Abstract
High levels of homocysteine (Hcy), known as hyperhomocysteinemia (HHcy), are associated with cerebrovascular diseases, such as vascualr dementia, stroke, and Alzheimer's disease. The -amino butyric acid (GABA) is a inhibitory neurotransmitter and a ligand of GABA-A receptor. By inhibiting excitatory response it may decrease complications associated with vascular dementia and stroke. Hcy specifically competes with the GABA-A receptors and acts as an excitotoxic neurotransmitter. Previously we have shown that Hcy increases levels of NADPH oxidase and reactive oxygen species (ROS), and decreases levels of thioredoxin and peroxiredoxin by antagonizing the GABA-A receptor. Hcy treatment leads to activation of matrix metalloproteinases (MMPs) in cerebral circualtion by inducing redox stress and ROS. The hypothesis is that Hcy induces MMPs and suppresses tissue inhibitors of metalloproteinase (TIMPs), in part, by inhibiting the GABA-A receptor. This leads to degradation of the matrix and disruption of the blood brain barrier. The brain cortex of transgenic mouse model of HHcy (cystathionine -synthase, CBS −/+) and GABA-A receptor null mice treated with and without muscimol (GABA-A receptor agonist) was analysed. The mRNA levels were measured by Q-RT-PCR. Levels of MMP-2, -9, -13, and TIMP-1, -2, -3, and -4 were evaluated by in situ labeling and PCR-gene arrays. Pial venular permeability to fluorescence-labeled albumin was assessed with intravital fluorescence microscopy. We found that Hcy increases metalloproteinase activity and decreases TIMP-4 by antagonizing the GABA-A receptor. The results demonstrate a novel mechanism in which brain microvascular permeability changes during HHcy and vascular dementias, and have therapeutic ramifications for microvascular disease in Alzheimer's patients.
Keywords: Pial venules, ROS, mitochondria, CBS, vascular dementia, Alzheimer's disease, stroke, muscimol
Introduction
Hyperhomocysteinemia (HHcy) is an important risk factor for the development of cerebrovascular diseases, thromboembolism, and stroke (1, 2). Reductions in Hcy levels are associated with reduced carotid artery restenotic events after angioplasty (3, 4). Interestingly, elevation in Hcy level induces seizure in animals (5) and it is associated with vascular dementia and Alzheimer's disease in humans (6). Treatment with a -amino butyric acid (GABA)-A receptor agonist decreases seizures in animals (5, 7). Hcy behaves as an antagonist to GABA-A receptor. Epidemiological studies have also indicated that HHcy is a contributory factor for the development of atherosclerotic lesions and arterial hypertension (8, 9). GABA-A receptor agonist decreases blood pressure and has been used as an antihypertensive agent (5). The decrease in blood pressure by a GABA-A agonist is associated with an increase in endothelial NO (10, 11). Hypertensive patients who had elevated Hcy had enhanced endothelial dysfunction (12).
GABA is a major inhibitory neurotransmitter. GABA-receptors are classified into ionotropic (GABA-A), metabotropic (GABA-B) and GABA-C on the basis of pharmacological and physiological studies (13, 14). GABA-receptors are present in the mammalian central nervous system (CNS) (15), heart (16) and endothelial cells (17). These receptors decrease neuronal membrane “excitability” i.e. by increasing chloride conductance resulting in the inhibitory actions of GABA (18–20). The antagonist to the N-methyl-D-aspartate (NMDA) receptor (21) protects Hcy-mediated toxic effects in neurons (5), suggesting that Hcy is an agonist to NMDA-receptor and an antagonist to GABA-receptor. Muscimol (MC, GABA-A receptor agonist) specifically competes with Hcy for binding to the GABA-A receptor (22).
The role of matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) in neuronal degeneration has been suggested (2, 5, 23, 24). Matrix degradation and proteolytic shedding of GABA/NMDA receptors have been implicated with tissue plasminogen activator (tPA) (25, 26). GABA receptors play a significant role in alteration of brain microvascular permeability which alters the cerebral microvascular environment leading to matrix degradation and edema (27–30). Ablation of the beta-1 integrin gene decreases GABA receptor-mediated cell-matrix interaction (31). Treatment with the GABA-A receptor antagonist, bicuculline, resulted in increased levels of MMP-9 (32). We showed that MC inhibits MMP activation in microvascular endothelial cells (33). Others have suggested that GABA inhibits cell migratory activity in 3-D collagen gels (34). MMPs play a significant role in regulation of microvascular permeability and edema (35–37). Hcy levels are associated with vascular dementias and seizures (5–7); however, it is unclear whether Hcy-mediated vascular edema is ameliorated by the GABA-A receptor via MMP activation.
