Abstract
We had previously suggested that women with endometriosis have increased oxidative stress in the peritoneal cavity. In order to assess whether antioxidant supplementation would ameliorate endometriosis associated symptoms, we performed a randomized, placebo controlled trial of antioxidant vitamins (Vitamin E and C) in women with pelvic pain and endometriosis. Fifty nine women, ages 19–41 years, with pelvic pain and history of endometriosis and/or infertility were recruited for this study. Patients were randomly assigned to two groups: vitamin E (1200 IU) and vitamin C (1000 mg) combination or placebo, daily for eight weeks before surgery. Pain scales were administered at baseline and bi-weekly. Inflammatory markers were measured in the peritoneal fluid obtained from both groups of patients at the end of therapy. Our results indicated that, after treatment with antioxidants, chronic pain (“everyday pain”) improved in 43% of patients in antioxidant treatment group (p=0.0055) as compared to the placebo group. In the same group, dysmenorrhea (“pain associated with menstruation”) and dyspareunia (“pain with sex”) decreased in 37% and 24% patients, respectively. In the placebo group, dysmenorrhea associated pain decreased in 4 patients and no change was seen in chronic pain or dyspareunia. There was significant decrease in peritoneal fluid inflammatory markers, RANTES (p≤0.002), interleukin-6 (p≤0.056) and monocyte chemotactic protein-1 (p≤0.016) after antioxidant therapy compared to patients not on antioxidants. In conclusion, results of this clinical trial show that administration of antioxidants reduces chronic pelvic pain in women with endometriosis and inflammatory markers in the peritoneal fluid.
INTRODUCTION
Endometriosis is a common gynecological disorder affecting around 10% of reproductive-age women [1–3]. Endometriosis is found in 25 to 40% of women with infertility [4, 5], and 40–87% of women with chronic pelvic pain have endometriosis [6]. The pathophysiology of endometriosis and the mechanisms responsible for its sequelae, i.e. infertility and pelvic pain, are not well understood. Sampson’s theory suggested retrograde menstruation is the cause for the presence of endometrial cells in the peritoneal cavity [7, 8]. Endometrial cells and undigested tissue in the peritoneal cavity may be a signal to the recruitment and activation of mononuclear phagocytes [9] leading to the growth and sustenance of endometrial cells outside the uterus.
The peritoneal fluid plays a dynamic role in the etiology of endometriosis. There is not only an increase in its volume during endometriosis, but there is also a concomitant increase in the levels of inflammatory, oxidative stress, growth promoting and pain-inducing factors in the fluid as the disease progresses. Several such factors that include, prostaglandin products [10, 11], interleukin-1 ( IL-1) [12], interleukin-6 (IL-6) [13], monocyte-chemotactic protein-1 (MCP-1) [14, 15], the chemokine-Regulated upon activation, normal T cell expressed and secreted (RANTES) [16, 17], tumor-necrosis factor- alpha (TNF-α) [15, 18], various growth-promoting factors [19–21], iron [22–24], hormones [25], and others [26, 27] are present at higher or lower levels in the peritoneal fluid of women with endometriosis and as a consequence, an association is suggested between their presence and the disease process itself. Many of these factors have NFκB or AP-1 response elements in the promoter region, which are oxidant sensitive [28–31].
Studies from our laboratory investigated the important etiological role of oxidative stress in the pathogenesis of endometriosis, which was later supported by studies from other investigators [32–36]. Activated macrophages in the peritoneal cavity generate an oxidative stress milieu, which consists of increased lipid peroxides, their degradation products and products formed from their interaction with low-density lipoprotein and other proteins [26, 36–39]. Such a stress also results in an increased inflammatory reaction with secretion of growth factors, cytokines and chemokines as seen in higher levels in the peritoneal fluid of women with endometriosis [32, 33, 35, 40]. This pro-oxidant environment (peritoneal fluid as well as activated macrophages) also promotes growth of ectopic endometrium [36, 39, 41].
In this present study, we investigated if antioxidant administration to patients with endometriosis would decrease peritoneal fluid biomarkers for oxidation and/or affect pelvic pain in these women.
