Abstract
Background/Objectives
Intake of the marine-based, n-3 fatty acids and engagement in physical activity are inversely related to cardiac morbidity and mortality. Among putative mechanisms, both n-3 fatty acids and physical activity may act through modulation of autonomic control of the cardiovascular system. This investigation examined the independent and interactive associations of n-3 fatty acids (eicosapentaenoic and docosahexanenoic acid; EPA, DHA) and physical activity with heart rate variability (HRV).
Methods
Subjects were 259 healthy 30–54 year-old adults. Serum phospholipid fatty acid composition was employed as biomarker of dietary n-3 fatty acid exposure. Physical activity based on the Paffenbarger questionnaire was coded as < or ≥ 2,000 kcal/week. Standard time-domain (standard deviation of normal-to-normal intervals and root-mean squared of successive differences; SDNN, RMSSD) and frequency domain (high frequency and low frequency power) measures of HRV were derived from resting electrocardiographic recordings.
Results
In linear regression models with covariate adjustment for age, gender and race, greater n-3 fatty acid exposure was associated with greater SDNN and RMSSD, and high physical activity was associated with greater RMSSD. n-3 fatty acid exposure also predicted variation in SDNN, RMSSD, and high-frequency power in interaction with physical activity. Specifically, n-3 fatty acid exposure covaried positively with these three HRV indices only among participants expending 2,000 kcal per week or more in physical activity. These latter findings were noted for DHA but not EPA.
Conclusions
These results suggest that the cardiovascular benefits of n-3 fatty acid consumption may be mediated by effects on cardiac autonomic control and may be dependent upon concomitant habitual exercise.
Index of last names: Harbaugh, Manuck, Jennings, Conklin, Yao, Muldoon
Background
Intake of n-3 long-chain, polyunsaturated fatty acids, naturally found in cold water fish, have documented utility in secondary prevention of cardiac death [1] and other coronary events [2]. The large, prospective US Physician’s Health Study [3] found that consumption of long-chain n-3 fatty acids was associated with reduced incidence of sudden cardiac death, but not total myocardial infarction. This suggests that n-3 fatty acids may specifically protect against ventricular arrhythmias. Nonetheless, trial data regarding effects on sudden cardiac death and ventricular arrhythmias have been mixed, and laboratory studies variously report both pro- and anti-arrhythmic associations with n-3 fatty acids [4, 5].
Anti-arrhythmic effects of n-3 fatty acids may be mediated by the autonomic nervous system, and several investigators have examined the association between n-3 fatty acid exposure and heart rate variability (HRV), a non-invasive measure of autonomic control of the heart. In vivo studies have demonstrated that low overall HRV, and particularly low variability in the frequency range of 0.15 to 0.40 hertz (Hz), correspond to low vagal, parasympathetic activity [6]. HRV itself is predictive of less risk of sudden cardiac death in people following acute myocardial infarctions [7–9]. In addition, low HRV indices are prognostic indicators for poor outcomes in people with diabetes mellitus, cardiac transplantation, myocardial dysfunction, ventricular arrhythmias, and end-stage renal disease [6]. Observational studies and randomized trials provide modest support for an association between greater n-3 fatty acid exposure and higher HRV [10–15]. However, findings are inconsistent as to which indices of HRV are affected. Additionally, most data were derived from select patient samples, leaving unclear whether n-3 fatty acids are related to HRV in the general population and, by extension, cardiovascular disease prevention.
Exercise is widely regarded as beneficial for cardiovascular health, based on ample evidence that greater habitual physical activity is associated with reduced risk of heart disease [16]. This benefit may be mediated through several mechanisms, such as obesity prevention, preserved insulin sensitivity, reduced vessel atherosclerosis, and increased lactic acid tolerance during ischemic insult. Additionally, physical activity may, like n-3 fatty acids, modulate autonomic control of the cardiovascular system. Exercise lowers resting heart rate while often increasing HRV [17]
However, it is unknown how habitual physical activity and n-3 fatty acid exposure interact as co-determinants of autonomic functioning. Fish oil appears to reduce heart rate and oxygen consumption during exercise [18]. Therefore, the present cross-sectional investigation of 259 healthy adults reports on the association between n-3 fatty acid exposure and standard indices of HRV and on potential interactions with habitual physical activity.