Methods
Animals were maintained at temperatures between 22°C and 24°C. A 12-hour light-dark cycle was maintained by artificial illumination. In accordance with the National Institute of Health Guidelines for animal research, all animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Louisville School of Medicine. The animals were fed standard chow and water ad libitum. Levels of water and food intake, and changes in body weight were measured every other day. Because results from other laboratories have suggested extensive genetic homology between mice and humans (38), we used mice. Mice were treated with or without MC and maintained accordingly in this study.
High methionine diet during the cystathionine -synthase (CBS) deficiency creates HHcy. Experimental animal models of HHcy have been created by infusing methionine or homocysteine thiolactone (22, 39). However, methionine overload may induce generalized protein synthesis and homocysteine-thiolactone may produce additional injury by lactone. On the other hand, CBS (−/+) mice have an endogenous elevation of Hcy due to CBS gene disruption. A breeding pair of heterozygous for CBS gene (C57BL/6J-Cbstm1Unc), and homozygote knockout for GABA-A receptor gene B6:129-GABrabtm1geh was obtained from Jackson Laboratories. The mice were bred in the mice breeding facility of the University of Louisville School of Medicine. CBS was inactivated using homologous recombination in C57BL/6J mouse embryonic stem cells to disrupt the coding sequence of the CBS gene as described (20). Heterozygous (−/+) CBS-deficient mice and littermate, wild type (WT, +/+) controls were used. The GABA-A −/− mice have a gene knockout due to specific mutation in the alpha subunit. MMP-9 −/− is a homozygote knockout.
The genotyping and phenotyping of offspring at the age of ~8 weeks that were weighing 20 3 grams were determined by collecting tail vein blood and tail tissue. CBS genotyping was performed by PCR using CBS specific primers and phenotype was done by evaluating plasma levels of Hcy (39). DNA was extracted and amplified by PCR for sequences in intron 3 and Neo insert in CBS−/+ mice (39). The PCR primers are as follows: 5'-GCCTCTGTCTGCTAACCTA-3'; 5'-GAGGTCGACGGTATCGATA-3' (20). The product was identified by two bands with differing electrophoretic mobility (39). The phenotype was determined by measuring Hcy levels in the blood obtained from the tail vein. Levels of Hcy in the plasma were measured by HPLC separation and spectrophometric titration with dithio-bis-nitrobenzoate with absorption measured at 412 nm, using ε412 nm of 13,600 M−1 cm−1 (40). Activity of CBS was measured using the methods of Kashiwamata and Greenberg (49). The mouse GABA-A receptor primers TTCTAGCCTCCTTCCAGTATGATTTG (sense) and TACTCAACAGTACTGCTCCACTTCC (antisense) (geneNank # X51986) were used (5). For GABA-A receptor genotyping, the product corresponding to nucleotides 250–963 of the alpha-6 subunit cDNA as described (5) was used as a marker. The wild type allele corresponding with Southern blot band at 9.4 KB and targeted mutant allele of 6.6 KB was used as described (5). Because Hcy levels vary with the levels of CBS enzyme, the mice were divided into four groups: 1) mice with Hcy levels between 3 and 5 μmol/L and one PCR product (WT) (7); 2) mice with Hcy levels between 15 to 25 μmol/L and two PCR products with CBS primers with different electrophoretic mobility in agarose gels (CBS−/+); 3) Mice with one PCR product of lower mobility than WT when using GABA-A primers (GABA-A−/−); 4) double knockout mice with Hcy levels between 15 to 25 μmol/L and two PCR products with CBS primers; and one PCR product with GABA-A primers with different electrophoretic mobility in agarose gels (CBS−/+/GABA-A−/−) (20). Primers for TIMP-4 were: sense 5'-GTGACGAGAAGGAGGTGGATTCC and anti-sense 5'-CTTGATGCAGGCAAAGAACTTGGC (GenBank No. U76456).
To activate the GABA-A receptor, MC (Sigma Chemical Co) was administered to CBS−/+ mice at 8 μg/ml in the drinking water. This amount of MC is soluble in aqueous conditions. The concentration was determined based on the fact that the binding constant between MC and GABA-A receptor is in the micromole range (22), mice have a blood volume of 7–8% of their body weight, and drink ~2.5 ml water/day. Therefore, each mouse ingested ~20 μg/day MC that resulted in a blood concentration of ~ 32 μMol/L MC, which is enough to saturate most binding sites on GABA receptors. To determine selectivity of MC in the absence of Hcy, MC was administered to WT (C57BL/6J) mice. To determine whether the MC treatment causes any change in food intake, food intake was measured every second day during the treatment period. Because previous studies demonstrated significant vascular dysfunction at 8–12 weeks of homocysteinemia (38), we administered MC for 8 weeks. After 8 weeks, mice were anesthetized with tribromoethanol (100 mg/kg i.p.), which has a minimal effect on cardiovascular function in mice (44). To determine plasma Hcy in each mouse, 0.5 ml blood was collected from each mouse by a catheter (PE-10) in the right common carotid artery.