MATERIALS AND METHODS
Participants
Fifty nine women, ages 19–41 years with pelvic pain and history of endometriosis and/or infertility were recruited from Emory Clinic and Crawford Long Hospital, affiliated to Emory University School of Medicine, Atlanta, GA. All women consented to be on trial for 2 months before surgical intervention. These patients were randomly assigned to two groups: Group A (n=46)-given vitamin E (1200 IU-3 capsules of 400 mg each) and vitamin C (1000 mg-2 tablets of 500 mg each) combinations or Group B (n=13)-placebo pills, daily for eight weeks prior to surgery. We had previously indicated that peroxidized lipids and lipoproteins might be involved in the pathology of endometriosis. Also, we speculated whether lipid oxidation-derived pseudo prostaglandin-like molecules would be involved in endometriosis associated pain. Vitamin E is a lipid-soluble, chain-breaking antioxidant that prevents the propagation of lipid peroxidation by forming a vitamin E radial [42, 43]. Vitamin C is generally recommended to be included along with Vitamin E supplementation since vitamin C helps in the recycling of Vitamin E radical back to Vitamin E [44]. We chose the stated concentrations because of clinical trials on cardiovascular diseases that used these levels [45] and our own unpublished studies suggested that at these concentrations they were nontoxic and decreased inflammatory markers. No pre-treatment with other medications were given a week before or during the antioxidant supplementation. At the end of eight weeks, peritoneal fluid was collected from patients during laparoscopic surgery. Women completed a pre-operative quality of life questionnaire and assessment of pain using a visual analogue scale for assessment of endometriosis associated pain including, dysmenorrhea, non-menstrual pelvic pain, dyspareunia, and dyschesia. Pain scores were obtained before, during (bi-weekly) and after treatment and assessment were performed using an arbitrary scale of “none, mild, moderate and severe” levels of pain. This HIPAA compliant clinical trial was approved by the Institutional Review Board of the Emory University School of Medicine and was carried out according to the principles of the Declaration of Helsinki. All patients were consented before the study.
ELISA for inflammatory markers
The peritoneal fluid collected at the end of the antioxidant treatment was used for the measurement of inflammatory markers. Markers such as RANTES, IL-6 and MCP-1 were measured using commercial ELISA kits (R&D systems, Minneapolis, MN) in the peritoneal fluid from all women. The cytokine levels in antioxidant supplemented women were compared to placebo controls using student’s t-test using unequal variances.
Statistics
Statistical comparisons of inflammatory markers between the two groups were analyzed using t-test using unequal variances. Analyses of pain scores were performed using Fisher’s exact test. Analysis was performed using Statistics Package for the Social Sciences (SPSS for Windows, Microsoft, USA). P < 0.05 was considered significant.
RESULTS
Antioxidant Treatment reduces Inflammatory Markers
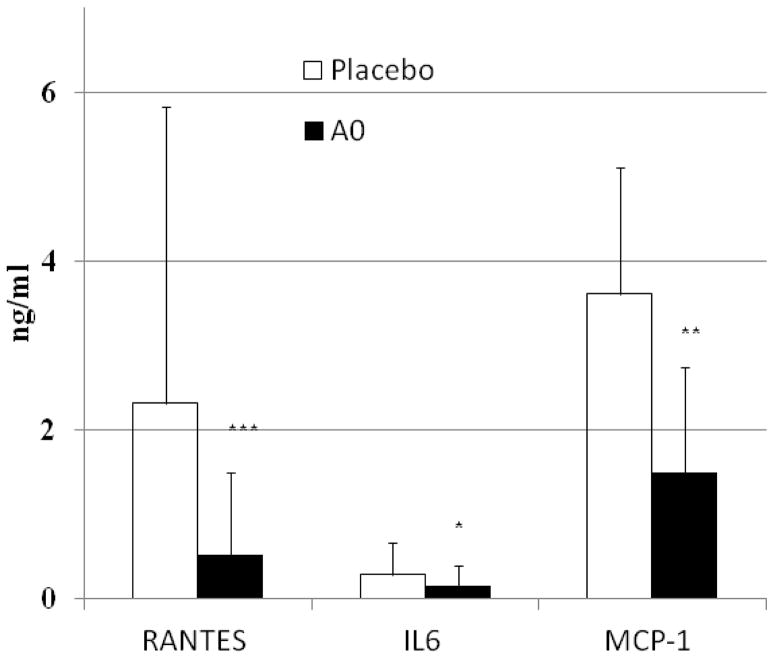
Inflammatory markers are present in high levels in the peritoneal fluid of women with endometriosis [46–49]. Our earlier studies suggested that oxidative stress plays an important role in increasing the inflammatory response in the peritoneal cavity [32, 39, 50]. In this study we investigated if antioxidant supplementation will decrease the levels of inflammatory markers in women with endometriosis. As shown in Figure 1, there was a significant reduction in the levels of inflammatory markers, RANTES (p≤0.002), IL-6 (p≤0.05) and MCP-1 (p≤0.01) after eight weeks of antioxidant supplementation (antioxidant treated patients with endometriosis (n=46) compared to those patients not on any antioxidant supplementation (n=13). Though, RANTES levels do not differ between eutopic and ectopic endometrium [51], but their levels in the peritoneal cavity correlated with the severity of endometriosis [52], the increased variations in the RANTES levels in this study might be due to the inclusion of women with varying severity of the disease in the two groups.