Methods
Subjects
The data analyzed in this report derive from the University of Pittsburgh Adult Health and Behavior study, which sampled adult volunteers between 35 and 54 years of age from Pittsburgh, Pennsylvania and surrounding communities (predominantly Allegheny County) via mass-mail solicitation. Participants attended several appointments for assessments of basic cardiovascular health parameters and health behaviors, psychosocial questionnaires and interviews, and cognitive testing. Exclusion criteria included a reported history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer, major neurological disorders, current pregnancy, and schizophrenia or other psychotic illness.
A portion of participants enrolled in a further investigation involving additional measures of cardiovascular risk. This protocol excluded persons with diabetes mellitus, severe hypertension (BP ≥ 180/110 mm Hg), and extreme obesity (body mass index ≥ 40 kg/m2). Additionally, persons taking anti-hypertensive, lipid-lowering, anti-arrhythmic, glucocorticoids or psychotropic medications were excluded. Of the 282 participants who met these criteria, 3 had fatty acid chromatogram peaks which were undetectable, leaving 279 subjects. Twenty of these participants did not have complete HRV information due to unwillingness to participate, equipment malfunction, excessive ectopy (≥ 15 premature beats per 5 minutes), erratic breathing/holding breath, or deviation from paced breathing. Only subjects who successfully completed both unpaced and paced electrocardiographic (ECG) recordings were included. Data on the remaining 259 subjects were used in analyses.
The study protocol was approved by the Institutional Review Board of the University of Pittsburgh (IRB numbers 0805006, 000535), and informed consent was obtained from all participants in accordance with the University IRB guidelines.
Heart Rate Variability
Participants were asked to refrain from caffeine for 4 hours, exercise and alcohol for 12 hours, and cold medications for 24 hours preceding the ECG recording. While seated in a temperature and sound-controlled chamber and after a 10-minute rest period, two successive 5-minute, resting ECG recordings were obtained. During the first, patients were instructed to relax and breathe at a comfortable rate. During the second recording period, the subjects’ respiratory rate was paced at 11 breaths per second by playing a high-pitched tone during the inhalation period and a low-pitched tone during the exhalation period. The resulting ECG recordings were sampled at 1,000 Hz, digitized and analyzed by PSPAT computer software [19]. The program labeled each R wave, and a trained technician reviewed each ECG for accuracy of R-wave identification. The program calculated two time-domain indices of HRV: standard deviation of normal-to-normal intervals (SDNN) and the square root of the mean of the squares of successive normal-to-normal interval differences (RMSSD). The sequential cardiac interbeat interval time series from the selected resting baseline was assessed to determine its component frequencies using a point process analysis developed at the University of Amsterdam, PSPAT [19]. This program yields results similar to a Fourier decomposition, but does not assume a continuous underlying generator function. Conceptually, the analysis is consistent with the integral-pulse frequency-modulation approach used in recent modeling of the neural basis of HRV [20]. High and low frequency HRV was defined as 0.15 Hz – 0.39 Hz, and 0.06 – 0.15 Hz, respectively. Respiratory sinus arrhythmia was defined as power at the participant’s respiratory frequency ± .015 hertz. The paced and un-paced HRV estimates correlated with one another at r > 0.6 and were averaged prior to data analysis to improve reliability [21].
Serum Phospholipid Fatty Acid Composition
A fasting sample of whole blood was centrifuged to separate red blood cells from protein-rich serum. Lipids were extracted and separated from the serum samples using a Strata NH2 500 mg/3ml column (P/N 8B-S009-HBJ). The phospholipid fraction was collected for analysis.
Quantification of the isolated phospholipids was done by gas chromatography. In order to make the phospholipids suitable for chromatographic analysis, a procedure known as FAME described by Shibahara, et al [22] was used to make the molecules volatile. Briefly, the phospholipids were cleaved into fatty acids and were then methyl-esterified. Analysis was performed by gas chromatography according to the method described by Yao, et al [23]. For the purposes of this study, we focused on serum phospholipid EPA and DHA (mole %).
Physical Activity
Physical activity was assessed using the Paffenbarger physical activity questionnaire [24]. This instrument queries daily walking habits and flights of stairs climbed, as well as exercise, sports and recreational activities. Weekly kilocaloric expenditure in all forms of physical activity was calculated for each participant.
The energy expended in physical activity was interpreted as an estimate given the limitations of the method. The frequency distribution of energy expenditure had a positive skew, and prior research indicates that the relationship between physical activity and health outcomes flattens at higher levels of exercise [25–27]. Because of these considerations and that fact that log transformation would obscure interpretation of levels of physical activity, energy expenditure was categorized as low (< 2,000 kcal/week) and high (≥ 2,000 kcal/week). This particular cut-point in the Paffenbarger Survey scores was felt to be suitable based on proximity to the median of the current sample (2,016 kcal/week) and its utility in differentiating rates of major cardiac events and all-cause mortality as function of physical activity [27, 28].
