Abstract
Bronchiolitis obliterans syndrome (BOS) is a condition of progressive airflow obstruction that affects a majority of lung transplant recipients and limits long-term post-transplant survival. Although epithelial injury appears central to the development of BOS, little is known regarding the specific epithelial cell types that are affected in this condition. We hypothesized that BOS would involve preferential injury to the secretory Clara cells that function in innate defense and epithelial repair. To test this hypothesis, we assessed tissue transcript, tissue protein, and lung fluid protein expression of Clara cell secretory protein (CCSP), a marker for Clara cells, in lung transplant recipients with BOS, BOS-free patients, and in donor controls. Our results demonstrate that CCSP tissue transcript and protein expression are significantly reduced in lung transplant recipients with BOS as compared to BOS-free or donor controls. In addition, we demonstrate that CCSP protein levels are significantly reduced in the lung fluid of patients with BOS as compared to BOS-free controls, in cross-sectional and longitudinal analysis. Collectively, these complementary results illustrate that BOS involves a selective alteration in the distribution and function of bronchiolar Clara cells.
Keywords: Clara cell, Clara cell secretory protein, lung transplantation, bronchiolitis Obliterans syndrome, epithelium
Introduction
Lung transplant is a viable treatment for select patients with advanced lung disease. Although immediate post-transplant survival has improved in recent years, long term survival remains disappointing. Within five years a majority of lung transplant recipients develop bronchiolitis obliterans syndrome (BOS), a condition of progressive airflow obstruction that correlates with fibrotic obliteration of the airways termed bronchiolitis obliterans (BO). Although the precise etiology of BO is uncertain, epithelial injury is thought to be central to its development.
The main cell types lining the bronchiolar epithelium are the ciliated and secretory cells that serve numerous functions including establishing the protective barrier against the environment. The secretory cells, known as Clara cells, contribute to host defense through production of the anti-inflammatory Clara cell secretory protein (CCSP) (1). For example, the absence of CCSP is associated with excessive inflammatory responses to LPS (2). In addition, Clara cells represent an abundant progenitor cell that functions to maintain distal airways in health and repair the epithelium after injury (3, 4). Reductions in CCSP have been noted in the serum or lung lining fluid in a number of chronic lung diseases, including COPD (5). Thus, altered Clara cell function may contribute to the inability of the epithelium to successfully repair or lead to excessive local pulmonary inflammation.
In the setting of lung transplantation, bronchiolar epithelial injury occurs as a result of alloreactive T-cells (6), allo-specific antibody (7), pulmonary infection (8), or repeated aspiration of gastroduodenal contents (9), all well-described risk factors for BOS. Limited previous data suggests a possible reduction in CCSP in the bronchoalveolar fluid (BAL) or serum of lung or bone marrow transplant patients with BOS (10–13). Because of the emerging importance of Clara cells in maintaining airway integrity and immune balance, we hypothesized that selective injury to bronchiolar Clara cells occurs in BOS resulting in changes in their cellular distribution or molecular properties. Therefore, to test the specific hypothesis that epithelial Clara cell function or distribution is altered in BOS, we assessed CCSP expression in tissue transcript, tissue protein, and BAL protein to varying degrees across multiple lung transplant patient cohorts that include BOS-free patients, early BOS patients, and advanced BOS patients, patients with acute rejection or acute infection, and donor controls.
Methods
Patient population
The independent study cohorts were drawn from patients that underwent lung transplantation at Duke University Medical Center. Patients received similar post-transplant management according to our center specific protocols, including triple immunosuppression with the calcineurin inhibitor tacrolimus, azathioprine, and corticosteroid from transplant onward. Additional details of our clinical management protocols have been described previously (14). BOS was defined and graded according to the most recent International Society of Heart and Lung Transplantation (ISHLT) guidelines using serial post-transplant pulmonary function test (PFT) measurements (15).
BOS cohort for transcript and cellular expression analyses
For transcript analysis, explant lung tissue was obtained from patients undergoing pulmonary retransplantation for BOS (n=5). Demographics of the patients with advanced BOS who underwent pulmonary re-transplantation are shown in Supplemental Table S1. Harvested but unused donor tissue (trimmed at the time of transplant due to size mismatch) was used as the BOS-free donor control tissue (n=5). Histopathological examination of the donor control lung tissue demonstrated normal lung tissue with no abnormalities. For protein expression studies, in addition to the samples described above, explanted lung tissue from 2 additional patients undergoing retransplantation for advanced BOS were used, as well as surgically obtained lung biopsy tissue from lung transplant recipients without BOS (n = 3), or early grades of BOS (BOS Grade 1, n=2 and BOS Grade 2, n=2).
Transcript analysis
Tissue RNA was preserved using RNALater (Ambion, Austin, TX). Total RNA was isolated from explanted or control lung tissue using 4RNAqueous RNA isolation kit from (Ambion, Austin, TX). The RNA concentration was measured by spectrophotometer (260/280), and quality was confirmed using Bio-Rad Experion bioanalyzer (Bio-Rad, Hercules, CA). RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription kit (ABI, Foster City, CA). 40 ng of cDNA was used in each assay of Taqman realtime PCR using validated Taqman probe and primer combinations (ABI, Foster City, CA); each assay was performed in triplicate. Taqman assays: CCSP (Hs01128580_m1, SCGB1A1), FoxJ1 (Hs00230964_m1, FOXJ1), and Beta Actin (4326315E, ACTB) as the endogenous control. Cycle conditions were: 95C for 10 minutes, and 40 cycles of 95C for 15 seconds and 60C for 1 minute. RT-PCR for target genes and endogenous genes were performed using Fam-labeled target probes and VIC-labeled endogenous probes, respectively. Ct values were determined using ABI 7500 RealTime PCR System (ABI, Foster City, CA) with SDS software version 1.3.1. Change in expression was calculated using the 2−ΔΔCt method.