FIGURE 1.
Antioxidant supplementation decreases inflammatory markers in women with endometriosis. Sixty patients with endometriosis were either given vitamin E/C supplementation (AO, n=46) or placebo pill (Placebo, n=13) for 8 weeks. Inflammatory markers were determined using ELISA, in the peritoneal fluid obtained during surgery at the end of the treatment. Student’s t-test was used to compare differences in responses between the two groups. (*** p value<0.005; ** p value <0.01, * p value <0.05).
Antioxidant Treatment reduces Pelvic Pain Scores
Everyday Pain
Eighteen of the 46 patients in Group A (43%) (p=0.0055) had a decrease in “everyday pain”, 22 patients (52%) had no change in “everyday pain”, 4 patients did not experience “everyday pain” and 2 of the women (5%) had an increase in “everyday pain”. (Table 1). Further analysis indicated that pain improved from severe to moderate in 3 patients, from severe to mild in 1, from severe to moderate-severe in 1, from moderate to mild in 6, from moderate to mild-moderate in 3, from moderate-severe to mild-moderate in 2, from mild-moderate to mild in 1 and from mild to none in 1 patient.
Table 1.
Effect of Antioxidant supplementation on “Everyday pain”
| Group: | Antioxidants | Placebo |
|---|---|---|
| Decreased pain | 18 (43%)a | 0 (0%) |
| No change | 22 (52%) | 11 (100%) |
| Increased pain | 2 (5%) | 0 (0%) |
| No pain at baseline | 4 | 2 |
| Total | 46 | 13 |
Fisher’s exact test, p=0.0055
Eleven out of 18 patients that had a decrease in “everyday pain” after antioxidant supplementation saw an improvement in pain associated with menstrual cycle (dysmenorrhea) as well. The other 6 patients did not experience any change with the cycle. Thirteen of the 18 patients who experienced relief from “everyday pain” ended up with having surgery. On surgery, 6 patients were diagnosed with endometriosis stage 1–2, and the other 5 had stage 3–4 endometriosis. In 2 patients there were no current endometriosis lesions found, however both of these patients had a past history of endometriosis.
From group A, 22 patients experienced no change with “everyday pain” while on treatment, but 5 of these women reported less pain with their cycle. 17 patients from the 22, who did not experience relief from “everyday pain”, underwent surgical treatment. In 2 patients out of 17 no current lesions were found, but both of them had prior history of endometriosis. Endometriosis staging of the rest of the 15 patients was: stage 1–2 in 5 patients, stage 3–4 in 10 patients.
Placebo group did not show any changes in “everyday pain” (100% of initial score). 8 patients out of 13 underwent surgery. Endometriosis was diagnosed in 6 cases, stage 1–2 in 2 and stage 3–4 in 4 patients. No lesions were found in one patient, who had a history of endometriosis and endometriosis window was seen in another one.
Dysmenorrhea and Dyspareunia
Among the 46 patients on antioxidant supplementation, 16 patients (37 %) had reported reduction in menstrual cycle associated pelvic pain, “dysmenorrhea”. Further analysis indicated decrease in pain from severe to moderate in 11 patients, from severe to moderate-severe in 1, from moderate to mild in 2, from moderate-severe to mild and severe in 1 and from moderate-severe to mild in 1 patient. Eleven of these 16 patients had also indicated a decrease in “everyday pain” at the same time.
