with Iowa winters and springs having low ambient spore concentrations and/or the low levels of fungal spores may suggest successful flood recovery efforts over the duration of time since the flood.
A 2.3-fold higher iPM concentration (p=0.012) was observed in the in-progress homes compared to the completed homes (Supplement Table 1). Figure 2B shows the range of iPM measurements. Over three-fourths of the in-progress homes had iPM exceeding 100 μg/m3 and the highest quartile exceeded 250 μg/m3.
Based on a previous study of Iowa homes, a level of household airborne endotoxin of 5 EU/m3 or above may be considered elevated (Thorne and Duchaine, 2007). Three in-progress homes and two completed homes exceeded this level. The in-progress homes had a GM of 1.55 EU/m3, 5-fold higher (p<0.0001) than the completed homes GM of 0.30 EU/m3. The concentrations of both linear and branched inhalable fungal β-D-glucan were almost 4-fold higher for the in-progress homes than the completed homes (p<0.001, Figure 2B).
EDC’s were deployed in a lower proportion of the in-progress than completed homes due to the residents declining to host the device citing their on-going remediation. Several of the in-progress homes lacked a suitable location for the EDC resulting in 15 of 24 in-progress homes hosting an EDC. All of the completed homes had an EDC deployed; however, one folder (out of 49) was uncollectable. The EDC endotoxin load extracted from the EDC for the in-progress homes was 1.90-fold higher (p=0.029) than the completed homes and none of these endotoxin values were below the LOD (0.0244 EU/mL) (Figure 3A). Glucan collected using the EDC showed no significant difference at the two remediation stages and demonstrated a large range of values for both linear (1→3)-β-D-Glucan (p = 0.083) and branched (1→3, 1→6)-β-D-Glucan (p=0.46).
Figure 3B depicts the concentration of allergens at the two remediation stages. Many households had undetectable levels of the 8 allergens sampled using the EDC. The fractions of homes above the LOD were: Der p1 (3/63), Der f1 (28/63), Mite Group 2 (16/63), Fel d1 (50/63), Can f1 (42/63), Mus m1 (16/63), Rat n1 (5/63), and Bla g2 (20/63). The high number of undetectable allergens suggests low allergen loads inside the homes and very few homes with rat allergens. Not surprisingly, some households had very high levels of cat, dog, and mite (Der f1) allergen with over 1 mg/m2. Forty-three households had allergens >1.0 mg/m2 (7 Der f 1, 11 Fel d 1, 20 Can f 1, 1 Mus m 1, 4 for Bla g 2) and 5 households had allergen concentration >10.0 mg/m2 (2 Fel d1, 3 Can f1).
Health Outcomes
Tables 5 and 6 display the health data for residents of the in-progress and completed homes. Health data were collected from 23 in-progress household residents and 48 completed household residents due to 2 separate participants having a secondary home sampled. These two individuals were categorized based on their primary residence. The prevalence of subject-reported, doctor-diagnosed asthma among the study participants was 26.1% and 14.6% for participants in the in-progress and completed homes, respectively (Table 4). The rate of doctor-diagnosed allergy was also higher for in-progress residents (56.5%) versus those in completed homes (29.2%). The asthma rate and prevalence of allergic rhinitis for the cohort was much higher than the rate reported in the National Survey of Endotoxin in United States Housing using the same questions which found a diagnosed asthma prevalence rate of 10.9% and a prevalence of allergic rhinitis of 18.2% (Thorne et al., 2005). The odds of having doctor diagnosed allergies were significantly higher in the in-progress homes compared to the completed homes with an adjusted odds ratio of 3.08 (95% CI: 1.05, 9.02). Thirty-nine percent of subjects in in-progress home and 31% of subjects in completed homes were taking prescription medications for respiratory problems (Table 4). Among those reporting asthma, most specified asthma of long duration suggesting that flood-associated exposures exacerbated existing asthma or asthma in remission. The higher asthma prevalence in this cohort compared to nationwide data likely reflects that this population was drawn from a low income self-selected group, with approximately 85% living within 2 km of a large grain processing facility.
Table 5.
Health responses and odds ratios for all participants.
| Health Data | Prevalence | ||||
|---|---|---|---|---|---|
| past 6months (post-flood) | 12 months ago (pre-flood) | Adjusted Odds Ratioa | 95% CI | pb | |
| Prescription for breathing problems, % | 29.5 | 23.9 | 1.38 | 1.01, 1.88 | 0.0421 |
| Self-reported: Ever wheeze, % | 38.0 | 15.5 | 3.77 | 2.06, 6.92 | <0.0001 |
The odds ratios have been adjusted for remediation stage, age, sex, and smoking status.