Histological and immunofluorescent analysis
Explant tissue was placed in formalin, paraffin embedded, and used for immunofluorescence or histopathological analysis with Hematoxylin and Eosin (H&E) or Masson Trichrome (MT). Immunofluorescence analysis used the following primary antibodies: Rat anti-human CCSP antibody (1:800; R&D Systems, Minneapolis, MN), Mouse IgG2B anti-acetylated tubulin (AT) antibody (1:24,000; Sigma, St Louis, MO), and Mouse IgG2a anti-smooth muscle actin antibody (1:500; Sigma, St Louis, MO). Secondary antibodies: Alexa Fluor 594 Goat anti Rat IgG, Alexa Fluor 488 Goat anti mouse IgG2b, and Alexa Fluor 488 Goat anti mouse IgG2a (1:500; Invitrogen, Carlsbad, CA), respectively. Antigen retrieval was performed by heating samples in a sodium citrate buffer followed by blocking in 5% bovine serum albumin (BSA)/1 × PBS to block non-specific antigen reactivity following citrate buffer retrieval. Primary and secondary antibodies were diluted in blocking solution and incubated on sections in a humid chamber overnight at 4C or room temperature for 90 minutes. Slides were extensively washed in PBS and cover-slipped with 4′,6-dianmidino-2-phenylindole (DAPI) (Sigma, St Louis, MO) in Fluouromont G mounting media (Southern Biotech, Birmingham, AL). Images for figures S1, 4 and 5 were obtained using Olympus Provis AX70 microscope (Center Valley, PA) equipped with a digital camera and processed using Image-Pro Plus (media Cybernetics, Bethesda, MD). Figure 3 images were obtained using a Zeiss Observer.Z1 inverted fluorescent microscope (Carl Ziess, Inc., Gottingen, Germany) equipped with a digitalcamera and processed using AxioVision Release 4.6.3 (Carl Ziess) Software.
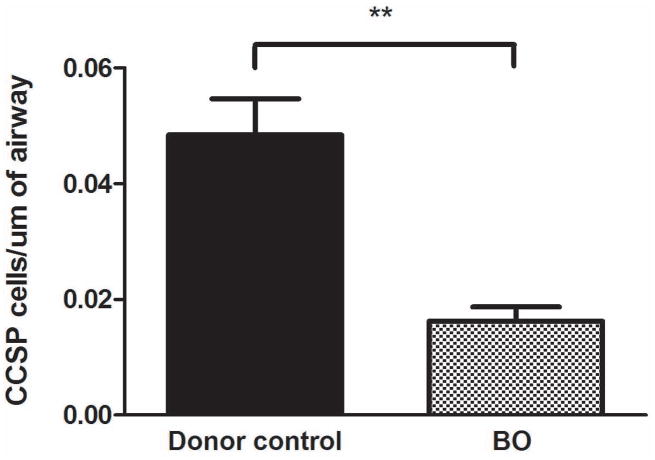
Figure 4.
Semi-quantitative analysis demonstrates that CCSP expression is reduced per length of airway in patients with advanced BOS as compared to BOS-free donors. The analysis used representative terminal bronchioles from BOS-free donors (n=3) compared to patients with advanced BOS (n=3) and assessed a comparable length of airway in each sample.
Figure 5.
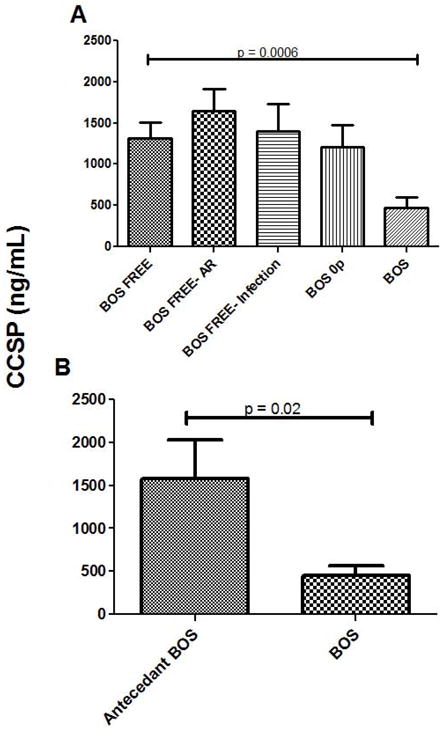
Depleted CCSP levels in lung transplant patients with BOS. A. A cross-sectional sampling of lavage fluid from lung transplant patients with BOS (n = 26) compared to patients who remained BOS-free (n = 62), BOS-free with acute rejection (n = 25), BOS-free with acute infection (n = 18), or were in BOS 0p (n = 24). B. A longitudinal sampling of lavage fluid from lung transplant with BOS compared to antecedent samples from the same patients obtained prior to BOS diagnosis (n = 12 pairs).
Figure 3.