Twenty six out of the 46 patients on antioxidant supplementation did not notice any change in pain associated with menstruation, i.e. dysmenorrhea, but 5 of them indicated relief in “everyday pain”. Of the 26 patients, 20 underwent surgery during the treatment. Endometriosis stage 1–2 was diagnosed in 8 patients and stage 3–4 had 11 patients. One patient had a prior history of endometriosis. There was 1 patient who did not have any menstrual pain, 2 women with no cycle and one patient who actually got worse during the treatment.
From Placebo group (group B), 4 out of 13 patients indicated decrease in dysmenorrhea, which on further analyses was a decrease in pain from severe to moderate in 3 patients and from moderate-severe to mild- moderate in 1 patient. The rest of the 6 patients did not report any changes in menstrual cycle associated pain, and 1 patient experienced more pain.
Patients from both groups were also asked to report on “dyspareunia” at both baseline and while on therapy. Result shown in Table 2 indicated that after antioxidant treatment, pain with intercourse decreased in 8 women (24%) and no change was seen in 24 patients. In the same group, pain increased in 1 patient, 9 did not have pain at baseline and 4 patients were not sexually active. No patients in the placebo group indicated either a decrease or increase in dyspareunia during the trial.
Table 2.
Effect of Antioxidant supplementation on Dysmenorrhea and Dyspareunia
| Group: | Antioxidants | Placebo | ||
|---|---|---|---|---|
| Dysmenorrhea | Dyspareunia | Dysmenorrhea | Dyspareunia | |
| Decreased pain | 16 (37%)b | 8 (24%)c | 4 (36%) | 0 (0%) |
| No change | 26 (60%) | 24 (73%) | 6 (55%) | 10 (100%) |
| Increased pain | 1 (2%) | 1 (3%) | 1 (9%) | 0 (0%) |
| No pain at baseline | 1 | 9 | 1 | 3 |
| N/A | 2 | 4 | 1 | 0 |
| Total | 46 | 46 | 13 | 13 |
Fisher’s exact test, p=0.2723;
Fisher’s exact test, p=0.0957
In summary, after treatment with antioxidants, chronic pelvic pain improved in 43% of patients in the antioxidant group. In the same group dysmenorrhea and dyspareunia decreased in 37% and 24%, respectively. In the placebo group, dysmenorrhea decreased in 36% of the subjects. No change was seen in chronic pain or dyspareunia in the placebo group.
DISCUSSION
Oxidative stress has been suggested in the etiology of chronic pelvic pain [53], abdominal pain [54, 55], fibromyalgia [56, 57] unstable angina [58, 59], idiopathic facial pain [60], lower back pain [61] and recently in chronic fatigue syndrome [62]. Oxidation products of polyunsaturated fatty acids such as 8-isoprostanes, 13- or 9- hydroperoxy eicosatetraenoic acid (HPETE) and hydroxyl eicosatetraenoic acid (HETE) are known to increase nociception [63–65]. Conversely, antioxidants have been used to alleviate pain in several conditions. Antioxidants alone or in combination, when given with known analgesics or independently have been shown to decrease the free radical mediated nociception [66]. Natural antioxidants such as alpha-lipoic acid and melatonin prevented oxidative stress induced neuropathic pain in animals models of diabetes or pain associated with pancreatitis [67, 68]. Aminoguanidine, by decreasing oxidative stress protected against peritoneal adhesion formation [69]. Recently, two studies conducted in Mexico showed an inverse correlation between antioxidant (vitamin E, vitamin C, zinc and selenium) intake and endometriosis pathology [70] and an improvement in antioxidant markers upon supplementation of an antioxidant rich diet [71].
Our study indicated that as short as two months of administration of antioxidants (vitamins E and C) to women with endometriosis not only lowered peritoneal inflammatory markers (which might be responsible for the generation of the pain inducing molecules), but also resulted in reduced chronic pelvic pain in these women. Although not statistically significant, patients on vitamins E and C also had clinically significant improvement in dysmenorrhea and dyspareunia. These latter two are expected outcomes as compared to chronic pain, dysmenorrhea and dyspareunia are associated with menstrual process and sexual intercourse. Perhaps, given adequate statistical power and longer duration of treatment, pain associated with dysmenorrhea also might have indicated significant differences. We anticipate that other antioxidants may also suppress oxidative stress and should be capable of affecting pain. Vitamin E was our antioxidant of choice, since it is a lipid-soluble antioxidant and has been used previously in decreasing inflammatory and oxidative markers in other disease conditions such as cardiovascular disease. It also has been extensively used on humans with little or no toxic effects at the concentrations used. We felt safe in using vitamin E/C combinations. However, it should be noted that vitamin E trials were not all successful in the treatment of atherosclerosis. Most other synthetic and natural antioxidants haven’t been tested in humans for their effectiveness to act as antioxidants. In the future, availability of more powerful antioxidants that are effective in humans will be tested for alleviating pain in women with endometriosis.