The p is comparing odds of experiencing the symptom in the past 6 months to odds of experiencing the symptom 12 months ago.
Table 4.
Health questionnaire assessment of asthma and allergy prevalence.
| Health data | Remediation In-Progress | Remediation Completed | Unadjusted Odds Ratio (95% CI)a | Adjusted Odds Ratio (95% CI)b |
|---|---|---|---|---|
| n | 23 | 48 | ||
| Dr. diagnosed asthma, % | 26.1 | 14.6 | 2.07(0.61, 7.06) | 2.16(0.59, 7.92) |
| Prescription for breathing problems, % | 39.1 | 31.3 | 1.41(0.51, 3.93) | 1.38(0.46, 4.14) |
| Dr. diagnosed allergies,% | 56.5 | 29.2 | 3.16(1.14, 8.75) | 3.08(1.05, 9.02) |
| Self-reported: | ||||
| Asthma, % | 26.1 | 16.8 | 1.77(0.55, 5.68) | 1.75(0.50, 6.15) |
| Asthma duration (year), mean ± SD | 24.7±19.3 | 19.4 ±15.0 | ||
| Ever wheeze, % | 39.1 | 37.5 | 1.07(0.39, 2.93) | 1.14(0.38, 3.40) |
| Trouble breathing, % | 34.8 | 20.8 | 2.03(0.69, 6.00) | 2.15(0.64, 7.30) |
| Allergic rhinitis, % | 56.5 | 31.3 | 2.86(1.04, 7.87) | 2.84(0.95, 8.41) |
CI: Confidence Interval
Odds Ratios calculated to compare odds of the health outcome for remediation in-progress subjects as compared to remediation completed subjects
Adjusted for age, sex, and smoking history
In an attempt to further differentiate the impact of the flood on respiratory conditions, health outcomes were classified as having been experienced in the past 6 months (post-flood) as compared to 12 months prior (pre-flood) (Table 5). Study subjects reported the odds of experiencing trouble breathing within the past six months to be 1.38 times higher than 12 months prior (p = 0.042). For all participants, 38% reported they ever wheezed in the past 6 months while 15.5% said they wheezed in the period of up to 12 months prior (adjusted OR = 3.77, p<0.0001).
Discussion
Despite extensive flood damage to the homes in this study and hot, humid conditions over the summer, levels of exposure to iPM and bioaerosols after remediation were low and within the range of typical homes without water damage. This was a result of careful clean-out of contaminated materials and extensive drying of wood framing resulting in lower bioaerosol concentrations. The low concentration of several bioaerosols suggests proper remediation techniques were completed. Public Health officials of Linn County Iowa disseminated pamphlets and provided several documents online educating the public on flood remediation with topics including: “Flood Cleanup Handout”, “Flood: Preventing mildew in your home”, “Flood: NIOSH Interim Guidance on PPE”, and many others (linncounty.org). The city of Cedar Rapids mandated a Contractor Certification Program to ensure that all contractors were reputable and prevented fraudulent contractors from performing remediation.
Levels of airborne bacteria, mold, iPM, endotoxin, and glucan were 1.5 to 5.1-fold higher in homes undergoing remediation versus those where remediation was completed. A factor that could contribute to the higher iPM in the in-progress homes was more dust being present due to the construction activities. Some of the in-progress homes were in the intermediate stages of the reconstruction process, potentially having construction debris inside the homes contaminating the IAQ. It is important to note that all homes were located in the same geographic area and were sampled from 29 November 2008 to 1 May 2009. As previously found in DeKoster and Thorne (1995), no significant seasonal variation exists in airborne bacteria and fungi concentrations during the months we sampled.
Overall, the homes had low inhalable endotoxin levels with only 7% exceeding 5 EU/m3, Figure 2B. Floodwaters carry bacteria and endotoxin into homes and sustained water activity levels above 0.98 foster the growth of Gram-negative bacteria. However, studies have suggested that water activities sustainable for mold growth (0.8) do not promote the presence of airborne endotoxin (WHO, 2009; Thorne et al., 2009). In our earlier study of New Orleans homes after Hurricane Katrina, we found much higher concentrations of culturable mold and endotoxin.4 In that study, we detected 353,000 CFU/m3 of culturable mold, roughly 800 times higher than the concentrations detected in the current study. This substantial difference in concentrations may be attributed to different lag times in sampling the homes, differences in climate, and duration of the submersion. Hurricane Katrina flooded over 75% of the city and homes remained underwater for several weeks whereas the Cedar River flooded 14% of the total land area of Cedar Rapids and on June 21, one week after the flood, the waters receded(Robinson, 2010; Chew et al., 2006). The ability to set up relief staging areas in un-flooded parts of Cedar Rapids and beginning the cleanup efforts one-week after the flood, may have contributed to the lower concentrations of bioaerosols detected in the homes impacted by the Cedar Rapids flood of 2008. This study did not ascertain bioaerosol concentrations immediately following the flood or during the initial mucking and gutting cleanup efforts so those exposures are unknown.