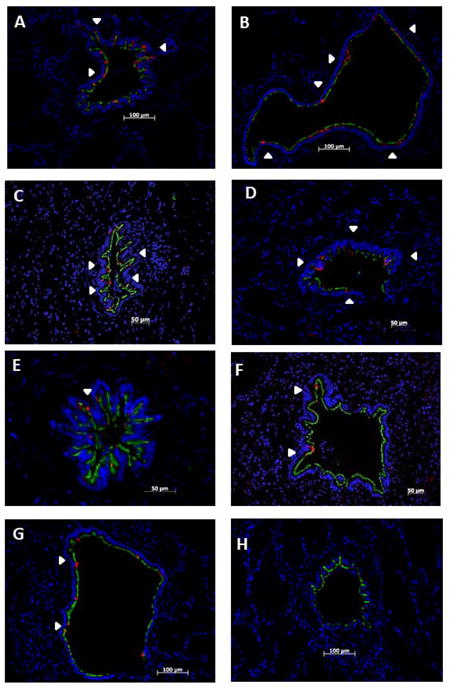
Depletion of epithelial CCSP occurs in BOS but is preserved in BOS-free and normal donor tissues: Tissue is co-immuno-stained for CCSP (red) and AT (green) and nuclear counter stained with DAPI (blue). White arrow heads show areas of CCSP expression throughout an airway. CCSP and AT were assessed in the airways of BOS-free donor tissue (A, B), BOS-free transplant tissue (C, D), early BOS tissue (E, F), and advanced BOS tissue (G, H). Representative airways showing normal CCSP expression in BOS-free donor tissue (A, B) and BOS-free transplant tissue (C, D). Representative airways demonstrating the depletion of CCSP in BOS 1(E) and BOS 2 (F). Similar CCSP loss in the airways of advanced BOS 3 (G,H). CCSP expression in the BOS free donor tissue is very similar to BOS free transplant tissue, while early BOS tissue and advanced BOS tissue show significant CCSP loss. Images A–D, F are presented at 200x, image E is presented at 400X.
Semi-quantitative analysis of CCSP in tissue
For these studies, representative terminal bronchioles of comparable size and length were identified from explanted lung tissue of patients undergoing retransplant for BOS or donor control tissue. Following staining for CCSP and acetylated tubulin the length of each terminal bronchiole was precisely measured using AxioVision Release 4.6.3 (Carl Ziess) software. CCSP positive cells were counted within the measured area and the CCSP positive cells/micrometer of airway were compared between the BOS and control tissue.
BAL study cohort
For these studies, BAL samples were prospectively and serially collected from first, cadaveric, adult lung transplant lung transplant recipients undergoing surveillance or clinically indicated bronchoscopies. BAL was performed in the right middle lobe or lingulae with 100cc fluid with the last 20cc of the return saved for analysis. BAL fluid was separated from the cellular content and stored at −80°C until testing was performed. BAL samples collected between 1–5 years posttransplantation were selected for this analysis to provide comparable followup-time among the cohort. Based on transbronchial biopsy and culture results, patients were then assigned to the following categories at the time of BAL collection: BOS free, BOS 0p, or BOS ≥ 1, BOS-free with acute rejection or BOS-free with acute infection. Rejection was defined by the presence of at least A≥ 1 and infection was defined by a positive culture on the BAL for a pathogenic organism. An additional inclusion criterion was imposed upon the BOS-free cohort to avoid misclassification: the recipient survived at least one year from sample collection date and did not subsequently develop BOS. These parameters generated a final cross-sectional cohort of 155 patients each with a unique sample with the following BOS grades at the time of collection: BOS free (n=62), BOS 0p (n=24) or BOS ≥ 1 (n=26), BOS-free with acute rejection (n=25), BOS-free with acute infection (n=18). In addition, further analysis was performed on a subset of the BOS patients with an antecedent BAL sample prior to the onset of BOS. Of the 26 BOS patients, 12 patients had a previously banked BAL prior to the onset of BOS permitting longitudinal comparison of CCSP levels in the BAL prior to and after the onset of BOS within the same patient.
CCSP protein analysis in BAL
CCSP protein was measured in the BAL fluid using ELISA (Biovendor, Candler, NC) and was performed in duplicate at a dilution of 1:1000. Out of the 155 samples tested, 16 fell below the standard curve, and were assigned a CCSP concentration of the lowest detectable value (1.04 ng/mL).
Statistical analysis
Transcript results were analyzed by nonparametric two tailed t-test (Mann-Whitney test). Unpaired T-test (with correction for unequal variances, as appropriate) was applied to assess the difference in mean BAL CCSP protein levels between BOS ≥ 1 patients and BOS-free patients and between BOS ≥ 1 patients and BOS 0p patients in the cross-sectional BAL cohort, while paired T-test was applied to assess the differences in mean BAL CCSP protein levels between antecedent BOS samples and BOS samples in the longitudinal BAL cohort The Spearman Rank test was applied to assess the correlation between neutrophil levels and CCSP levels in the cross-sectional BAL cohort. Statistical analysis was done using Graph Pad Prism 5.0 (GraphPad Software Inc, La Jolla, CA) and p-values <0.05 were considered statistically significant.
Human subjects approvals
All studies were approved by the Duke University Internal Review Board. BOS tissue was obtained under protocol Pro00011676. Control tissue was obtained under protocol Pro0008725. BAL samples and analysis were performed under protocol Pro00013378.
Results
Histological examination of the explanted lung tissue from patients with advanced BOS
H&E and Masson Trichrome staining demonstrated the presence of characteristic BO lesions in the lung tissue from patients undergoing pulmonary retransplantation for BOS (Figure 1). As shown in Figure 1A and B, typical concentric BO lesions were evident with some degree of epithelial dysplasia even in early affected airways. As shown in Figures 1C and D, in severely affected, nearly obliterated airways only small remnants of intact epithelial remain.
Figure 1.