Better therapeutics to relieve pain associated with endometriosis is one of the high priorities in endometriosis research. Suggestions to include alternate strategies to relieve symptoms of endometriosis was recommended by the International Consensus group [72]. The use of Chinese herbal medicines to relieve pain is now part of the standard treatment for endometriosis [73–75]. Though, the major limitation of our study is the patient number, the study does suggest that antioxidant vitamins are efficacious in decreasing chronic pelvic pain in women with endometriosis. Our study also suggested that natural antioxidants such as vitamin E and C at low doses, are highly efficient alternative therapy to relieve chronic pelvic pain in women with endometriosis. The current study also provided “in vivo” evidence for our global hypothesis that endometriosis is a disease of oxidative stress.
Acknowledgments
The authors acknowledge funding from NIH-NICHD PO1-HD-35276-01A1. The manuscript is based on a clinical trial that was performed and completed prior to 2003 and was not registered under the clinical trial registry at that time. None of the authors have any financial or personal relationship with any organizations that would potentially influence the research. All authors have read the journal’s policy on disclosure of potential conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–8. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 2.May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17:637–53. doi: 10.1093/humupd/dmr013. [DOI] [PubMed] [Google Scholar]
- 3.Adamson GD. Endometriosis classification: an update. Curr Opin Obstet Gynecol. 2011;23:213–20. doi: 10.1097/GCO.0b013e328348a3ba. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90:247–57. doi: 10.1016/j.fertnstert.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 5.Allaire C. Endometriosis and infertility: a review. J Reprod Med. 2006;51:164–8. [PubMed] [Google Scholar]
- 6.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17:327–46. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–69. [Google Scholar]
- 8.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–4. [PubMed] [Google Scholar]
- 9.Cao X, Yang D, Song M, Murphy A, Parthasarathy S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: Implications for endometriosis. Fertil Steril. 2004;82 (Suppl 3):999–1007. doi: 10.1016/j.fertnstert.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Hu J, Shen W, Wang J, Chen C, Han J, et al. Peritoneal fluid of patients with endometriosis promotes proliferation of endometrial stromal cells and induces COX-2 expression. Fertil Steril. 2011;95:1836–8. doi: 10.1016/j.fertnstert.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood) 2010;235:668–77. doi: 10.1258/ebm.2010.009321. [DOI] [PubMed] [Google Scholar]
- 12.Akoum A, Al-Akoum M, Lemay A, Maheux R, Leboeuf M. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil Steril. 2008;89:1618–24. doi: 10.1016/j.fertnstert.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Boutten A, Dehoux M, Edelman P, Seta N, Menard A, Madelenat P, et al. IL6 and acute phase plasma proteins in peritoneal fluid of women with endometriosis. Clin Chim Acta. 1992;210:187–95. doi: 10.1016/0009-8981(92)90204-4. [DOI] [PubMed] [Google Scholar]
- 14.Na YJ, Lee DH, Kim SC, Joo JK, Wang JW, Jin JO, et al. Effects of peritoneal fluid from endometriosis patients on the release of monocyte-specific chemokines by leukocytes. Arch Gynecol Obstet. 2011;283:1333–41. doi: 10.1007/s00404-010-1583-1. [DOI] [PubMed] [Google Scholar]
- 15.Tao Y, Zhang Q, Huang W, Zhu H, Zhang D, Luo W. The peritoneal leptin, MCP-1 and TNF-alpha in the pathogenesis of endometriosis-associated infertility. Am J Reprod Immunol. 2011;65:403–6. doi: 10.1111/j.1600-0897.2010.00920.x. [DOI] [PubMed] [Google Scholar]
- 16.Nishida M, Nasu K, Narahara H. Role of chemokines in the pathogenesis of endometriosis. Front Biosci (Schol Ed) 2011;3:1196–204. doi: 10.2741/220. [DOI] [PubMed] [Google Scholar]