Chew et al. found higher concentrations of culturable mold and endotoxin during the work period as compared to ‘prework’ and ‘postwork’; which corresponds to our findings that the in-progress homes had higher concentrations than the completed homes. The study of Rao et al. found lower levels of bioaerosols inside flooded homes than Chew et al. however; their results were still higher than the levels detected in the homes after the Cedar Rapids flood of 2008. In the current study, and the studies of Chew et al. and Rao et al. the endotoxin measurements were performed in the same laboratory (P.S. Thorne) using the same methodology (Chew et al., 2006; Rao et al., 2007).
Active air samples collected over a 24 hr period were compared with 14 day passive EDC sampling. The Pearson correlation coefficient for endotoxin concentration was 0.40 (p =0.0013). This degree of correlation is reasonable given the different duration of sampling and the fact that the samplers were not side-by-side but simply co-located in a room. Glucan measurements from active air sampling were not significantly correlated with EDC values.
Overall, 13 homes were identified with radon concentrations above the recommended 4.0 pCi/L. Having homes with elevated radon is expected in Iowa. The Iowa Department of Public Health has listed the average radon level from 1990 to 2010 inside Linn County homes to be 4.4 pCi/L (Iowa Department of Public Health, 2010). As noted in the CO2 concentrations, the in-progress homes exchanged more air allowing for less accumulation of radon inside the home. Importantly, no significant exposures to asbestos, lead, or CO were found.
Dampness contributes to the growth of molds and dust mites which may result in increased concentrations of fungal spores, allergens, and fungal glucan (WHO, 2009). During the flooding of 2008 the waters receded uniformly (within 1–2 weeks); residences closer to the river generally did not experience more prolonged flooding. No spatial autocorrelation was found among the locations, which supports the finding that the concentrations measured inside the homes were due to indoor conditions (contamination from flood and residual moisture) and not from outdoor sources.
Potential confounding factors were assessed via a questionnaire that could contribute to the presence of pulmonary ailments, including: current and past smoking exposures, occupation, age, sex, and co-morbidities. Our study was relatively modest and the evaluation of health outcomes was limited to questionnaire responses. Significant differences between the two groups were observed for self-reported allergic rhinitis and doctor-diagnosed allergies. Further, there were increased prevalences of self-reported wheeze and use of prescription medications for breathing problems. An increase in the odds of experiencing several of the measured health symptoms in the past six months as compared to the 12 months prior suggest that the residents of these flooded homes experienced more frequent occurrences of adverse health effects as a result of their exposures during the flood and subsequent remediation efforts.
Participation bias may be present in the study since study subjects were drawn from a list of people who had contacted the county health department. The demographic data collected in the questionnaires display differences in the two groups that may cause bias. The questionnaire ascertained information pertaining to the past 6 months, 12 months ago, and total duration they were experiencing the symptom or condition in question. This could potentially lead to recall bias and bias due to self-reporting. This format was used rather than querying pre- and post-flood symptoms to avoid associating the questions directly with the flood. Health outcomes were assessed at the same time as the IAQ evaluation; therefore exposures prior to the development of the health outcomes are unknown. In addition, as noted in Tables 1 and 2, the in-progress homes reported completing more extensive clean-up. For example 96% of in-progress homes were yellow placard whereas only 84% of the completed homes were so designated. More severe water damage could potentially confound the levels of bioaerosols measured inside the in-progress homes. However, this difference between groups was small and all homes sustained significant flood damaged.
Among the in-progress residents, 11 participants had not moved back into their homes full-time. These participants were performing the remediation on their homes. IAQ measurements were not sampled at the locations where they were sleeping; however, all participants reported no flood-damage at that location. Therefore, their sleeping location would be expected to have a lower concentration of biocontaminants than their in-progress remediation home potentially decreasing their exposures. This could potentially produce a bias toward the null for any health outcomes. Seven of these individuals reported living in Federal Emergency Management Agency (FEMA) trailers. All remediation-completed residents were living in their homes.