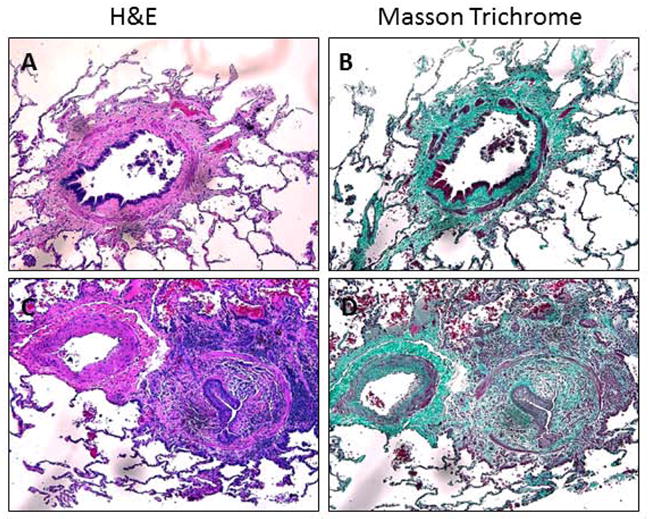
H&E and MT staining of advanced BO airways. Tissue stained for H&E (A, C) and Masson Trichrome (B,D). Images are representative of BO-affected airways from explanted lungs. Images A and B show a concentric lesion with areas of damaged epithelium (A) with subepithelial fibrosis (B). Images C and D show diffuse fibrosis from an advanced lesion with epithelial remnants (C) in the center of the fibrosis (D). All images are presented at 200x.
Expression of CCSP transcript in BOS
CCSP, a marker of non-ciliated epithelial Clara cells, and FoxJ1, a useful transcript marker for ciliated epithelial cells, were used to identify the main cell types of the bronchiolar epithelium. Transcript levels of CCSP and FoxJ1 were assessed in tissue obtained from patients with advanced BOS versus BOS-free donor controls (Figure 2). CCSP mRNA expression was significantly down-regulated in BO tissue compared to BOS-free control tissue (0.2636 fold +/− 0.1636 vs 1.42 +/− 0.43 p=0.03; Figure 2A). Interestingly, FoxJ1 mRNA expression was also down-regulated in BO tissue compared to control tissue but non-significantly, (0.6618 fold +/−0.2294, vs (1.17 fold +/− 0.24, p=0.22; Figure 2B). This transcript data shows diffuse injury to both ciliated and Clara cells, with a more pronounced effect on Clara cells.
Figure 2.

CCSP expression is significantly affected by BOS. Relative fold change in mRNA from BOS explant lung tissue (n=5) compared to BOS-free control lung tissue (n=5) using taqman validated primers and probes. A. CCSP, a marker for Clara cells. B. FoxJ1, marker for ciliated cells. BO-affected tissue shows a significant decrease in CCSP transcript compared to normal BOS-free control tissue, while FoxJ1 transcript remains relatively unchanged.
Immunofluorescence analysis of Clara Cells in transplant patients with varying degrees of BOS and BOS-free donor controls
To extend the PCR results and define the spatial context of changes in airway CCSP expression, we performed immunofluorescence analysis on lung tissue from patients with varying degrees of BOS and BOS-free donor controls. First to understand the normal distribution of ciliated and non-ciliated cells, we stained airways from BOS-free donor controls as well as BOS-free lung transplant patients for acetylated tubulin (AT), a useful protein marker of ciliated cells, and CCSP. Normal donor airway tissue (Figure 3A,B) was shown to express AT immunoreactivity apically across all the airway epithelium consistent with a predominance of ciliated airway epithelial cells. CCSP expression was also observed to occur regularly throughout normal donor airways. A similar pattern of CCSP expression, evenly distributed throughout the airways, was observed in the tissue obtained from BOS free lung transplant recipients (Figure 3C, D, illustrated by the white arrowheads).
Next, to determine the effects of BOS on Clara and ciliated cells, CCSP and AT expression were assessed in tissue obtained from patients with varying degrees of BOS (Figure 3E–H). In patients with early BOS (Figure 3E, F), we observed consistent decreases in CCSP immunoreactivity, compared to BOS free transplant tissue or normal donor tissue controls (Figure 3A–D). Patients with advanced BOS (Figure 3G, H), also show significant decrease in CCSP immunoreactivity, similar to early BOS tissue. Interestingly, we do not observe much of a difference in early BOS CCSP staining compared to advanced BOS CCSP staining, as all the samples from patients with BOS demonstrated reduced CCSP expression relative to the BOS-free or normal donor control tissue, illustrated by the white arrowheads.
To quantify the decreased CCSP immunoreactivity in advanced BOS tissue compared to BOS-free donors, we assessed the number of CCSP positive cells within the terminal bronchioles across multiple samples. Terminal bronchioles were chosen for this analysis to control for morphometric differences in cell types and numbers. The number of CCSP positive cells per μm of terminal bronchiole was significantly decreased in BOS tissue compared to BOS-free control tissue (0.016 +/− 0.002 vs 0.048 +/− 0.0063 p=0.009; Figure 4). For this analysis, comparable mean length of airway was assessed in BO and donor control tissues (267.8 +/− 167.4um vs 425.9 +/− 45.19 μm, p=0.42, respectively)
Finally, to understand the decrease in CCSP expression further in the context of BO, we performed an additional dual immunofluorescence analysis with SMA (smooth muscle actin), a marker of myofibroblasts that contribute to airway obliteration in BO, and CCSP. As shown in supplemental figure 1, in the lung tissue obtained from patients with advanced BOS, increased SMA immuno-reactivity in the peribronchial and subepithelial regions is evident in the BO affected airways. Consistent with the prior immunofluorescence results (Figure 3), markedly reduced expression of CCSP is noted in these airways, particularly in regions of active fibrosis, providing some evidence for a spatial context to the changes in CCSP relative to fibrosis.