- 17.Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, et al. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010;45:291–9. doi: 10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- 18.Lv D, Song H, Shi G. Anti-TNF-alpha treatment for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2010:CD008088. doi: 10.1002/14651858.CD008088.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Fan R, Huang X, Xu H, Zhang X. Decreased concentrations of pigment epithelium-derived factor in peritoneal fluid of patients with endometriosis. Fertil Steril. 2011;95:1798–800. doi: 10.1016/j.fertnstert.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 20.Barcz E, Kaminski P, Marianowski L. [VEGF concentration in peritoneal fluid of patients with endometriosis] Ginekol Pol. 2001;72:442–8. [PubMed] [Google Scholar]
- 21.Mahnke JL, Dawood MY, Huang JC. Vascular endothelial growth factor and interleukin-6 in peritoneal fluid of women with endometriosis. Fertil Steril. 2000;73:166–70. doi: 10.1016/s0015-0282(99)00466-5. [DOI] [PubMed] [Google Scholar]
- 22.Polak G, Wertel I, Tarkowski R, Kotarski J. [Peritoneal fluid iron levels in women with endometriosis] Ginekol Pol. 2010;81:20–3. [PubMed] [Google Scholar]
- 23.Defrere S, Lousse JC, Gonzalez-Ramos R, Colette S, Donnez J, Van Langendonckt A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008;14:377–85. doi: 10.1093/molehr/gan033. [DOI] [PubMed] [Google Scholar]
- 24.Van Langendonckt A, Casanas-Roux F, Donnez J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil Steril. 2002;78:712–8. doi: 10.1016/s0015-0282(02)03346-0. [DOI] [PubMed] [Google Scholar]
- 25.De Leon FD, Vijayakumar R, Brown M, Rao CV, Yussman MA, Schultz G. Peritoneal fluid volume, estrogen, progesterone, prostaglandin, and epidermal growth factor concentrations in patients with and without endometriosis. Obstet Gynecol. 1986;68:189–94. [PubMed] [Google Scholar]
- 26.Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T- lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab. 1998;83:2110–3. doi: 10.1210/jcem.83.6.4823. [DOI] [PubMed] [Google Scholar]
- 27.Gill K, Kirma N, Gunna VS, Santanam N, Parthasarathy S, Tekmal RR. Regulation of colony stimulating factor-1 (CSF-1) in endometrial cells: glucocorticoids and oxidative stress regulate the expression of CSF-1 and its receptor c-fms in endometrial cells. Fertil Steril. 2001;76:1005–11. doi: 10.1016/s0015-0282(01)02735-2. [DOI] [PubMed] [Google Scholar]
- 28.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I. Regulation of nuclear factor-kappa B, activator protein-1, and glutathione levels by tumor necrosis factor-alpha and dexamethasone in alveolar epithelial cells. Biochem Pharmacol. 2000;60:1041–9. doi: 10.1016/s0006-2952(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 30.Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol. 2000;60:1075–83. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 31.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–32. [PubMed] [Google Scholar]
- 32.Murphy AA, Santanam N, Parthasarathy S. Endometriosis: a disease of oxidative stress? Semin Reprod Endocrinol. 1998;16:263–73. doi: 10.1055/s-2007-1016286. [DOI] [PubMed] [Google Scholar]
- 33.Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002;77:861–70. doi: 10.1016/s0015-0282(02)02959-x. [DOI] [PubMed] [Google Scholar]
- 34.Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79:1288–93. doi: 10.1016/s0015-0282(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13:126–34. doi: 10.1016/s1472-6483(10)62026-3. [DOI] [PubMed] [Google Scholar]
- 36.Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol Endocrinol. 2009;25:75–81. doi: 10.1080/09513590802485012. [DOI] [PubMed] [Google Scholar]
