Abstract
Objectives
To determine if the spontaneous reinnervation that characteristically ensues after recurrent laryngeal nerve (RLN) injury could be selectively promoted and directed to certain laryngeal muscles with the use of neurotrophic factor (NF)-secreting muscle stem cell (MSC) vectors while antagonistic reinnervation is inhibited with vincristine (VNC).
Study Design
Basic science investigations involving primary cell cultures, gene cloning/transfer, and animal experiments.
Methods
(i.) MSC survival assays were used to test multiple individual NFs in vitro. (ii.) Motoneuron outgrowth assays assessed the trophic effects of identified NF on cranial nerve X-derived (CNX) motoneurons in vitro. (iii.) Therapeutic NF was cloned into a lentiviral vector, and MSCs were tranduced to secrete NF. 60 rats underwent left RLN transection injury, and at 3 weeks received injections of either MSCs (n=24), MSCs secreting NF (n=24), or saline (n=12) into the left thyroarytenoid muscle complex (TA); half of the animals in the MSC groups simultaneously received left posterior cricoarytenoid (PCA) injections of vincristine (VNC) while half the animals received saline.
Results
(i.) Ciliary-derived neurotrophic factor (CNTF) had the greatest survival-promoting effect on MSCs in culture. (ii.) Addition of CNTF (50 ng/mL) to CN X motoneuron cultures resulted in enhanced neurite outgrowth and branching. (iii.) In the animal model, the injected MSCs fused with the denervated myofibers, immunohistochemistry demonstrated enhanced reinnervation based on motor endplate to nerve contact, and RT-PCR confirmed stable CNTF expression at longest follow-up (4 months) in the CNTF-secreting MSC treated groups.
Conclusions
MSC therapy may have a future role in selectively promoting and directing laryngeal reinnervation after RLN injury.
Level of evidence: NA
Keywords: larynx, recurrent laryngeal nerve, vocal fold paralysis, laryngeal paralysis, muscle stem cells
INTRODUCTION
Loss of vocal fold function due to neurological injury with incomplete recovery can result in dysphonia and dysphagia. If there is bilateral loss of function then airway compromise can result as well.1,2 Current therapies for vocal fold paralysis (VFP) fail to reliably restore functional motion, and therefore are suboptimal. Thus, the search for a new treatment for VFP is warranted. The goal of this thesis is to investigate muscle stem cell (MSC)-based therapy for the treatment of VFP. These studies are preliminary in nature, to determine if neural regeneration can be influenced with such an approach. While a MSC-based model is unlikely to restore full vocal fold motion as a primary therapy for RLN injury, such an approach may serve as a minimally invasive adjuvant therapy for directing reinnervation after VFP reinnervation procedures, or for promoting reinnervation of laryngeal replacement models such as laryngeal transplantation or the conceptual stem cell-based tissue engineered larynx.
Recent studies suggest that persistent vocal fold immobility after recurrent laryngeal nerve (RLN) injury is not due to lack of reinnervation, but due to the aberrant, spontaneous reinnervation that ensues after nearly all RLN injuries.3–4 Despite spontaneous reinnervation after RLN injury being widely recognized, the source of reinnervation is debatable. Sources such as the ipsilateral RLN,4,5 autonomic nerves (adrenergic and non-adrenergic),6,7 and the superior laryngeal nerve (SLN)8 have independently been implicated as primary sources of laryngeal reinnervation after RLN injury. Certainly the functional outcome attained with spontaneous reinnervation after RLN injury depends not only on the on the primary source of reinnervation, but also on the relative degree of reinnervation to the denervated adductor and abductor muscles. The importance of this relationship is probably best exemplified in studies attempting to reanimate the larynx via surgical reinnervation after RLN injury. For example, proximal RLN transection followed by primary neurorrhaphy consistently fails to result in normal vocal fold motion (abduction and adduction) due to misdirected axonal regeneration of the adductor and abductor fibers resulting in synkinesis.9 In contrast, surgically guiding phrenic to posterior cricoarytenoid (PCA) muscle reinnervation with simultaneous non-phrenic laryngeal adductor muscle reinnervation is an approach that has been investigated for years,10–11 and recently shown to lead to restoration of abductor motion in select cases.11 Thus, based on the conceptual need to promote differential reinnervation to the adductor and abductor muscles after RLN injury, the goal of this study was to use minimally invasive techniques to enhance physiologic pathways involved in neural regeneration to selected laryngeal muscles, while preventing functional antagonistic reinnervation.