CCSP protein levels in BAL
To further extend the idea that functional changes of Clara cells occurs in BOS, CCSP protein levels were measured in a cross-sectional cohort of lung transplant recipients, independent from those patients undergoing pulmonary re-transplantation considered in the previous tissue analyses. Table 1 defines the demographics and clinical characteristics of this BAL study cohort showing comparable demographic and clinical characteristics among the patients that were BOS-free, BOS 0p, BOS-free with acute rejection, BOS-free with acute infection or BOS at the time of sample collection. CCSP levels were significantly decreased in the BAL fluid of patients classified as BOS versus those who were BOS-free (475 +/− 123 ng/mL vs. 1309 +/− 201 ng/mL, p=.0006), as shown in Figure 5A. Similar CCSP levels were observed in patients with BOS 0p (1205 +/− 267.4 ng/mL), BOS-free with AR (1649 +/−261.1 ng/mL), and BOS-free with acute infection (1397 +/− 322.7 ng/mL) when compared to BOS-free patients.
Table 1.
Demographics and Clinical Characteristics of BAL study cohort
| BOS-free | BOS 0p | BOS | BOS-free, rejection† | BOS-free, infection | ||
|---|---|---|---|---|---|---|
| N | 62 | 24 | 26 | 25 | 18 | |
| Median Age at Transplant, years (IQR) | 55.5 [44.8, 63.0] | 58.0 [54.2, 61.8] | 57.0 [42.8, 65.0] | 55.0 [47.5, 64.0] | 61.0 [46.5, 66] | |
| Median time to BAL collection, month (IQR) | 36.0 (24.0,44.5) | 37.5 [29.2, 47.7] | 42.5 [30.2, 54.0] | 22.0 [9.5, 35.5] | 6.5 [3.8, 25.2] | |
| Caucasian Race* | 53/62 (85.4%) | 24/24 (100.0%) | 21/26 (80.8%) | 23/25 (92.0%) | 17/18 (94.4%) | |
| Female Sex* | 27/62 (43.5%) | 8/24 (33.3%) | 7/26 (26.9%) | 10/25 (40.0%) | 5/18 (27.8%) | |
| Indication for Lung Tx:* | COPD+ | 22/62 (35.4%) | 16/24 (66.7%) | 10/26 (38.5%) | 10/25 (40.0%) | 7/18 (38.9%) |
| Pulmonary Fibrosis | 23/62 (37.1%) | 7/24 (29.1%) | 10/26 (38.5%) | 7/25 (28.0%) | 4/18 (22.2%) | |
| Cystic Fibrosis | 9/62 (14.5%) | 0/24 (0.0%) | 4/26 (15.4%) | 5/25 (20.0%) | 6/18 (33.3%) | |
| Other | 8/62 (12.9%) | 1/24 (4.2%) | 2/26 (7.7%) | 3/25 (12.0%) | 1/18 (5.6%) | |
| Bilateral Lung Transplant* | 62/62 (100.0%) | 24/24 (100.0%) | 24/26 (92.3%) | 25/25 | 25/25 (100.0%) | |
number (%)
chronic obstructive pulmonary disease
Acute Rejection (Grade A ≥ 1 or B ≥ 1)
In order to better understand the serial changes in CCSP within individual patients, we then conducted a longitudinal analysis of CCSP levels in patients with BOS using an antecedent BAL sample obtained prior to the onset of BOS. In this analysis shown in Figure 5B, we demonstrate that CCSP levels were significantly higher in these patients prior to the onset of BOS (1575 +/− 451.1 ng/mL vs. 446.3 +/− 121.8 ng/mL, p = 0.02).
Finally, we considered the relationship of BAL CCSP levels to the degree of BAL neutrophilia. First, we considered the extent to which the composition of the BAL varied with BOS, and found that BOS was associated with significantly increased neutrophilia as compared to BOS-free patients (17.6 +/− 1.1% vs. 7.0 +/− 4.1%, p = 0.019), as shown in Supplemental Figure 2A. Next, we considered if the elevated neutrophilia correlated with the reduction in CCSP. Interestingly, when considered across either the entire cohort or within only the patients with BOS, CCSP levels correlated poorly with the degree of airway neutrophilia (r = 0.12, p = 0.19 for entire cohort; r = 0.36, p = 0.08 for patients with BOS), as shown in Supplemental Figure 2B–C Finally, we considered the potential confounding effects of azithromycin on BAL CCSP levels in patients with BOS. While about one-fourth of the BOS patients were on azithromycin, CCSP levels did not differ between BOS patients treated with azithromycin as compared to those not treated with azithromycin (465.3 +/− 327.3 ng/mL vs. 711.6 +/− 257.9 ng/mL, p = 0.67), as shown in Supplemental Figure 3
Discussion
Although BOS is thought to involve epithelial cell injury, the extent to which selective injury occurs with bronchiolar Clara cells in this condition has not been previously well-studied. Using complementary and translational analysis of tissue transcript, tissue protein expression, and BAL protein expression, we make the novel observation that BOS involves selective alteration in the distribution and function of epithelial Clara cells. Strengths of this analysis include the multimodality approach used to test our hypothesis and inclusion of a range of clinical samples including normal donor tissue, BOS-free lung transplant recipients, and patients with advanced BOS undergoing retransplantation. Our BAL analysis is particularly notable for comparably matched samples with explicit well-defined clinical phenotypes and provides important validation and extension of the observations made at a tissue transcript and protein level in the BAL.