cDNA Gene Expression Array (GEArray) analysis
The expression of neurotransmitter receptor response genes was analyzed using a non-radioactive GEArray Q-series mouse neurotransmitter receptors and regulators finder Gene Array (SuperArray, Inc, Bethesda, MD). Briefly, 4 μg total RNA was used as template for biotinylated cDNA probe synthesis. RNA was reverse-transcribed by gene-specific primers with biotin-16-dUTP. Biotinylated cDNA probes were denatured and hybridized to obtain gene-specific cDNA fragments spotted on the membranes. The GEArray membranes were then washed and blocked with GEArray blocking solution, and incubated with alkaline phosphatase–conjugated streptavidin. The hybridized biotinylated probes were detected by chemiluminescent method using the alkaline phosphatase substrate, CDP-Star. The results were analyzed using Kodak Molecular Imaging Software. Each array membrane comprises 96 marker genes in quadruplicate; four positive controls including ß-actin, glyceraldehyde-3-phosphate dehydrogenase, cyclophilin A, and ribosomal protein L13a; and a negative control, bacterial plasmid pUC18. The relative expression levels of different genes were estimated by comparing its signal intensity with that of internal control ß-actin.
In vivo fluorescence imaging of MMP activity
To conduct in vivo imaging of MMP activity, we injected DQ-gelatin (Molecular Probes Inc) into tail vein. The probe was injected into tail vein 24-hours prior to measurements. MMP substrate fluoresces was assessed at Ex/Em 495/515 (green) after MMP cleavage. To avoid fluorescence interference due to hair, whole body was saved and isolated brain fluorescence was measured by Kodak MM-4000 (43). The serial tissue sections were analyzed by FV-1000 confocal microscope (Olympus Corp). To estimate the variation in measurements of MMP activity among similarly treated animals, the intensity of the green fluorescence generated due to MMP activation was quantitated using Image Pro-Plus Software (Media Cybernetics Inc).
Brain microvascular preparation
Vascular leakage from mouse pial venules was studied in 1) WT; 2) CBS−/+; 3) GABA-A −/−; 4) GABA-A −/− / CBS−/+; 5) CBC−/+ + MC; 6) WT + MC mice. After anesthesia, a carotid artery was cannulated to measure mean arterial blood pressure and to infuse fluorescein isothiocyanate (FITC)-conjugated bovine serum albumin (BSA). The pial circulation at the cortical surface was exposed via a craniotomy. The mouse was positioned on the heating pad placed on the modified stage of the upright microscope (BXG61WI, Olympus, Japan). After stabilization of mice, FITC-BSA (0.2 ml/100g of body wt.) probe was injected intra-arterially and allowed to circulate for 5 min. Epi-illumination with blue light (450–490 nm) from a mercury arc lamp was used to observe the vascular network. A neutral density filter 4 (ND4) was used, to limit fluorescence intensity. Prior to Hcy infusion (50 μl of 1.25 mM), the image of the pial circulation segment was recorded using a 10× objective and data acquisition system. Five minutes and 40 minutes after Hcy infusion, images of the same vascular area were recorded (50). The fluorescence intensity in the interstitial area adjacent to the brain pial venule of interest was measured using Image Pro Plus image analysis software. The area of interest was kept the same during analysis. Data are presented as fluorescence intensity units (FIU) in the range of 0–250 FIU (50).
Statistical Analysis
Statistical analysis was performed using GraphPad InStat to compare data collected from groups. Differences between groups and controls were determined by one-way analysis of variance (ANOVA). If a significant difference was indicated, the Tukey post-hoc test was used to identify groups that were significant. Differences were considered significant if P < 0.05. All values are presented as mean ± SEM.