Statistical Analysis
All statistical analyses were performed using SPSS (Version 17.0, SPSS Inc., USA). Serum n-3 fatty acid levels and HRV data were natural logarithm transformed for regression analyses to normalize distributions. Race of the subjects was categorized into two groups: Caucasians (N=231), and other (N = 28). Linear regression models were then created using n-3 fatty acids and physical activity as independent variables, age, race and sex as covariates, and indices of HRV as dependent variables. In each regression model, the interaction between serum n-3 fatty acids and physical activity was tested. The interaction term was calculated by the arithmetic product of the mean-centered n-3 fatty acid variable and dichotomized physical activity (coded as −0.5, +0.5). Distributions of power indices of HRV after log transformation showed extreme values to be present. In order to ensure that results were not unduly influenced by these values distributions were trimmed by elimination of the bottom and top 2.5% of the data [29].
Results
As shown in Table 1, the study population was comprised of mid-life adults who, on average, were normotensive and slightly overweight. The low and high physical activity groups were similar in age, body mass index (BMI) and blood pressure, whereas differences in the expected direction were noted for heart rate. With respect to cardiac autonomic control, time-domain measures of HRV (RMSSD and SDNN) were somewhat greater in the high, compared to low, exercise groups, whereas other indices were comparable across groups. Participants reporting high physical activity tended to have higher circulating levels of EPA and DHA.
Table 1.
Characteristics of study population1
| Low physical activity2 | High physical activity2 | p value3 | |
|---|---|---|---|
| N | 127 | 132 | |
| Gender (% female) | 55 | 48 | 0.35 |
| Age | 45.3 ± 6.6 | 44.3 ± 6.8 | 0.20 |
| BMI (kg/m2) | 26.5 ± 4.4 | 26.0 ± 4.1 | 0.38 |
| Physical activity (kcal/week) | 1113 ± 493 | 3516 ± 1534 | <0.001 |
| Heart Rate | 69.3 ± 9.3 | 64.9 ± 9.0 | <0.001 |
| Systolic BP | 114.5 ± 12.8 | 114.0 ± 9.9 | 0.76 |
| Diastolic BP | 75.32 ± 9.1 | 75.37 ± 7.9 | 0.96 |
| Serum phospholipid EPA (mol%)4 | 0.44 ± 0.27 | 0.51 ± 0.45 | 0.09 |
| Serum phospholipid DHA (mol%)4 | 1.42 ± 0.63 | 1.56 ± 0.63 | 0.05 |
| SDNN (ms)4 | 45.90 ± 14.29 | 51.68 ± 22.18 | 0.06 |
| RMSSD (ms)4 | 31.80 ± 16.12 | 38.46 ± 25.56 | 0.03 |
| High frequency power (ms2)4 | 42268 ± 46014 | 47467 ± 79196 | 0.71 |
| Low frequency power (ms2)4 | 24659 ± 19066 | 27095 ± 34719 | 0.49 |
| Low freq:high freq ratio4 | 1.18 ± 1.51 | .97 ± 1.02 | 0.32 |
Mean values ± standard deviation unless specified otherwise
Low and high physical activity defined as < 2,000 or ≥ 2,000 kcal/week, respectively
Groups were compared by t-test except in the case of gender for which the Chi-square test for independence was used.
Presented in raw form whereas data were transformed by natural logarithm for statistical analyses.
Table 2 shows the results of analyses based upon the sum of EPA and DHA. Each linear regression model included covariate adjustment for age, sex, and race. Models 1 through 3 revealed associations between n-3 fatty acids and heart rate, SDNN and RMSSD, and an association between physical activity and both heart rate and RMSSD. Significant n-3 fatty acid–X–physical activity interactions were found for both SDNN and RMSSD (p values ≤ 0.02). Among the frequency domain HRV indices, linear regression of high frequency power also revealed a significant n-3 fatty acid–X–physical activity interaction. Similar regression analyses were conducted using respiratory sinus arrhythmia (i.e., power assessed solely in a .3 Hz band encompassing each participant’s measured respiratory rate). Here also, the interaction between n-3 fatty acids and physical activity was significant at P = .01). To test for the potential effects of extreme values, linear regression models for each of the HRV parameters were repeated after trimming the low and high 2.5% of values. These re-analyses of SDNN, RMSSD, HF and RSA yielded interaction term P values of .06, .01, 01, and .02, respectively.