Although several previous studies have suggested that CCSP protein levels are reduced in BOS, our current results extend these previous BAL studies in several ways. First, we confirm reduced levels of CCSP protein occurs in BOS across an adequately sized cohort of BOS and BOS-free patients. A notable limitation of the few previous studies in this area is a relatively small sample size, particularly among the BOS patients. For example, in one study, Nord and colleagues found that both BAL and serum levels of CCSP were reduced in lung transplant recipients with BOS(10); that analysis, however, included only 22 subjects, 6 of whom developed BOS. Second, we explicitly measured CCSP levels in the BAL of all patients. Interestingly, while studies that analyzed the BAL from patients with BOS using a proteomic approach have implicated CCSP changes, results were not always confirmed. For example, Zhang and colleagues followed 57 patients, 30 of whom developed BOS, and associated the development of BOS with a lower ratio of proteins suggestive of CCSP to lysozyme(11). Due to the pilot nature of that study, however, confirmation of the proteins identified in the MALDI-TOF profile was not performed. More recently, Wolf and colleagues generated BAL proteome profiles for 82 lung transplant recipients, 48 of whom developed BOS (12). Among the panel of significant markers was CCSP; however, this finding was validated by ELISA in only 13 patients, 8 of whom had BOS. Third, through analysis of CCSP expression at a tissue transcript and protein level, we provide the first direct evidence that reduced BAL CCSP levels reflect actual depletion of epithelial Clara cells.
Interestingly, in our current analysis, the CCSP levels in the BAL were comparable among patients that were BOS-free, BOS-free with acute rejection, BOS-free with acute infection, or in BOS 0p. All of these groups, however, had higher BAL CCSP levels than those in BOS. This suggests that CCSP, while reduced in BOS, may be less useful as a biomarker for early disease identification, in contrast to the preliminary results observed in the prior studies (10–13). In part, these results might reflect the primarily cross-sectional nature of our study (15). We did confirm through a longitudinal analysis of a subset of the study patients with BOS that their antecedent BAL (a median of 9.5 months prior to the BOS sample) had significantly higher CCSP levels than that observed in the same patients once BOS had occurred. Thus, additional prospective studies would be useful to better understand serial changes in BAL CCSP levels to determine its true value as a predictive biomarker for BOS. Alternatively, early changes noted at the tissue transcript and protein expression level might not translate into the BAL until later stages of disease. Further study of CCSP transcript or protein expression seems worthwhile and could provide a novel and early biomarker for BOS.
The mechanism of selective injury to epithelial Clara cells relative to ciliated cells in BOS is uncertain. In the post-transplant setting, the entire pulmonary epithelium is subject to a range of injuries including alloimmune cellular and humoral immune responses, gastric reflux, and pulmonary infections (7, 16–18). More recently, autoimmune responses to selected self-antigens such as type V collagen and k-alpha tubulin have been implicated in the development of BOS (19, 20). Potentially, there is increased expression of AlloMHC or unique self-antigens on Clara cells that are targeted in transplant leading to their selective depletion. Alternatively, differences in intracellular metabolism between Clara cells and ciliated cells may lead to increased vulnerability of Clara cells in this allotransplant setting, as has been observed with certain toxin exposures in murine models (21).
Loss of Clara cells adversely affects pulmonary homeostasis and could contribute to BOS development through several mechanisms. First, recent evidence suggests an important anti-inflammatory role of these cells in response to several environmental stimuli (22) or infections (23). Plopper and colleagues found that when exposed to 1.0 ppm ozone, CCSP deficient mice had a greater loss of both ciliated and non-ciliated epithelial cells as well as increased necrosis. Harrod found that CCSP deficient mice had a more exacerbated response to RSV, leading to more BAL cells as well as increased cytokine and chemokine levels (24). In addition, recent work found that CCSP deficient mice demonstrate an excessive response to lipopolysaccharide (LPS) (25). Given that our prior work has implicated LPS response genes in acute rejection or BOS, this latter observation is particularly relevant to the lung transplant patient population (26). Thus, loss of Clara cells and associated reductions in lung fluid CCSP levels could contribute to excessive inflammatory responses in the transplant allograft.
In addition, recent evidence suggests non-ciliated luminal cells that express CCSP function as epithelial progenitor cells responsible for maintaining epithelial homeostasis and regenerating injured epithelium (27). Failure of epithelial repair has been implicated in the development of obliterative airways disease after allogeneic, as compared to syngeneic, murine heterotopic tracheal transplants (28). Similarly, in human BOS it is possible that constant injury post lung transplant depletes the airway progenitor cells over time, and by depleting the reparative capacity of the epithelium results in dysfunctional repair and ultimately BO. As such, early depletion at a cellular level could represent a critical step in the progression of BOS if normal epithelial repair is retarded.
Although our study was the first to examine selective injury to Clara cells in BOS, there are several limitations. Our transcriptional analysis compared tissue from patients undergoing retransplant for BOS to BOS-free donor controls that were obtained from excessive donor tissue trimmed at the time of lung transplantation, reflecting the challenges in obtaining suitable quality and quantity tissue samples from normal healthy lung transplant recipients for transcript analysis. We addressed this point directly by obtaining additional tissue for immunofluorescence analysis. By using tissue from surgical lung biopsies, we were able to confirm normal expression of CCSP in BOS-free lung transplant recipients, demonstrating that changes in CCSP expression are not simply a consequence of lung transplantation but rather are specifically associated with BOS. We also leveraged samples from patients with early stage BOS to determine that these changes are evident early on in the development of BOS, supporting a potential role for CCSP depletion in disease pathogenesis. Second, we also acknowledge that a limitation of our study is an inability to assess if these changes in Clara cell distribution and function are a cause or consequence of the events that lead to BOS. Instead, our study was designed to determine if BOS involves selective Clara cell injury. Based on the multiple lines of evidence that support alterations in Clara cells occur in BOS, our work justifies more in-depth in vitro and in vivo studies to better understand how Clara cells could contribute to the pathogenesis of BOS.