Results
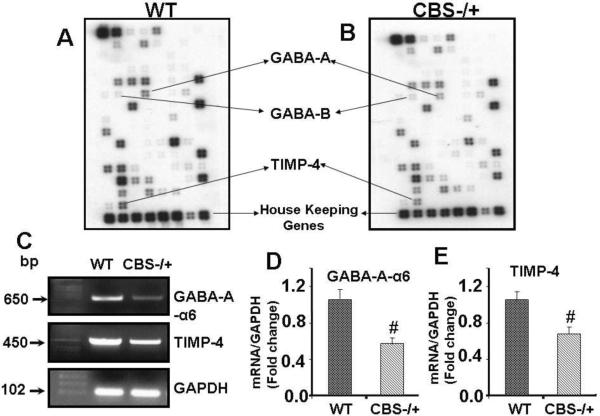
The cDNA gene array and RT-PCR analyses of the brains of CBS−/+ mice suggests significant decrease in the mRNA expression of GABA-A receptor in CBS −/+ mice as compared to WT controls. There was no significant (p<0.05) change in the mRNA levels of GABA-B receptor. The levels of TIMP-4 mRNA were also decreased significantly (p<0.05) in the brains of CBS−/+ mice as compared to controls (Figure 1). These data suggest down-regulation of GABA-A receptor in CBS−/+ HHcy mice as compared to WT controls. They also suggest a decrease in TIMP-4 levels and thus significant alteration in subendothelial matrix.
Figure 1. Gene array and RT-PCR analyses of GABA receptors and TIMP-4 in WT and CBS−/+ mouse brain.
A&B: The RNA was isolated, and equal amounts of poly(A)+ RNA from WT and CBS−/+ mouse brain was hybridized to cDNA array membranes. Affected genes are pointed with arrows. C: RT-PCR analysis of GABA receptors and TIMP-4 mRNA expression in WT and CBS−/+ mouse brain. The GAPDH housekeeping gene was used as the loading control. D&E: Densitometric analysis of GABA receptors and TIMP-4 mRNA expressions as represented in the bar diagram. #P<0.05 vs WT, n=4 animals/ group.
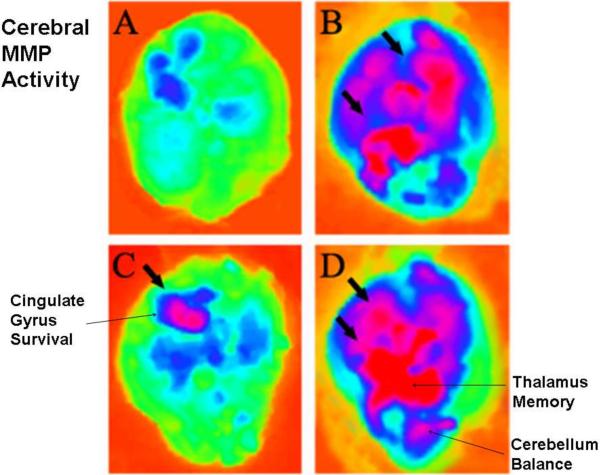
In vivo MMP activity was increased in brain cortex of CBS−/+, GABA-A−/− and double knockout CBS−/+/GABA-A−/− mice as compared to WT controls (large arrows pointing to the red spots indicate MMP activity, Figure 2A). The results from cross sections of frozen tissue suggest robust MMP activation in both CBS−/+ and double knockouts as compared to WT controls. There was significant (p<0.05) increase in MMP activity in GABA-A null mice as compared to WT controls. The activity of MMP in the microvessels (arrows, Figure 2B) of CBS−/+ and double knockouts as compared to GABA-A−/− and WT controls was also increased (Figure 2C). These data suggested that although there was an increase in MMP activity in GABA-A−/− null, Hcy further induced MMP activation in GABA-A−/−, indicating an additional role of GABA-B receptor in Hcy-mediated MMP activation (Figure 2).
Figure 2. In situ Zymography in WT, CBS, GABA-A and double KO mice.
A: DQ- gelatin (a MMP substrate) was injected in tail vein 24- hours prior to in vivo imaging of the brain. In addition, FITC-labeled gelatin was infused through carotid artery prior to sacrifice the animal. The in fluorescence image of isolated brain was obtained in (A) WT; (B) CBS−/+; (C) GABA-A−/−; (D) CBS−/+ / GABA-A−/−. The arrows indicate MMP activity. B: in situ MMP activity in the brain. Cross-sections were prepared and MMP activiy was measured in tissue sections under confocal microscopein WT; CBS−/+; GABA-A−/−; CBS−/+ / GABA-A−/−. The arrows indicate capillaries and MMP activity. C: Densitometric analysis of MMP activity. *P<0.05 vs WT, **P<0.05 vs CBS−/+ ; n=4 animals/group.