- 37.Murphy AA, Palinski W, Rankin S, Morales AJ, Parthasarathy S. Macrophage scavenger receptor(s) and oxidatively modified proteins in endometriosis. Fertil Steril. 1998;69:1085–91. doi: 10.1016/s0015-0282(98)00088-0. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran S, Song M, Murphy AA, Parthasarathy S. Expression of scavenger receptor class B1 in endometrium and endometriosis. J Clin Endocrinol Metab. 2001;86:3924–8. doi: 10.1210/jcem.86.8.7706. [DOI] [PubMed] [Google Scholar]
- 39.Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci. 2002;955:183–98. doi: 10.1111/j.1749-6632.2002.tb02779.x. discussion 19–200, 396–406. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 2006;18:325–32. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- 41.Mier-Cabrera J, Jimenez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernandez-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118:6–16. doi: 10.1111/j.1471-0528.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- 42.Ricciarelli R, Zingg JM, Azzi A. Vitamin E: protective role of a Janus molecule. FASEB J. 2001;15:2314–25. doi: 10.1096/fj.01-0258rev. [DOI] [PubMed] [Google Scholar]
- 43.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 44.Altan ZM, Denis D, Kagan D, Grund EM, Palmer SS, Nataraja SG. A long-acting tumor necrosis factor alpha-binding protein demonstrates activity in both in vitro and in vivo models of endometriosis. J Pharmacol Exp Ther. 2010;334:460–6. doi: 10.1124/jpet.110.166488. [DOI] [PubMed] [Google Scholar]
- 45.Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–45. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 46.Berbic M, Fraser IS. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J Reprod Immunol. 2011;88:149–55. doi: 10.1016/j.jri.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Montagna P, Capellino S, Villaggio B, Remorgida V, Ragni N, Cutolo M, et al. Peritoneal fluid macrophages in endometriosis: correlation between the expression of estrogen receptors and inflammation. Fertil Steril. 2008;90:156–64. doi: 10.1016/j.fertnstert.2006.11.200. [DOI] [PubMed] [Google Scholar]
- 48.Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123:217–26. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 49.Schroder W, Gaetje R, Baumann R. Interleukin-6 and soluble interleukin-6 receptor in peritoneal fluid and serum of patients with endometriosis. Clin Exp Obstet Gynecol. 1996;23:10–4. [PubMed] [Google Scholar]
- 50.Murphy AA, Palinski W, Rankin S, Morales AJ, Parthasarathy S. Evidence for oxidatively modified lipid-protein complexes in endometrium and endometriosis. Fertil Steril. 1998;69:1092–4. doi: 10.1016/s0015-0282(98)00087-9. [DOI] [PubMed] [Google Scholar]
- 51.Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol Hum Reprod. 2001;7:163–8. doi: 10.1093/molehr/7.2.163. [DOI] [PubMed] [Google Scholar]
- 52.Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169:1545–9. doi: 10.1016/0002-9378(93)90433-j. [DOI] [PubMed] [Google Scholar]
- 53.Shahed AR, Shoskes DA. Oxidative stress in prostatic fluid of patients with chronic pelvic pain syndrome: correlation with gram positive bacterial growth and treatment response. J Androl. 2000;21:669–75. [PubMed] [Google Scholar]
- 54.Chi CH, Shiesh SC, Lin XZ. Total antioxidant capacity and malondialdehyde in acute abdominal pain. Am J Emerg Med. 2002;20:79–82. doi: 10.1053/ajem.2002.30102. [DOI] [PubMed] [Google Scholar]
- 55.Wereszczynska-Siemiatkowska U, Dabrowski A, Siemiatkowski A, Mroczko B, Laszewicz W, Gabryelewicz A. Serum profiles of E-selectin, interleukin-10, and interleukin-6 and oxidative stress parameters in patients with acute pancreatitis and nonpancreatic acute abdominal pain. Pancreas. 2003;26:144–52. doi: 10.1097/00006676-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Ozgocmen S, Ozyurt H, Sogut S, Akyol O. Current concepts in the pathophysiology of fibromyalgia: the potential role of oxidative stress and nitric oxide. Rheumatol Int. 2006;26:585–97. doi: 10.1007/s00296-005-0078-z. [DOI] [PubMed] [Google Scholar]
- 57.Ozgocmen S, Ozyurt H, Sogut S, Akyol O, Ardicoglu O, Yildizhan H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: etiologic and therapeutic concerns. Rheumatol Int. 2006;26:598–603. doi: 10.1007/s00296-005-0079-y. [DOI] [PubMed] [Google Scholar]