The development of this minimally invasive model was based on previous work by Flint and colleagues demonstrating that spontaneous reinnervation can be enhanced with delivery of certain NF at the denervated laryngeal muscle studies,12–15 and the work by Paydarfar and Paniello that showed VNC can inhibit reinnervation to selected muscles.16 While the studies by Flint and colleagues directly injected NF-expressing plasmid vector into the denervated hemilarynx and demonstrated successful enhancement of reinnervation when the plasmid was expressed, we chose not to use plasmid alone because of its innate limitations of unreliable and inefficient sustained gene expression. Based on our laboratory’s previous investigations demonstrating that autologous MSCs will survive in a denervated hemilarynx in an animal model for at least a two month period,17,18 we anticipated that using MSC vectors for NF delivery would lead to selectively increased spontaneous reinnervation if a stable gene transfer vector were identified for the MSCs. Furthermore, based on prior work by Paydarfar and Paniello,16 our laboratory had studied vincristine (VNC) as a neurotoxin to prevent antagonistic reinnervation, and an optimal dose and concentration of VNC was identified that could be injected to the rat laryngeal muscles without histologically affecting reinnervation of adjacent muscles.19 Thus, we anticipated being able to use these minimally invasive approaches to selectively enhance reinnervation to certain muscles after RLN injury, while chemically inhibiting functional reinnervation to antagonistic abductor muscles. In the proposed model, it was anticipated that application of VNC to inhibit spontaneous PCA reinnervation to the posterior cricoarytenoid (PCA) would thereby unmask the innate spontaneous reinnervation to the adductor complex; furthermore, NF-secreting MSCs in the adductor complex may selectively augment innate adductor reinnervation. Of course, outcomes would be expected to be directly related to the source of adductor spontaneous reinnervation. For example, tone and midline migration alone would be anticipated with predominantly autonomic reinnervation, adduction during phonation/deglutition with adductor RLN fiber reinnervation, inspiratory adduction with abductor RLN fiber reinnervation, and some combination of functions may result if a variety of reinnervation source(s) were involved. Thus, the overall goal of the study was not to restore normal vocal fold motion, but rather to determine if neural regeneration after RLN injury can be selectively guided with a minimally invasive MSC-based approach and to determine outcomes in an animal model.
Hypothesis
It was hypothesized that a neurotrophic factor (NF) with the potential to enhance RLN recovery could be identified using MSC survival assays and vagus motoneuron outgrowth testing; furthermore, it was hypothesized that therapeutic delivery of identified NF via autologous MSC vectors could be used to selectively enhance reinnervation after RLN injury, and, when antagonistic reinnervation (synkinesis) is simultaneously inhibited, vocal fold adductor activity may be detectable.
Thus, this thesis involved 3 specific aims: (Please see Figure 1 for overview):