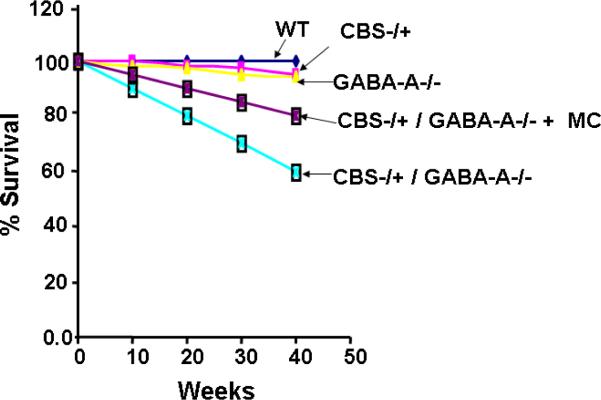
The GABA-A−/− knockout allele is not lethal (45). We had measured the survival rate of GABA-A receptor null and double knockout mice (Figure 3). The results showed that CBS−/+ and GABA-A receptor null mice lifespan is similar to WT mice, however, 40% of double CBS−/+ / GABA-A receptor null mice died at 40 weeks. Interestingly, the treatment with muscimol at birth improved the survival to 80%. It was also noted that there was no link between the survival rate of CBS/GABA-A knockout mice and vascular defects.
Figure 3. Survival Rate.
Survival of double knockout (CBS−/+ / GABA-A−/−) mice. The mice were administered with muscimol or vehicle at birth (n=4 in each group).
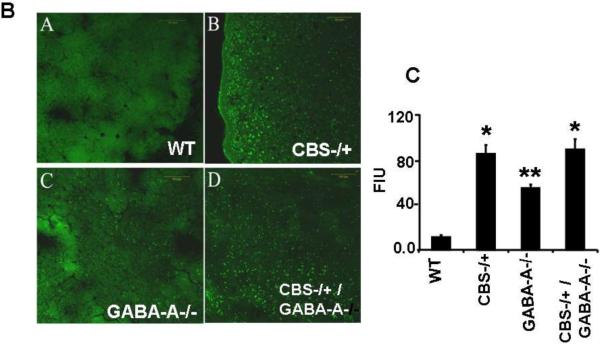
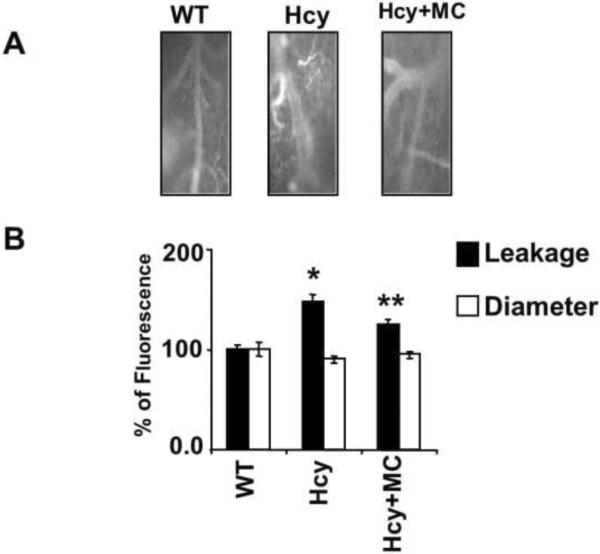
Pial venular permeability and diameters were measured in HHcy mice treated with or without MC. The results showed that there was 1.5-fold higher leakage in the pial vessels of HHcy mice as compared to WT controls. Pial vascular diameters of HHcy mice tended to be lesser than those in WT mice. The treatment with MC ameliorated the leakage in the brains of HHcy mice and normalized the vascular diameters (Figure 4). No toxic effect of MC was noticed in these animals.
Figure 4. Muscimol ameliorates brain microvascular leakage in HHcy mice.
A: examples of images recorded in of WT, Hcy-treated (Hcy), and HHcy mice treated with MC (Hcy+MC). Pial venular leakage was assessed by measuring fluorescence intensity in the area of interest adjacent to the venuales.
B: The bar graphs represent mean±SEM of leakage and diameter from 4 animals in each group. *p<0.05 vs WT; ** p<0.05 vs Hcy, n=4 animals/ group.
Discussion
The majority of genetic causes of HHcy are due, primarily, to the heterozygote deficiency in CBS enzyme. The heterozygous (−/+) mice demonstrated a 4-fold increase in the levels of Hcy (20), analogous to the hyperhomocysteinemic human. Furthermore, there is cerebral arteriolar stiffness in CBS−/+ mice (46). We previously showed an increase in brain microvascular permeability in HHcy mice and that the ablation of MMP-9 attenuated the Hcy-mediated increase in cerebrovascular permeability (50).