Table 2.
Linear regression analyses of combined n-3 fatty acids and physical activity as predictors of indices of heart rate variability
| Model | Heart rate | SDNN | RMSSD | High frequency | Low frequency | Low-to-high frequency ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | ||
| 1 | EPA+DHA | .206 | .001 | .133 | .03 | .138 | .02 | .032 | .52 | .056 | .53 | −.008 | .89 |
| 2 | Physical activity | .216 | <.001 | .095 | .12 | .133 | .03 | .015 | .78 | −.084 | .14 | −.096 | .10 |
| 3 | EPA+DHA | .178 | .003 | .122 | .04 | .122 | .04 | .031 | .55 | .049 | .39 | .007 | .91 |
| Physical activity | .189 | .002 | .077 | .21 | .114 | .06 | .010 | .85 | −.091 | .11 | −.097 | .11 | |
| 4 | EPA+DHA | .175 | .004 | .117 | .05 | .117 | .05 | .027 | .60 | .048 | .40 | .010 | .87 |
| Physical activity | .189 | .002 | .076 | .21 | .114 | .05 | .010 | .85 | −.091 | .11 | −.097 | .11 | |
| Interaction | .087 | .14 | .147 | .01 | .137 | .02 | .125 | .01 | .033 | .56 | −.087 | .13 | |
Covariates were age, race, and sex in all models.
SDNN = standard deviation of inter-beat intervals. RMSSD = root mean square of differences in successive interbeat intervals DHA = docosahexanenoic acid. EPA = eicosapentaenoic acid.
As displayed in Table 3, regression analyses based upon DHA alone yielded very similar results. DHA was positively related to heart rate, SDNN and RMSSD, and significant interactions were noted between DHA and physical activity with respect to SDNN, RMSSD and high frequency power (p values < 0.01). DHA and physical activity also tended to interact in predicting low frequency/high frequency ratio (p=0.08). In contrast, analyses based on EPA revealed a positive association with heart rate and RMSSD, but no interactions between EPA and physical activity (Table 4). Additional covariate adjustment for body mass index did not materially alter the results reported in Tables 2–4.
Table 3.
Linear regression analyses of DHA and physical activity as predictors of indices of heart rate variability
| Model | Heart rate | SDNN | RMSSD | High frequency | Low frequency | Low-to-high frequency ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | ||
| 1 | DHA | 0.188 | 0.002 | 0.124 | 0.04 | 0.121 | 0.04 | 0.022 | 0.67 | 0.027 | 0.63 | −0.012 | 0.84 |
| 2 | Physical activity | 0.216 | <0.001 | 0.095 | 0.12 | 0.133 | 0.03 | 0.015 | 0.78 | −0.084 | 0.14 | −0.097 | 0.10 |
| 3 | DHA | 0.162 | 0.007 | 0.114 | 0.06 | 0.105 | 0.08 | 0.020 | 0.69 | 0.039 | 0.49 | 0.001 | 0.99 |
| Physical activity | 0.194 | 0.002 | 0.079 | 0.19 | 0.118 | 0.05 | 0.012 | 0.82 | −0.089 | 0.12 | −0.097 | 0.11 | |
| 4 | DHA | 0.159 | 0.008 | 0.109 | 0.07 | 0.100 | 0.08 | 0.016 | 0.75 | 0.039 | 0.50 | 0.004 | 0.94 |
| Physical activity | 0.194 | 0.001 | 0.079 | 0.19 | 0.118 | 0.05 | 0.012 | 0.82 | −0.089 | 0.12 | −0.097 | 0.10 | |
| Interaction | 0.107 | 0.07 | 0.169 | 0.004 | 0.156 | 0.007 | 0.131 | 0.009 | 0.016 | 0.77 | −0.104 | 0.08 | |
Table 4.