In conclusion, our results demonstrate that functional changes in Clara cell expression occur in BOS reflected at a transcript, cellular and lung fluid level. Recent identification of the anti-inflammatory role for Clara cells and the role of non-ciliated cells in the repair of the injured epithelium support the idea that such changes could play a mechanistic role in the development of BO, although further mechanistic studies are needed to better understand the significance of our observation and the relevance of these changes in Clara cells expression to the pathogenesis of BOS. Given the general failure of existing treatments for BOS, manipulation of epithelial Clara cells might represents a promising strategy to pursue in future studies to improve long-term outcomes after lung transplantation.
Supplementary Material
Supplemental Figure 1: Depletion of CCSP relative to SMA expression in BO-affected airways: Advanced BO tissue is co-immuno-stained for CCSP (red) and SMA (green) or AT (green) and nuclear counter stained with DAPI (blue). CCSP and AT (A,C) in BO-affected ariways. The same airways were co-stained with CCSP and SMA (B,D). In all images, the relative preservation of AT compared to CCSP is striking. All images are presented at 200x.
Supplemental Figure 2: Relationship of BAL CCSP levels to the severity of the BAL neutrophilia. A. A cross-sectional sampling of lavage fluid from lung transplant patients with BOS (n = 26) compared to patients who remained BOS-free (n = 62). B. Correlation between CCSP levels and percentage of neutrophils for all patients. C. Correlation between CCSP levels and percentage of neutrophils for all patients with BOS.
Supplemental Figure 3: Effect of azithromycin on BAL CCSP levels in lung transplant patients with BOS. A cross-sectional sampling of lavage fluid from BOS patients on azithromycin at time of BAL (n = 5) compared to BOS patients not on azithromycin at time of BAL (n = 22).
Acknowledgments
This study was funded by the Roche Organ Transplant Research Foundation and NHLBI (5K24-HL09 1140-03).
Abbreviations
- BOS
bronchiolitis obliterans syndrome
- BO
bronchiolitis obliterans
- CCSP
Clara cell secretory protein
- BAL
bronchoalveolar fluid
- AT
acetylated tubulin
- SMA
smooth muscle actin
- LPS
lipopolysaccharide
Footnotes
Disclosure: This manuscript was not prepared or funded in any part by a commercial organization. The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional supporting information may be found in the online version of this article.
Supplemental Table 1: Demographics for patients undergoing re-transplantation due to advanced BOS.
Supplemental Figure 1: Cellular depletion of CCSP in BOS airways relative to expression of SMA.
Supplemental Figure 2: BAL CCSP protein levels do not correlate with BAL neutrophila.
Supplemental Figure 3: Azithromycin does not alter BAL CCSP protein levels in BOS.
References
- 1.Johnston CJ, Finkelstein JN, Oberdorster G, Reynolds SD, Stripp BR. Clara cell secretory protein-deficient mice differ from wild-type mice in inflammatory chemokine expression to oxygen and ozone, but not to endotoxin. Experimental lung research. 1999;25(1):7–21. doi: 10.1080/019021499270394. Epub 1999/02/23. [DOI] [PubMed] [Google Scholar]
- 2.Snyder JC, Reynolds SD, Hollingsworth JW, Li Z, Kaminski N, Stripp BR. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. American journal of respiratory cell and molecular biology. 2010;42(2):161–71. doi: 10.1165/rcmb.2008-0353OC. Epub 2009/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. The American journal of pathology. 2002;161(1):173–82. doi: 10.1016/S0002-9440(10)64169-7. Epub 2002/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proceedings of the American Thoracic Society. 2008;5(3):328–33. doi: 10.1513/pats.200711-167DR. Epub 2008/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Winkle LS, Johnson ZA, Nishio SJ, Bronn CD, Plopper CG. Early events in naphthalene-induced acute Clara cell toxicity - Comparison of membrane permeability and ultrastructure. American journal of respiratory cell and molecular biology. 1999;21(1):44–53. doi: 10.1165/ajrcmb.21.1.3630. [DOI] [PubMed] [Google Scholar]
- 6.Snyder LD, Palmer SM. Immune mechanisms of lung allograft rejection. Seminars in respiratory and critical care medicine. 2006;27(5):534–43. doi: 10.1055/s-2006-954610. Epub 2006/10/31. [DOI] [PubMed] [Google Scholar]
- 7.Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74(6):799–804. doi: 10.1097/00007890-200209270-00011. Epub 2002/10/05. [DOI] [PubMed] [Google Scholar]
- 8.Martinu T, Chen DF, Palmer SM. Acute rejection and humoral sensitization in lung transplant recipients. Proceedings of the American Thoracic Society. 2009;6(1):54–65. doi: 10.1513/pats.200808-080GO. Epub 2009/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadjiliadis D, Duane Davis R, Steele MP, Messier RH, Lau CL, Eubanks SS, et al. Gastroesophageal reflux disease in lung transplant recipients. Clinical transplantation. 2003;17(4):363–8. doi: 10.1034/j.1399-0012.2003.00060.x. Epub 2003/07/19. [DOI] [PubMed] [Google Scholar]
- 10.Nord M, Schubert K, Cassel TN, Andersson O, Riise GC. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation. 2002;73(8):1264–9. doi: 10.1097/00007890-200204270-00013. Epub 2002/05/01. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomics. 2006;6(3):1001–10. doi: 10.1002/pmic.200500105. Epub 2006/01/10. [DOI] [PubMed] [Google Scholar]