| Aim 1 | To use an MSC survival assay followed by vagus motoneuron outgrowth testing to identify an individual NF that promotes muscle stem cell survival and enhances vagus motoneuron regeneration. |
| Aim 2. | To construct a gene transfer vector encoding therapeutic NF, with resultant NF secretion from transduced primary MSCs in vitro. |
| Aim 3. | To determine post-RLN injury therapeutic outcomes in a time-dependent fashion after NF-secreting autologous MSCs are injected into acutely denervated laryngeal adductor muscles to promote reinnervation and localized neurotoxin is injected into the posterior cricoarytenoid muscle (PCA) to inhibit antagonistic reinnervation. |
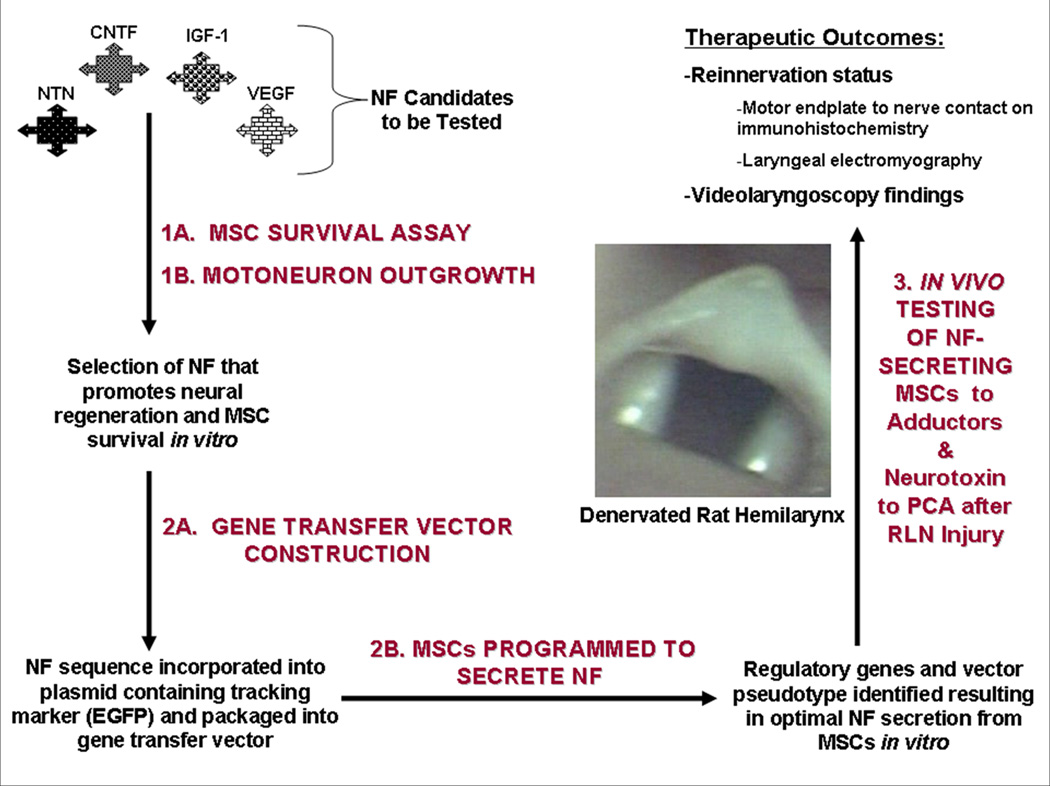
Figure 1. Overview of Specific Aims.
NFs of interest are tested with MSC survival assays and motoneuron outgrowth testing (1A and 1B) to identify a NF that promotes MSC survival and enhances vagus motoneuron outgrowth. The identified NF is encoded into a lentiviral vector (2A); MSCs are then transduced with the lentiviral vector encoding the NF, and NF secretion from primary MSCs is optimized (2B). Finally, RLN transection injury is treated by introduction of the NF-secreting MSCs into the denervated adductor complex, while the posterior cricoarytenoid (PCA) muscle is injected with a neurotoxin (vincristine) to prevent antagonistic reinnervation (3). NF= neurotrophic factor, MSC=muscle stem cell, EGFP=enhanced green fluorescent protein, RLN=recurrent laryngeal nerve.
METHODS
Experiments to Address Specific Aim 1
1A: MSC Culture Technique
MSC survival assays were developed to test the impact that the NFs of interest have on MSC survival. First, MSC cultures were established as previously described.17,18 In brief, starting with 1 gram of rat skeletal muscle, connective tissue was trimmed and muscle dissected into 1 mm pieces or smaller. Excess D-PBS was aspirated and 4ml of 0.2% collagenase type I (Worthington Biochemical, Lakewood, NJ) was added, incubating it at 37°C for 2 hours. The cells were then titurated, rinsed in D-PBS, and distributed among the wells of a 6-well 0.2% Matrigel-coated plate (Fisher Sci, Chicago, IL) containing myogenic medium consisting of Dulbeco’s Modified Eagle Medium (DMEM) (Fisher-Scientific, Chicago, IL), 1% Penicillin and Streptomycin (P/S) (Gibco Invitrogen Cell Culture, Carlsbad, CA), 10 ng/mL IGF-1 (Gibco Invitrogen Cell Culture, Carlsbad, CA), 2.5µg/ml Amphotericin B (Sigma-Aldrich, St Louis, MO), 20% Heat-Inactivated Fetal Bovine Serum (FBS) (Valley Biomedical, Winchester, VA), 10% Heat-Inactivated Horse Serum (HS) (Gibco Invitrogen Cell Culture, Carlsbad, CA), 0.1% Chick Embryo Extract (CEE) (Accurate Chemicals, Westbury, NY). Cells were grown at 37°C and 5% CO2. Preplating steps were done as each passage to maintain a balanced population of muscle progenitor cells. Passaging of the cultures (1:3 split) was carried out at subconfluency (70% confluency) to avoid maturation of the cells and myotube formation. Homogeneity of MSC cultures was characterized by examining CD56 cell surface expression employing fluorescence activated cytometric analysis (FACS) (Becton Dickinson, San Jose, CA).