Linear regression analyses of EPA and physical activity as predictors of indices of heart rate variability
| Model | Heart rate | SDNN | RMSSD | High frequency | Low frequency | Low-to-high frequency ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | ||
| 1 | EPA | 0.172 | 0.007 | 0.110 | 0.07 | 0.126 | 0.04 | 0.035 | 0.49 | 0.040 | 0.48 | 0.016 | 0.79 |
| 2 | Physical activity | 0.216 | <0.001 | 0.095 | 0.12 | 0.133 | 0.03 | 0.015 | 0.78 | −0.084 | 0.14 | −0.097 | 0.10 |
| 3 | EPA | 0.147 | 0.02 | 0.100 | 0.10 | 0.111 | 0.06 | 0.034 | 0.51 | 0.051 | 0.37 | 0.028 | 0.64 |
| Physical activity | 0.198 | 0.001 | 0.083 | 0.17 | 0.119 | 0.05 | 0.010 | 0.84 | −0.090 | 0.12 | −0.100 | 0.10 | |
| 4 | EPA | 0.147 | 0.02 | 0.100 | 0.10 | 0.111 | 0.06 | 0.035 | 0.50 | 0.052 | 0.37 | 0.028 | 0.65 |
| Physical activity | 0.198 | 0.001 | 0.083 | 0.18 | 0.119 | 0.05 | 0.010 | 0.85 | −0.091 | 0.11 | −0.100 | 0.10 | |
| Interaction | 0.00 | 0.99 | 0.040 | 0.51 | 0.039 | 0.50 | 0.071 | 0.16 | 0.067 | 0.23 | −0.025 | 0.67 | |
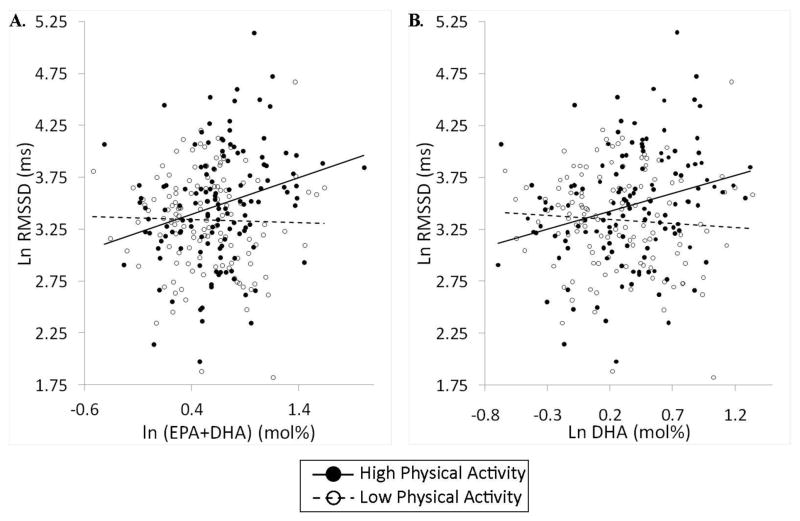
To investigate the noted interactions, regression analyses of SDNN, RMSSD and high frequency power were conducted separately in the low and high physical activity groups. The findings reported in Table 5 indicate that the sum of EPA and DHA, as well as DHA individually, predicted all three measures of HRV in the high but not the low physical activity groups. Figure 1 displays scatter plots of RMSSD as a function of EPA plus DHA and DHA alone for both low- and high-physical activity groups. This illustrates the positive relationship between n-3 fatty acids and HRV among individuals expending at least 2,000 kcal per week in physical activity.
Table 5.
Linear regression analyses of n-3 fatty acids as predictors of heart rate variability in low- and high-physical activity groups
| Independent variable | Dependent variable | Low Physical activity | High Physical activity | ||
|---|---|---|---|---|---|
| β | p | β | p | ||
| EPA+DHA | SDNN | −0.040 | 0.64 | 0.250 | 0.003 |
| RMSSD | −0.026 | 0.75 | 0.257 | 0.002 | |
| High frequency power | −0.100 | 0.15 | 0.157 | 0.03 | |
| DHA | SDNN | −0.077 | 0.36 | 0.263 | 0.002 |
| RMSSD | −0.065 | 0.42 | 0.260 | 0.002 | |
| High frequency power | −0.119 | 0.08 | 0.155 | 0.04 | |
Covariates were age, race, and sex in all models.
Fig. 1.
Scatterplots and regression lines of heart rate variability (HRV) in relation to serum phospholipid n-3 fatty acid composition as a function of self-reported physical activity. Panel A displays root mean sum of squares of successive differences (RMSSD) in relation to eicosapentaenoic acid (EPA) plus docosahexanenoic acid (DHA). Panel B shows the same relationship concerning DHA alone. As is evident, HRV was directly related to n-3 fatty acid content among participants in the high, but not the low, physical activity group. Values were adjusted for age, race and sex.
High frequency power specifically quantifies cardiac parasympathetic control whereas other HRV indices reflect mixed autonomic and possibly other influences. To determine if the findings for SDNN and RMSSD in the high exercise group were driven by cardiac parasympathetic control, analyses were repeated with high frequency power as an additional covariate. The n-3 fatty acids remained significant predictors of both SDNN and RMSSD in the high exercise group.