Conclusion
This study represents a health and comprehensive exposure assessment of homes undergoing two stages of remediation after experiencing devastating river flooding. Flooded homes that had completed remediation had low levels of indoor bioaerosols and had significantly lower levels than homes that were still in-progress of remediation. This indicates that the owners of completed homes and the contractors had taken correct steps in remediation. In the future, health departments encountering flooding similar to that seen in Cedar Rapids, Iowa can advise residents that have been impacted by floods that proper remediation is necessary and effective to return their homes to a suitable living environment. Significant differences were seen for several of the measured health outcomes reported temporally and between the two remediation stage groups, indicating that exposures individuals experienced from flood-damage homes may have adversely affected their health. Thus, flooded homes should not be reoccupied too soon and residents should be under the care of medical practitioners.
The number of severe natural disasters is on the rise and health departments need to be informed on how to advise their citizens in the case of such disasters (NOAA, 2011). Our study suggests that remediation of flood-damaged homes does decrease the amount of bio-contaminants inside the home. These bio-contaminants are known to cause respiratory complications among individuals exposed to them, especially individuals with pre-existing conditions and co-morbidities.
Supplementary Material
Practical Implications.
The number and severity of floods is on the rise and health departments need evidence-based information to advise homeowners on recovery after such disasters. Our study suggests that proper remediation of flood-damaged homes can reduce bioaerosols to acceptable levels but exposures are significantly increased while remediation is in-progress leading to an increased burden of allergy and allergic rhinitis.
Acknowledgments
Funding: NIH P30 ES005605-S. The authors gratefully acknowledge the assistance of Jessica Magwood, Mahmoud Metwali, Nancy Wyland, Ruby Perin, Laura Slimak, Craig Taylor, and the study participants.
References
- ASHRAE. Ventilation for Acceptable Air Quality. Atlanta, USA: ASHRAE; 1989. pp. 62–1999. [Google Scholar]
- Baxter DM, Perkins JL, McGhee CR, Seltzer JM. A regional comparison of mold spore concentrations outdoors and inside “clean” and “mold contaminated” Southern California buildings. J of Occup Envir Hyg. 2005;2:8–18. doi: 10.1080/15459620590897523. [DOI] [PubMed] [Google Scholar]
- Chew GL, Wilson J, Rabito FA, Grimsley F, Iqbal S, Reponen T, Muilenberg ML, Thorne PS, Dearborn DG, Morley RL. Mold and endotoxin levels in the aftermath of Hurricane Katrina: a pilot project of homes in New Orleans undergoing renovation. Environ Health Persp. 2006;114:1883–1889. doi: 10.1289/ehp.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKoster JA, Thorne PS. Bioaerosol concentrations in noncompliant, compliant, and intervention homes in the Midwest. Am Ind Hyg Assoc J. 1995;56:573–580. [Google Scholar]
- Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2003;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- Field RW, Steck DJ, Smith BJ, Brus CP, Neuberger JS, Fisher EF, Neuberger JS, Platz CE, Robinson RA, Woolson RF, Lynch CF. Residential radon gas exposure and lung cancer: The Iowa Radon Lung Cancer Study. Am J Epidemiol. 2000;151:1091–1102. doi: 10.1093/oxfordjournals.aje.a010153. [DOI] [PubMed] [Google Scholar]
- Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D. Airborne fungal fragments and allergenicity. Med Mycol. 2006;44:245–55. doi: 10.1080/13693780600776308. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Institute of Medicine. Climate Change, the Indoor Environment, and Health. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- Intergovernmental Panel on Climate Change. Fourth assessment report. Geneva, Switzerland: Intergovernmental Panel on Climate Change; 2008. Climate Change 2007: Synthesis Report. [Google Scholar]
- Iowa Climate Change Impacts Committee. Report to the Governor and the Iowa General Assembly. 2010. Climate change impacts on Iowa 2010. [Google Scholar]
- Iowa Department of Public Health. Iowa Radon Map by County Datasheet. Des Moines, IA: Iowa Department of Public Health; 2010. [Google Scholar]
- Kurup VP, Shen SHD, Banerjee B. Respiratory fungal allergy. Microbes Infect. 2000;2:1101–1110. doi: 10.1016/s1286-4579(00)01264-8. [DOI] [PubMed] [Google Scholar]