Since most of the oxidative stress is caused by peroxidase the levels of peroxidase depend on the level of thioredoxin. In addition, the level of thioredoxin is a strong predictor of oxidative stress (42, 47). Therefore we measured tissue level of thioredoxin and found an increased oxidative stress (38, 42, 48, 49). Previous studies from our laboratory showed that Hcy increases oxidative stress by generating ROS and nitrotyrosine (38). We and others have suggested competition for binding between GABA and Hcy (22, 33, 51). To determine whether the increase in ROS by Hcy is due, in part, to the increase in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity secondary to GABA-A we measured NADPH oxidase activity in CBS−/+, GABA-A receptor null, and CBS−/+/GABA-A−/− mice treated with or without MC. In tissues NADPH oxidase is a primary source of ROS generation and treatment with MC decreases ROS formation.
Structural alteration in cerebral microcirculation of CBS −/+ mice has been demonstrated (46). Previous studies from our laboratory have shown that Hcy induces MMP (39, 40, 50). Activation of MMPs causes an increase in microvascular permeability (35–37). In an acute experiment, we demonstrated that Hcy induced brain microvascular leakage secondary to activation of MMP-9 (50). Although an antagonist to GABA-A receptor activates MMPs (32), and GABA-A receptor is associated with inhibitory neurotransmission, however, role of GABA-A receptor in vascular permeability was unclear. An observed reduction in GABA-A receptor mRNA levels in CBS+/− mice suggest a possible down regulation of GABA-A receptor indicating its possible role in cerebrovascular permeability.
Summary
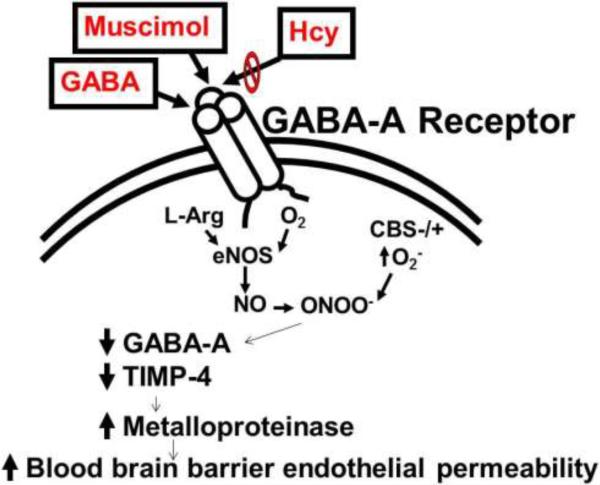
The relationship between hyperhomocysteinemia and vascular permeability was examined, as it relates to the blood-brain barrier permeability. The premise of the study is that homocysteine affects endothelial cells, thus altering its normal property and function. Specifically, homocysteine leads, via inhibition of the GABA-A receptor, to elevations in MMP-9 activity through a NO-dependent decrease in TIMP levels. As a result, there is a breakdown of the extracellular matrix which affects the blood-brain barrier. A reduction in TIMP-4 correlated with a higher MMP-9 activity. Based on the previous studies from our laboratory and the present data, we conclude that the chronic hyperhomocysteinemia causes brain microvascular leakage and treatment with MC ameliorates this condition in HHcy mice, attenuating the effects of Hcy overload (Figure 5).
Figure 5.
The schametic of hypothesis that Hcy activates metalloproteinases, causes cerebral microvascular leakage, secondary to antagonizing GABA-A receptor, and decreasing TIMP-4.
Footnotes
This work was supported in part by NIH grants: HL-71010 and NS-51568 (to SCT) and HL-80394 (to DL).