In additional supplementary analyses treating Paffenbarger scores as a continuous measure, physical activity was unrelated to any index of HRV. Additional sensitivity analyses were conducted to test whether the reported findings might be spurious due to values near the physical activity cut point. Excluding from analyses the 20% of subjects closest to 2,000 kcal/week in energy expenditure did not alter the results. Finally, in separate regression models using measures of HRV collected during paced and unpaced respiration the noted findings were most evident in data collected during paced respiration.
Discussion
This cross-sectional study examined the manner in which n-3 fatty acid exposure and physical activity are related to autonomic control of the cardiovascular system. We found that among 259 community-dwelling and generally healthy adults, the long-chain n-3 fatty acids were associated with the time domain HRV measures of SDNN and RMDD. Additionally, physical activity was found to moderate the association of n-3 PUFAs with time domain HRV and also high frequency power. Specifically, higher levels of n-3 fatty acids in serum phospholipids were associated with greater HRV only in persons expending 2,000 or more calories per week. The results also indicate that among the long-chain fatty acids, DHA is the nutrient most closely related to HRV. By studying generally healthy adults, the study avoids confounding effects of sinus node disease, peripheral neuropathy and prescription medications, and may be interpreted in relation to primary prevention of CVD. Overall, the results suggest that parasympathetic or vagal tone and other, presumably sympathetic influences on sinoatrial function vary in relation to n-3 fatty acid exposure and do so in a manner that is influenced by concurrent exercise habits.
Whereas many clinical studies indicate that long-chain n-3 fatty acids are protective against cardiovascular disease, the mechanisms for such effects are unclear. Increased n-3 fatty acid exposure may lower blood pressure [30], heart rate and catecholamine release, and may improve baroreceptor sensitivity [31–33] An anti-arrhythmic action has been found in animal studies and some, but not all, clinical trials [34].
The cardiovascular benefits of n-3 fatty acids may be mediated, in part, via the autonomic nervous system. This hypothesis is supported by the current findings. Low values of the particular HRV indices associated with n-3 fatty acids, SDNN, RMSSD and high frequency power, themselves predict greater atherosclerosis progression, cardiovascular disease events and total mortality [35–37]. Associations between those indices and n-3 fatty acids suggest that n-3 fatty acid exposure increases cardiac control by both sympathetic and parasympathetic nervous input.
Regular aerobic activity is a primary component of healthy lifestyle, particular with respect to cardiovascular disease prevention [16]. Its physiologic benefits include lowered blood pressure and resting heart rate, weight control, improved cardiorespiratory fitness and metabolic and lipid parameters, and increased HRV. Effects of exercise on HRV generally involves augmentation of parasympathetic activity in conjunction with reduced cardiac adrenergic activity [17]. Changes in HRV parallel and may well mediate the cardiovascular protection afforded by regular physical activity (viz. ventricular arrhythmias;[38]. Nonetheless, individuals notably differ in their autonomic responses to aerobic activity [39]. Explication of such response heterogeneity has identified age and baseline HRV as moderators. In the presented analyses, physical activity is primarily related to HRV in interaction with n-3 fatty acids, encouraging future consideration of n-3 fatty acid exposure as an additional moderator.
The current findings also offer new interpretations of the existing research on n-3 fatty acids and HRV. Among over 1,000 unselected US adults, self-reported fish consumption correlated positively with RMSSD and high frequency power, whereas these analyses did not distinguish between EPA and DHA intake [40]. Much smaller reports on healthy, young adults suggest that found that serum DHA, but not EPA, is proportional to SDNN though perhaps only among men [14, 41]. The current report corroborates this finding across gender. In contrast, randomized trials supplementing either healthy or heart disease participants with fish oil for 2–4 months have not consistently observed changes in HRV [10, 12–14].
The inconsistency of the above trial findings could be due to a truly absent treatment effect, the small sample sizes employed (generally less than 25 subjects per group), issues related to dose and duration, or the existence of a moderating factor. The current findings indicate that effects of n-3 fatty acids may be specific to DHA and may be moderated by concomitant physical activity. The published trials have not considered physical activity in their analyses and likely studied relatively inactive participants, as many enrolled heart disease patients [10, 12, 14]. An additional, small, 2-x-2 trial randomized obese subjects to DHA-rich fish oil, exercise, both or neither for 12 weeks [11]. Fish oil increased HRV but no interactions between the two interventions were noted. However, the exercise did not affect either heart rate at rest or during exercise, or high frequency power, questioning the adequacy of the exercise intervention.