- Macher JM. Positive-hole correction of multiple-jet impactors for collecting viable microorganism. Am Ind Hyg Assoc J. 1989;50:561–568. doi: 10.1080/15298668991375164. [DOI] [PubMed] [Google Scholar]
- NIOSH. Asbestos: Method 7400, NIOSH Manual of Analytical Methods (NMAM) 4. Ohio: National Institute for Occupational Safety and Health; 1994. [Google Scholar]
- NIOSH. Lead: Method 7300, NIOSH Manual of Analytical Methods (NMAM) 4. Ohio: National Institute for Occupational Safety and Health; 2003. [Google Scholar]
- National Oceanic and Atmospheric Administration. Billion Dollar US Weather/Climate Disasters. Asheville, NC: National Climatic Data Center; 2011. [Google Scholar]
- Noss I, Wouters IM, Visser M, Heerderik DJ, Thorne PS, Brunekreef B, Doekes G. Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl Environ Microb. 2008;74:5621–5627. doi: 10.1128/AEM.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. National Center for Health Statistics. Vital Health Stat. 2010;10(249) [PubMed] [Google Scholar]
- OSHA. OSHA Fact Sheets: Asbestos Fact Sheet. Washington, DC: Occupational Safety and Health Administration; 2002. [Google Scholar]
- Rao CY, Riggs MA, Chew GL, Muilenberg ML, Thorne PS, Van Sickle D, Dunn KH, Brown C. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl Environ Microb. 2007;73:1630–1634. doi: 10.1128/AEM.01973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP. Regional economic impacts of the 2008 Cedar Rapids Flood. Fairfax, VA: George Mason University; 2010. [Google Scholar]
- Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman J. American Thoracic Society: Respiratory health hazards in agriculture. Am J Resp Crit Care. 1998;158:S1 S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- Siroux V, Boudier A, Anto JM, Cazzoletti L, Accordini S, Alonso J, Cerveri I, Corsico A, Gulsvik A, Jarvis D, de Marco R, Marcon A, Marques EA, Bugiani M, Janson C, Leynaert B, Pin I. Quality-of-life and asthma-severity in general population asthmatics: results of the ECRHS II study. Allergy. 2008;63:547–554. doi: 10.1111/j.1398-9995.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- Sigsgaard T, Hoffmann HJ, Thorne PS. The role of innate immunity in occupational allergy; recent findings. Curr Opinion in Allergy & Clin Immunol. 2008;8:120–125. doi: 10.1097/ACI.0b013e3282f82492. [DOI] [PubMed] [Google Scholar]
- Tak S, Bernard BP, Driscoll RJ, Dowell CH. Floodwater exposure and the related health symptoms among firefighters in New Orleans, Louisiana 2005. Am J Ind Med. 2007;50:277–382. doi: 10.1002/ajim.20459. [DOI] [PubMed] [Google Scholar]
- Thorne PS. Inhalation toxicology models of endotoxin and bioaerosol-induced inflammation. Toxicology. 2000;152:13–23. doi: 10.1016/s0300-483x(00)00287-0. [DOI] [PubMed] [Google Scholar]
- Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes AJ, Zeldin DC. Endotoxin exposure is a risk factor for asthma: The National Survey of Endotoxin in U.S. Housing. Am J Resp Crit Care. 2005;172:1371–1377. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, Adamčaková-Dodd A, Kelly KM, O’Neill ME, Duchaine C. Metalworking fluid with mycobacteria and endotoxin induces hypersensitivity pneumonitis in mice. Am J Resp Crit Care. 2006;173:759–768. doi: 10.1164/rccm.200405-627OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, Chrischilles EA, Kuehl AKW, Kelly KM, Metwali N, Harris LM, O’Neill ME, Quella AK, Walker RE. Reduction of endotoxin and glucan exposures in the Louisa/Keokuk Environmental Invention Project (LEIP) for rural childhood asthma. J Allergy Clin Immu. 2006;117:S215–17. [Google Scholar]
- Thorne PS, Duchaine C. Airborne bacteria and endotoxin. In: Hurst CJ, Crawford RL, Garland JL, Lipson DA, Mills AL, Stezenbach LD, editors. Manual of Environmental Microbiology. 3. ASM Press; Washington, DC: 2007. pp. 989–1004. [Google Scholar]
- Thorne PS, Cohn R, Mav D, Arbes SJ, Zeldin DC. Predictors of endotoxin levels in U.S. housing. Environ Health Persp. 2009;117:763–771. doi: 10.1289/ehp.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS. Air Quality Hazards. In: Mutel CF, editor. A Watershed Year Anatomy of the Iowa Flood of 2008. Iowa Press; Iowa City, IA: 2010. p. 163. [Google Scholar]
- U.S. Environmental Protection Agency. Mold Resources. Cincinnati, Ohio: National Service Center for Environmental Publications; 2012. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- World Health Organization. WHO Guidelines for Indoor Air Quality: Dampness and Mould. Copenhagen: WHO Regional Office for Europe; 2009. [PubMed] [Google Scholar]
- Yang CS, Heinsohn P. Sampling and analysis of indoor microorganisms. John Wiley & Sons; New Jersey, US: 2007. Microscopic analytical methods for fungi. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.