MSC Survival Assays
While neurotrophic factors (NFs) are defined as secreted proteins that promote the survival, growth and/or differentiation of neurons, the NFs selected for these studies were selected based on their known potential for dual myotrophic and neurotrophic effects. Based on these criteria, and a previous investigation on adjuvant therapies for optimizing MSC survival in the denervated larynx,18 vascular endothelial growth factor (VEGF), insulin like growth factor-1 (IGF-1), ciliary derived neurotrophic factor (CNTF), and neurturin (NTN) were selected for the MSC survival investigations.
After primary MSC cultures had been established (as described above), MSCs were cultured in serum-free medium for 24 hours after which 5×105 cells were plated in 6-well culture plates. Serum-free medium was then supplemented with individual growth factors including recombinant human insulin-like growth factor (IGF-1) (100 ng/mL) (Invitrogen, Carlsbad, CA); recombinant rat vascular endothelial growth factor (VEGF) (100ng/mL) (Biosource International, Inc., Camarillo, CA); recombinant ciliary neurotrophic factor (CNTF) (100 ng/mL) (Biosource International, Inc., Camarillo, CA); recombinant human neurturin (NTN) (100 ng/mL) (Biosource International, Inc., Camarillo, CA); or saline. Cell cultures were maintained in a 37°C, 5% CO2, humidified incubator. Cells were harvested 48 hours later and cell survival assessed by trypan blue exclusion. All experiments were conducted in triplicate, using three different primary cell lines. Student’s t-test was used to compare NF-mediated MSC survival to that of controls.
1B: Motoneuron Culture Technique
Six well plates of rat motoneuron cultures derived from tenth cranial motor nuclei were created by incorporating methodology of Kivell and colleagues20 with significant modifications.21 In brief, rat motoneurons were purified from the ventral brainstem of rats on post-natal day one (P1) with dissection including motoneurons from the nuclei of cranial nerve X, with minimal extension into IX and XII to ensure that all CN X motoneurons were encompassed in the dissection. The specimen was immediately incubated in Hibernate-A (Hib-A) (Brainbits, Springfield, IL) with 1X B27 (2% v/v; Invitrogen, Carlsbad, CA) on ice. Brainstem tissue was dissociated after trypsinization (0.05%) (Sigma, St Louis, MO) with 0.1 mg/mL DNAse I (Roche, Indianapolis, IN) added, repetitive centrifuging in Hib-A, and trituration with fire-polished Pasteur-pipets until a single cell suspension is obtained. Motoneuron enrichment over a 10% OptiPrep (Accurate Chemical Co, Westbury, NY) density gradient in Hib-A was then performed, centrifuging at 400g for 25 min, removing the upper fraction, and then centrifuging at 700g for 10 min to pellet. Cells were resuspended in Hib-A, centrifuged, and then resuspended in 1.5 mL growth media [Neurobasal-A with 1X glutamine (Invitrogen),1X B27 supplement, 0.45X 2-mercaptoethanol (Invitrogen), 50 U/mL penicillin/streptomycin (Invitrogen)] and plated on 22mm polyornithine (Sigma, St. Louis, MO) and laminin (Invitrogen)-coated tissue 5mm culture-treated coverslips. For additional purification, the cells were allowed to adhere while incubating at 37°C for 30 minutes. The coverslips were then removed and washed in pre-warmed 37°C plain Neurobasal-A (Invitrogen), placed into a 96-well dish pre-filled with 3 mL growth media and incubated at 37°C.
Motoneuron Outgrowth Assessment
Because CNTF demonstrated the greatest impact on MSC survival, this series of experiments focused on CNTF at differing concentrations, to determine if there was an optimal concentration of CNTF that would induce CN X outgrowth effects in vitro. Recombinant rat CNTF was added at physiologic concentrations (0.5, 5, 50, or 500 ng/mL) every 24 hours for 3 days. The negative control group had saline alone added every 24 hours. Phase-contrast images were captured using the Nikon Upright Spotcam, with Image J® software used for analysis. Neurite length (summation of the lengths of all neurites per neuron) was measured at three days. Only those neurons with a neurite length at least twice the diameter of the soma were included in this analysis, involving six motoneurons per treatment group. In addition to analyzing neurite length, the number of side branches (primary branches) and the longest neurite per motoneuron was determined (Figure 2). Experiments were performed in triplicate.