The neural components regulating cardiovascular function include sympathetic and parasympathetic afferents from dispersed, peripheral mechano- and chemo-receptors, brainstem and hypothalamic nuclei, modulating input from corticolimbic structures, and sympathetic and parasympathetic efferents to the heart and vasculature. While n-3 fatty acid intake and physical activity may directly affect myocardial cells or cardiac beta-receptor function [34], their apparent cardiac autonomic effects likely involves altered central integration of cardiovascular afferent information [17, 38]. Angiotensin II and nitric oxide have been proposed as mediators of the central effects of n-3 fatty acids [17, 38]. Intake of n-3 fatty acids and physical activity appear to also affect cognitive and affective processes [42–44], raising the possibility that corticolimbic modulation of cardiac autonomic control underlies the associations reported here.
The current study is limited by its cross-sectional design and reliance on self-reported physical activity. These considerations may be offset by the relatively large sample and use of serum phospholipid composition to quantify n-3 exposure, thus avoiding recall bias in self-reported diet data. Physical activity was dichotomized in analyses at 2,000 kcal as done elsewhere, and the reported interactions were robust in sensitivity analyses. In conclusion, we observed in generally healthy volunteers a relationship between long-chain, n-3 fatty acids and cardiac autonomic control that was specific to DHA and was moderated by concurrent exercise habits. These findings pertain to the putative role of these health behaviors in primary cardiovascular disease prevention and may inform the design and interpretation of randomized clinical trials.
Acknowledgments
Support: US Public Health Service Awards T32 HL07560, P01 HL40962, and R21 HL081282
MFM, SBM, and JRJ designed the research; MFM, JKY, SBM, and SMC conducted research; MPH and MFM analyzed data and wrote the paper; MFM had primary responsibility for final content. All authors read and approved the final manuscript. MFM received fish oil capsules and matching placebo as a gift from NourishLife LLC for an investigation conducted between 2008 and 2011.
Footnotes
The authors have no other potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354(9177):447–455. [PubMed] [Google Scholar]
- 2.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–8. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 3.Albert CM, Hennekens CH, O’Donnell CJ, et al. Fish consumption and risk of sudden cardiac death. Jama. 1998;279(1):23–8. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Albert CM. Omega-3 fatty acids and ventricular arrhythmias: nothing is simple. Am Heart J. 2008;155(6):967–70. doi: 10.1016/j.ahj.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf RG, Sanders P, James MJ, Cleland LG, Young GD. Effect of dietary n-3 polyunsaturated fatty acids on the inducibility of ventricular tachycardia in patients with ischemic cardiomyopathy. Am J Cardiol. 2008;101(6):758–61. doi: 10.1016/j.amjcard.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 7.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 8.Malik M, Farrell T, Cripps T, Camm AJ. Heart rate variability in relation to prognosis after myocardial infarction: selection of optimal processing techniques. Eur Heart J. 1989;10(12):1060–74. doi: 10.1093/oxfordjournals.eurheartj.a059428. [DOI] [PubMed] [Google Scholar]
- 9.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–71. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 10.Villa B, Calabresi L, Chiesa G, Rise P, Galli C, Sirtori CR. Omega-3 fatty acid ethyl esters increase heart rate variability in patients with coronary disease. Pharmacol Res. 2002;45(6):475. doi: 10.1006/phrs.2002.0989. [DOI] [PubMed] [Google Scholar]
- 11.Ninio DM, Hill AM, Howe PR, Buckley JD, Saint DA. Docosahexaenoic acid-rich fish oil improves heart rate variability and heart rate responses to exercise in overweight adults. Br J Nutr. 2008;100(5):1097–103. doi: 10.1017/S0007114508959225. [DOI] [PubMed] [Google Scholar]
- 12.O’Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol. 2006;97(8):1127–30. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Dyerberg J, Eskesen DC, Andersen PW, et al. Effects of trans- and n-3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr. 2004;58(7):1062–70. doi: 10.1038/sj.ejcn.1601934. [DOI] [PubMed] [Google Scholar]
- 14.Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr. 1999;70(3):331–7. doi: 10.1093/ajcn/70.3.331. [DOI] [PubMed] [Google Scholar]
- 15.Hamaad A, Kaeng Lee W, Lip GY, MacFadyen RJ. Oral omega n3-PUFA therapy (Omacor) has no impact on indices of heart rate variability in stable post myocardial infarction patients. Cardiovasc Drugs Ther. 2006;20(5):359–64. doi: 10.1007/s10557-006-0295-z. [DOI] [PubMed] [Google Scholar]