- 12.Wolf TOT, Gottlieb J, Pich A, Brors B, Eils R, Haverich A, Schlegelberger B, Welte T, Zapatka M, von Neuhoff N. Proteomic bronchiolitis obliterans syndrome risk monitoring in lung transplant recipients. Transplantation. 2011;92(4):477–85. doi: 10.1097/TP.0b013e318224c109. Epub August 27, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Mattsson J, Remberger M, Andersson O, Sundberg B, Nord M. Decreased serum levels of clara cell secretory protein (CC16) are associated with bronchiolitis obliterans and may permit early diagnosis in patients after allogeneic stem-cell transplantation. Transplantation. 2005;79(10):1411–6. doi: 10.1097/01.tp.0000158354.39635.ab. Epub 2005/05/25. [DOI] [PubMed] [Google Scholar]
- 14.Snyder LD, Finlen-Copeland A, Hartwig MG, Lin SS, Davis RD, Palmer SM. Lung transplantation at Duke University Medical Center. Clinical transplants. 2007:99–111. Epub 2008/07/22. [PubMed] [Google Scholar]
- 15.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. The Journal of Heart and Lung Transplantation. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 16.Martinu T, Howell DN, Palmer SM. Acute cellular rejection and humoral sensitization in lung transplant recipients. Seminars in respiratory and critical care medicine. 2010;31(2):179–88. doi: 10.1055/s-0030-1249113. Epub 2010/04/01. [DOI] [PubMed] [Google Scholar]
- 17.Castor JM, Wood RK, Muir AJ, Palmer SM, Shimpi RA. Gastroesophageal reflux and altered motility in lung transplant rejection. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2010;22(8):41–50. doi: 10.1111/j.1365-2982.2010.01522.x. Epub 2010/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinu T, Howell DN, Davis RD, Steele MP, Palmer SM. Pathologic correlates of bronchiolitis obliterans syndrome in pulmonary retransplant recipients. Chest. 2006;129(4):1016–23. doi: 10.1378/chest.129.4.1016. Epub 2006/04/13. [DOI] [PubMed] [Google Scholar]
- 19.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. The Journal of clinical investigation. 2007;117(11):3498–506. doi: 10.1172/JCI28031. Epub 2007/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiriveedhi V, Angaswamy N, Weber J, Mohanakumar T. Lipid raft facilitated ligation of K-alpha1-tubulin by specific antibodies on epithelial cells: Role in pathogenesis of chronic rejection following human lung transplantation. Biochemical and biophysical research communications. 2010;399(2):251–5. doi: 10.1016/j.bbrc.2010.07.063. Epub 2010/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. The American journal of pathology. 2000;156(1):269–78. doi: 10.1016/S0002-9440(10)64727-X. Epub 2000/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plopper CG, Mango GW, Hatch GE, Wong VJ, Toskala E, Reynolds SD, et al. Elevation of susceptibility to ozone-induced acute tracheobronchial injury in transgenic mice deficient in Clara cell secretory protein. Toxicology and applied pharmacology. 2006;213(1):74–85. doi: 10.1016/j.taap.2005.09.003. Epub 2005/10/18. [DOI] [PubMed] [Google Scholar]
- 23.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. The American journal of physiology. 1998;275(5 Pt 1):L924–30. doi: 10.1152/ajplung.1998.275.5.L924. Epub 1998/11/14. [DOI] [PubMed] [Google Scholar]
- 24.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171(2):1051–60. doi: 10.4049/jimmunol.171.2.1051. Epub 2003/07/09. [DOI] [PubMed] [Google Scholar]
- 25.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. American journal of respiratory cell and molecular biology. 2009;40(6):633–42. doi: 10.1165/rcmb.2008-0334OC. Epub 2008/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer SM, Burch LH, Trindade AJ, Davis RD, Herczyk WF, Reinsmoen NL, et al. Innate immunity influences long-term outcomes after human lung transplant. American journal of respiratory and critical care medicine. 2005;171(7):780–5. doi: 10.1164/rccm.200408-1129OC. Epub 2005/01/11. [DOI] [PubMed] [Google Scholar]
- 27.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(23):9286–91. doi: 10.1073/pnas.0900668106. Epub 2009/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalermskulrat W, Neuringer IP, Brickey WJ, Felix NJ, Randell SH, Ting JP, et al. Hierarchical contributions of allorecognition pathways in chronic lung rejection. American journal of respiratory and critical care medicine. 2003;167(7):999–1007. doi: 10.1164/rccm.200209-1099OC. Epub 2002/11/26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Depletion of CCSP relative to SMA expression in BO-affected airways: Advanced BO tissue is co-immuno-stained for CCSP (red) and SMA (green) or AT (green) and nuclear counter stained with DAPI (blue). CCSP and AT (A,C) in BO-affected ariways. The same airways were co-stained with CCSP and SMA (B,D). In all images, the relative preservation of AT compared to CCSP is striking. All images are presented at 200x.
Supplemental Figure 2: Relationship of BAL CCSP levels to the severity of the BAL neutrophilia. A. A cross-sectional sampling of lavage fluid from lung transplant patients with BOS (n = 26) compared to patients who remained BOS-free (n = 62). B. Correlation between CCSP levels and percentage of neutrophils for all patients. C. Correlation between CCSP levels and percentage of neutrophils for all patients with BOS.
Supplemental Figure 3: Effect of azithromycin on BAL CCSP levels in lung transplant patients with BOS. A cross-sectional sampling of lavage fluid from BOS patients on azithromycin at time of BAL (n = 5) compared to BOS patients not on azithromycin at time of BAL (n = 22).