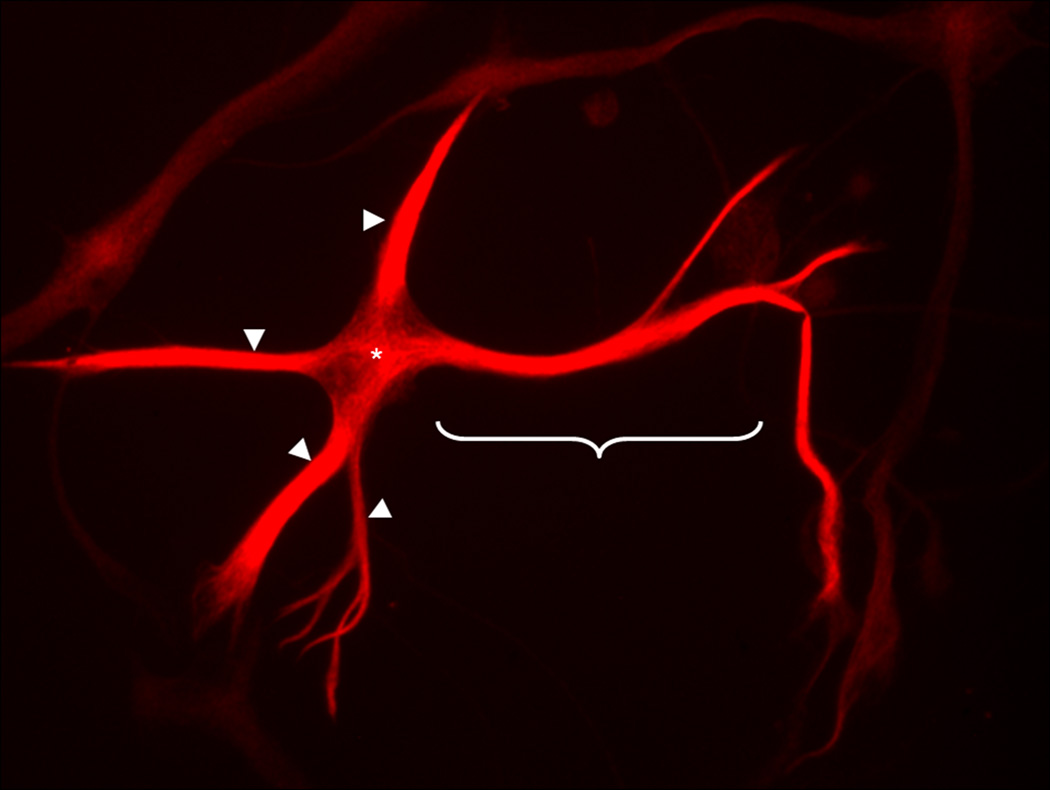
Figure 2. CN X Motoneuron in Culture.
A motoneuron in culture under 40X fluorescent microscope demonstrates positive synaptophysin (fluorescent red) staining. For the experiments, the longest neurite (bracket) was measured, and the number of primary and secondary branches. In addition, the sum of the total length of all neurites (arrowheads and bracket) was determined from the periphery of the soma (*).