- 16.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122(7):743–52. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 17.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–12. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peoples GE, McLennan PL, Howe PR, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol. 2008;52(6):540–7. doi: 10.1097/FJC.0b013e3181911913. [DOI] [PubMed] [Google Scholar]
- 19.Weber EJM, Molenaar PCM, van der Molen MW, et al. Computers in Psychology: Applications in Education, Research, and Psychodiagnostics. Amsterdam: Swets & Zeitlinger; 1991. PSPAT: aA program for spectral analysis of point events including a test for stationarity; pp. 132–139. [Google Scholar]
- 20.Pyetan E, Akselrod S. Do the high-frequency indexes of HRV provide a faithful assessment of cardiac vagal tone? A critical theoretical evaluation. IEEE Trans Biomed Eng. 2003;50(6):777–83. doi: 10.1109/TBME.2003.812158. [DOI] [PubMed] [Google Scholar]
- 21.Pinna GD, Maestri R, Torunski A, et al. Heart rate variability measures: a fresh look at reliability. Clin Sci (Lond) 2007;113(3):131–40. doi: 10.1042/CS20070055. [DOI] [PubMed] [Google Scholar]
- 22.Ichihara K, Shibahara A, Yamamoto K, Nakayama T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids. 1996;31(5):535–539. doi: 10.1007/BF02522648. [DOI] [PubMed] [Google Scholar]
- 23.Yao JK, van Kammen DP, Welker JA. Red blood cell membrane dynamics in schizophrenia. II. Fatty acid composition. Schizophr Res. 1994;13(3):217–26. doi: 10.1016/0920-9964(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 24.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 25.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Jama. 1995;273(5):402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 26.Lee IM, Paffenbarger RS., Jr Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;29(10):2049–54. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- 27.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 28.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 29.Rothenberg T, Fisher F, Tilanus C. A note on estimation from a cauchy sample. Journal of the American Statistical Association. 1966;59:460–463. [Google Scholar]
- 30.Liu JC, Conklin SM, Manuck SB, Yao JK, Muldoon MF. Long-chain omega-3 Fatty acids and blood pressure. Am J Hypertens. 2011;24(10):1121–6. doi: 10.1038/ajh.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamazaki K, Itomura M, Huan M, et al. Effect of omega-3 fatty acid-containing phospholipids on blood catecholamine concentrations in healthy volunteers: a randomized, placebo-controlled, double-blind trial. Nutrition. 2005;21(6):705–10. doi: 10.1016/j.nut.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112(13):1945–52. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 33.Radaelli A, Cazzaniga M, Viola A, et al. Enhanced baroreceptor control of the cardiovascular system by polyunsaturated Fatty acids in heart failure patients. J Am Coll Cardiol. 2006;48(8):1600–6. doi: 10.1016/j.jacc.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 34.London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias. prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116(10):e320–35. doi: 10.1161/CIRCULATIONAHA.107.712984. [DOI] [PubMed] [Google Scholar]
- 35.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88(3):927–34. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 37.Huikuri HV, Jokinen V, Syvanne M, et al. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19(8):1979–85. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 38.Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol. 2009;297(4):H1171–93. doi: 10.1152/ajpheart.00534.2009. [DOI] [PubMed] [Google Scholar]
- 39.Hautala AJ, Kiviniemi AM, Tulppo MP. Individual responses to aerobic exercise: the role of the autonomic nervous system. Neurosci Biobehav Rev. 2009;33(2):107–15. doi: 10.1016/j.neubiorev.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117(9):1130–7. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 41.Brouwer IA, Zock PL, van Amelsvoort LG, Katan MB, Schouten EG. Association between n-3 fatty acid status in blood and electrocardiographic predictors of arrhythmia risk in healthy volunteers. Am J Cardiol. 2002;89(5):629–31. doi: 10.1016/s0002-9149(01)02314-1. [DOI] [PubMed] [Google Scholar]
- 42.Smith PJ, Blumenthal JA, Babyak MA, Georgiades A, Hinderliter A, Sherwood A. Effects of exercise and weight loss on depressive symptoms among men and women with hypertension. J Psychosom Res. 2007;63(5):463–9. doi: 10.1016/j.jpsychores.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blumenthal JA, Sherwood A, Rogers SD, et al. Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study--rationale, design, and methodological issues. Clin Trials. 2007;4(5):548–59. doi: 10.1177/1740774507083388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68(2):140–7. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]