LITERATURE CITED
- 1.Loscalzo J. Homocysteine and dementias. N.Engl.J.Med. 2002;346:466–468. doi: 10.1056/NEJM200202143460702. [DOI] [PubMed] [Google Scholar]
- 2.Fridman O. [Hyperhomocysteinemia: atherothrombosis and neurotoxicity] Acta Physiol Pharmacol.Ther.Latinoam. 1999;49:21–30. [PubMed] [Google Scholar]
- 3.Hackam DG, Peterson JC, Spence JD. What level of plasma homocyst(e)ine should be treated? Effects of vitamin therapy on progression of carotid atherosclerosis in patients with homocyst(e)ine levels above and below 14 micromol/L. Am.J.Hypertens. 2000;13:105–110. doi: 10.1016/s0895-7061(99)00180-6. [DOI] [PubMed] [Google Scholar]
- 4.Schnyder G, Roffi M, Pin R, Flammer Y, Lange H, Eberli FR, Meier B, Turi ZG, Hess OM. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N.Engl.J.Med. 2001;345:1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- 5.Folbergrova J. NMDA and not non-NMDA receptor antagonists are protective against seizures induced by homocysteine in neonatal rats. Exp.Neurol. 1994;130:344–350. doi: 10.1006/exnr.1994.1213. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N.Engl.J.Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 7.Sieklucka M, Bortolotto Z, Heim C, Block F, Sontag KH. Decreased susceptibility to seizures induced by bicuculline after transient bilateral clamping of the carotid arteries in rats. J.Neural Transm.Gen.Sect. 1991;83:127–137. doi: 10.1007/BF01244459. [DOI] [PubMed] [Google Scholar]
- 8.Malinow MR, Levenson J, Giral P, Nieto FJ, Razavian M, Segond P, Simon A. Role of blood pressure, uric acid, and hemorheological parameters on plasma homocyst(e)ine concentration. Atherosclerosis. 1995;114:175–183. doi: 10.1016/0021-9150(94)05481-w. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Bostom A, Selhub J, Zeigler-Johnson C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997;96:1745–1749. doi: 10.1161/01.cir.96.6.1745. [DOI] [PubMed] [Google Scholar]
- 10.Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901. [PubMed] [Google Scholar]
- 11.Unger T, Becker H, Dietz R, Ganten D, Lang RE, Rettig R, Schomig A, Schwab NA. Antihypertensive effect of the GABA receptor agonist muscimol in spontaneously hypertensive rats. Role of the sympathoadrenal axis. Circ.Res. 1984;54:30–37. doi: 10.1161/01.res.54.1.30. [DOI] [PubMed] [Google Scholar]
- 12.Huang PL. Neuronal and endothelial nitric oxide synthase gene knockout mice. Braz.J.Med.Biol.Res. 1999;32:1353–1359. doi: 10.1590/s0100-879x1999001100005. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi SC, Smiley LM, Mujumdar VS. Homocyst(e)ine impairs endocardial endothelial function. Can.J.Physiol Pharmacol. 1999;77:950–957. [PubMed] [Google Scholar]
- 14.Taylor C, Fricker AD, Devi LA, Gomes I. Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal. 2005;17:549–557. doi: 10.1016/j.cellsig.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier J, Akyeli J, Kirischuk S, Grantyn R. GABA(A) receptor activity and PKC control inhibitory synaptogenesis in CNS tissue slices. Mol.Cell Neurosci. 2003;23:600–613. doi: 10.1016/s1044-7431(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HY, McPherson BC, Liu H, Baman TS, Rock P, Yao Z. H(2)O(2) opens mitochondrial K(ATP) channels and inhibits GABA receptors via protein kinase C-epsilon in cardiomyocytes. Am.J.Physiol Heart Circ.Physiol. 2002;282:H1395–H1403. doi: 10.1152/ajpheart.00683.2001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Liu G. A novel method to determine the localization of high and low-affinity GABA transporters to the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res.Brain Res.Protoc. 1999;4:288–294. doi: 10.1016/s1385-299x(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 18.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol.Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 19.Chebib M, Johnston GA. The 'ABC' of GABA receptors: a brief review. Clin.Exp.Pharmacol.Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- 20.Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol.Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 21.Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths R, Williams DC, O'Neill C, Dewhurst IC, Ekuwem CE, Sinclair CD. Synergistic inhibition of [3H]muscimol binding to calf-brain synaptic membranes in the presence of L-homocysteine and pyridoxal 5'-phosphate. A possible mechanism for homocysteine-induced seizures. Eur.J.Biochem. 1983;137:467–478. doi: 10.1111/j.1432-1033.1983.tb07850.x. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J, Ribo M, Santamarina E, Quintana M, Monasterio J, Montaner J. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 24.Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Hollander GA. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin.Infect.Dis. 2000;31:80–84. doi: 10.1086/313922. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 26.Frey U, Muller M, Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. J.Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WS, Limmroth V, Ayata C, Cutrer FM, Waeber C, Yu X, Moskowitz MA. Peripheral GABAA receptor-mediated effects of sodium valproate on dural plasma protein extravasation to substance P and trigeminal stimulation. Br.J.Pharmacol. 