- 58.Dubois-Rande JL, Artigou JY, Darmon JY, Habbal R, Manuel C, Tayarani I, et al. Oxidative stress in patients with unstable angina. Eur Heart J. 1994;15:179–83. doi: 10.1093/oxfordjournals.eurheartj.a060473. [DOI] [PubMed] [Google Scholar]
- 59.Cipollone F, Ciabattoni G, Patrignani P, Pasquale M, Di Gregorio D, Bucciarelli T, et al. Oxidant stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation. 2000;102:1007–13. doi: 10.1161/01.cir.102.9.1007. [DOI] [PubMed] [Google Scholar]
- 60.Haque MF, Aghabeighi B, Wasil M, Hodges S, Harris M. Oxygen free radicals in idiopathic facial pain. Bangladesh Med Res Counc Bull. 1994;20:104–16. [PubMed] [Google Scholar]
- 61.Borenstein DG. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 1999;11:151–7. doi: 10.1097/00002281-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJ. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. 2005;39:584–9. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Evans AR, Junger H, Southall MD, Nicol GD, Sorkin LS, Broome JT, et al. Isoprostanes, Novel Eicosanoids That Produce Nociception and Sensitize Rat Sensory Neurons. J Pharmacol Exp Ther. 2000;293:912–20. [PubMed] [Google Scholar]
- 64.Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des. 2005;11:2687–98. doi: 10.2174/1381612054546789. [DOI] [PubMed] [Google Scholar]
- 65.Trang T, McNaull B, Quirion R, Jhamandas K. Involvement of spinal lipoxygenase metabolites in hyperalgesia and opioid tolerance. Eur J Pharmacol. 2004;491:21–30. doi: 10.1016/j.ejphar.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 66.Rokyta R, Holecek V, Pekarkova I, Krejcova J, Racek J, Trefil L, et al. Free radicals after painful stimulation are influenced by antioxidants and analgesics. Neuro Endocrinol Lett. 2003;24:304–9. [PubMed] [Google Scholar]
- 67.Ambriz-Tututi M, Rocha-Gonzalez HI, Cruz SL, Granados-Soto V. Melatonin: a hormone that modulates pain. Life Sci. 2009;84:489–98. doi: 10.1016/j.lfs.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 68.Cameron NE, Jack AM, Cotter MA. Effect of alpha-lipoic acid on vascular responses and nociception in diabetic rats. Free Radic Biol Med. 2001;31:125–35. doi: 10.1016/s0891-5849(01)00564-0. [DOI] [PubMed] [Google Scholar]
- 69.Ara C, Karabulut AB, Kirimlioglu H, Yilmaz M, Kirimliglu V, Yilmaz S. Protective effect of aminoguanidine against oxidative stress in an experimental peritoneal adhesion model in rats. Cell Biochem Funct. 2006;24:443–8. doi: 10.1002/cbf.1245. [DOI] [PubMed] [Google Scholar]
- 70.Hernandez Guerrero CABML, de la Jara Diaz J, Mier Cabrera J, Bouchan Valencia P. Endometriosis and deficient intake of antioxidants molecules related to peripheral and peritoneal oxidative stress. Ginecol Obstet Mex. 2009;74:20–8. [PubMed] [Google Scholar]
- 71.Mier-Cabrera J, Aburto-Soto T, Burrola-Mendez S, Jimenez-Zamudio L, Tolentino MC, Casanueva E, et al. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol. 2009;7:54. doi: 10.1186/1477-7827-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers PA, D'Hooghe TM, Fazleabas A, Gargett CE, Giudice LC, Montgomery GW, et al. Priorities for endometriosis research: recommendations from an international consensus workshop. Reprod Sci. 2009;16:335–46. doi: 10.1177/1933719108330568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flower A, Lewith GT, Little P. A feasibility study exploring the role of Chinese herbal medicine in the treatment of endometriosis. J Altern Complement Med. 2011;17:691–9. doi: 10.1089/acm.2010.0073. [DOI] [PubMed] [Google Scholar]
- 74.Dobos G, Tao I. The model of Western integrative medicine: the role of Chinese medicine. Chin J Integr Med. 2011;17:11–20. doi: 10.1007/s11655-011-0601-x. [DOI] [PubMed] [Google Scholar]
- 75.Jiang H, Shen Y, Wang XG. Current progress of Chinese medicinal treatment of endometriosis. Chin J Integr Med. 2010;16:283–8. doi: 10.1007/s11655-010-0283-9. [DOI] [PubMed] [Google Scholar]