1995;116:1661–1667. doi: 10.1111/j.1476-5381.1995.tb16388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limmroth V, Lee WS, Moskowitz MA. GABAA-receptor-mediated effects of progesterone, its ring-A-reduced metabolites and synthetic neuroactive steroids on neurogenic oedema in the rat meninges. Br.J.Pharmacol. 1996;117:99–104. doi: 10.1111/j.1476-5381.1996.tb15160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fruscella P, Sottocorno M, Di BM, Diomede L, Piccardi N, Cagnotto A, Grossi G, Romano M, Mennini T, Roma G. 1,5-Benzodiazepine tricyclic derivatives exerting anti-inflammatory effects in mice by inhibiting interleukin-6 and prostaglandinE(2)production. Pharmacol.Res. 2001;43:445–452. doi: 10.1006/phrs.2001.0800. [DOI] [PubMed] [Google Scholar]
- 30.Lazzarini R, Malucelli BE, Palermo-Neto J. Reduction of acute inflammation in rats by diazepam: role of peripheral benzodiazepine receptors and corticosterone. Immunopharmacol.Immunotoxicol. 2001;23:253–265. doi: 10.1081/iph-100103864. [DOI] [PubMed] [Google Scholar]
- 31.Andressen C, Arnhold S, Puschmann M, Bloch W, Hescheler J, Fassler R, Addicks K. Beta1 integrin deficiency impairs migration and differentiation of mouse embryonic stem cell derived neurons. Neurosci.Lett. 1998;251:165–168. doi: 10.1016/s0304-3940(98)00535-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JW, Deb S, Gottschall PE. Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. Eur.J.Neurosci. 1998;10:3358–3368. doi: 10.1046/j.1460-9568.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 33.Shastry S, Tyagi N, Moshal KS, Lominadze D, Hayden MR, Tyagi SC. GABA receptors ameliorate Hcy-mediated integrin shedding and constrictive collagen remodeling in microvascular endothelial cells. Cell Biochem.Biophys. 2006;45:157–165. doi: 10.1385/CBB:45:2:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph J, Niggemann B, Zaenker KS, Entschladen F. The neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002;62:6467–6469. [PubMed] [Google Scholar]
- 35.Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, Wang X, Rosenberg GA, Lo EH, Moskowitz MA. Cortical spreading depression activates and upregulates MMP-9. J.Clin.Invest. 2004;113:1447–1455. doi: 10.1172/JCI21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 37.Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- 38.Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, Mi ZP, Wang XH, Grayson DR, Firestone LL. Gene knockout of the alpha6 subunit of the gamma-aminobutyric acid type A receptor: lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol.Pharmacol. 1997;51:588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- 39.Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, Givvimani S, Tyagi SC. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem.Int. 2008;53:214–219. doi: 10.1016/j.neuint.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller A, Mujumdar V, Shek E, Guillot J, Angelo M, Palmer L, Tyagi SC. Hyperhomocyst(e)inemia induces multiorgan damage. Heart Vessels. 2000;15:135–143. doi: 10.1007/s003800070030. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi ST, Skamene E, Malo D. Comparative genomics and host resistance against infectious diseases. Emerg.Infect.Dis. 1999;5:36–47. doi: 10.3201/eid0501.990105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc.Natl.Acad.Sci.U.S.A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci. 1993;43:189–192. [PubMed] [Google Scholar]
- 45.Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, Malinow MR, Heistad DD. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J.Clin.Invest. 1996;98:24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthias D, Becker CH, Riezler R, Kindling PH. Homocysteine induced arteriosclerosis-like alterations of the aorta in normotensive and hypertensive rats following application of high doses of methionine. Atherosclerosis. 1996;122:201–216. doi: 10.1016/0021-9150(95)05740-4. [DOI] [PubMed] [Google Scholar]
- 47.Wong CM, Zhou Y, Ng RW, Kung Hf HF, Jin DY. Cooperation of yeast peroxiredoxins Tsa1p and Tsa2p in the cellular defense against oxidative and nitrosative stress. J.Biol.Chem. 2002;277:5385–5394. doi: 10.1074/jbc.M106846200. [DOI] [PubMed] [Google Scholar]
- 48.Tyagi SC, Smiley LM, Mujumdar VS, Clonts B, Parker JL. Reduction-oxidation (Redox) and vascular tissue level of homocyst(e)ine in human coronary atherosclerotic lesions and role in extracellular matrix remodeling and vascular tone. Mol.Cell Biochem. 1998;181:107–116. doi: 10.1023/a:1006882014593. [DOI] [PubMed] [Google Scholar]
- 49.Kashiwamata S, Greenberg DM. Studies on cystathionine synthase of rat liver. Properties of the highly purified enzyme. Biochim.Biophys.Acta. 1970;212:488–500. doi: 10.1016/0005-2744(70)90255-x. [DOI] [PubMed] [Google Scholar]
- 50.Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am.J.Physiol Heart Circ.Physiol. 2006;290:H1206–H1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shastry S, Moning L, Tyagi N, Steed M, Tyagi SC. GABA receptors and nitric oxide ameliorate constrictive collagen remodeling in hyperhomocysteinemia. J.Cell Physiol. 2005;205:422–427. doi: 10.1002/jcp.20416. [DOI] [PubMed] [Google Scholar]