Experiments to Address Specific Aim 2
2A: Construction of lentiviral vector encoding NF
NF-encoding constructs were based on the self-inactivating CMV-driven CNTF lentiviral vector kindly provided to our laboratory by Dr. Bas Blits.22 Dr. Kenneth Cornetta, the director of the Indiana University Vector Production Facility, kindly provided our laboratory with lentivirus of differing pseudotypes, with the lenti-adapted envelopes 4070A and RD114 are generated by Dr. Cornetta using plasmids kindly provided by Derek Person and Arthur Nienhuis of Memphis, TN.23 Transduction efficiencies with primary MSCs were then established with recombinant lentiviral vector pseudotyped with VSVG, pCAG-LPPP2, RD114, or amphotropic (pCAG 4 AMP) envelopes. The CNTF sequence of interest was cloned into the Bam HI site, just upstream from the EGFP reporter gene, such that EGFP expression would function as a marker of transduction. As previously described,24 recombinant lentivirus was produced by transient triple transfection of 293T cells. After seeding cells in 75-cm2 tissue culture flasks 24 hours before transfection in D-10 medium, and refeeding with D-10 medium 2 hours before transfection, 293T cells were transfected with the NF-encoding transfer plasmid, packaging plasmid, and amphotropic envelope plasmid at a ratio of 1:1:0.2 using calcium phosphate method (Profection kit; Promega, Madison, WI). Approximately 16 hours after transfection cells were refed with fresh medium, and 48 hours after transfection vector supernatants were collected, centrifuged, and pellets resuspended in PBS. Vector was filtered, suspended in 1 ml of D-10 and stored at −80°C. Viral titers were determined as previously described.21 In brief, twenty-four hours after 1×105 D17 cells were grown in a 6-well plate, and serial dilutions of concentrated viral stock were placed on cells and centrifuged at 2600 rpm for 1 hour at 32°C. The percentage of EGFP positive cells was determined with fluorescence activated cytometric analysis (FACS) (Becton Dickinson, San Jose, CA), and based on the percentage of positive cells and viral dilutions, viral titers were determined.
2B: Optimize NF Secretion from Transduced Primary MSCs In Vitro
In order to attain this goal, MSC cultures were established as described in Experiments to Address Specific Aim 1 above, and previously.17,18 Transduction was performed with MSCs at 50% confluency in 100mm plates. MSCs were incubated in lentiviral supernatant containing polybrene (8 µg/ml) (Sigma) for 4 hours at 37°C. All infections will be done in triplicate. When the cells demonstrate EGFP expression by fluorescent microscopy (approximately 72 hours post-transduction), cells were sorted by FACS for MSC-specific marker, CD56, and transduction marker, EGFP. Sorted cells were plated on 100mm plates, and expression of CNTF was determined with quantitative ELISA when cells reached 70% confluency as previously described by Hu et al.25 In brief, approximately 106 transduced MSCs were treated with protease inhibitor (Sigma), centrifuged, and the supernatant collected. After reaction with protein assay agent (Bio-Rad, Richmond, CA), protein concentration of each sample was measured by spectrophotometer. Ninety-six-well miniplates were coated with monoclonal mouse CNTF-antibody diluted in PBS buffer overnight at 4°C. The plates were incubated overnight at room temperature with blocking solution (1% BSA, 5% sucrose and 0.05% NaN3 in PBS). With interceding washes (0.05% Tween 20 in PBS, pH 7.4), the plates underwent 2 hour incubations with double aliquots of conditioned medium, protein extract, biotinylated polyclonal goat anti-CNTF factor antibody, and ABC Reagent (Vectastain; Vector Laboratories, Burlingame, USA). Horseradish peroxidase activity was detected using 3,3’,5,5’-tetramethylbenzidine (MP Biomedicals, Irvine, USA). After 30 min incubation, color reaction was stopped by adding H2SO4. Absorbance at 450nm was measured using an ELISA reader (Bio-Rad). Using serial dilutions of known amounts of CNTF, the color reaction was used to construct a standard curve, and CNTF levels in the samples were determined. All experiments were repeated in triplicate.
Experiments to Address Specific Aim 3
Animal Model of RLN Injury
To address specific aim three, 60 Fischer 344 rats were anesthetized with intraperitoneal (IP) ketamine (75 mg/kg) and xylazine (10 mg/kg). The left RLN was identified and transected, with 1 cm nerve segment removed. One gram of sternocleidomastoid muscle was harvested at the time of denervation, and placed immediately in myogenic medium. Before the animals awoke from anesthesia, unilateral vocal fold immobility was confirmed. The muscle biopsy was then used to culture autologous MSCs which were tranduced with lentiviral vector (see Experiments to Address Specific Aims 1 & 2 for MSC culture technique and lentiviral transduction methods, respectively). At approximately three weeks after the denervation procedure, lentiviral-transduced MSCs were sorted with FACS (Becton Dickinson, San Jose, CA) for expression of EGFP (a marker of transduction) and CD56 (a marker of myogenic lineage-committed myoblasts), with sorted MSCs used for injection. This time period was selected because it was ample time for therapeutic levels of transduced MSCs to be developed, it is an ideal time to administer a neurotoxin to inhibit ongoing reinnervation as demonstrated by our previous studies.19 For the injection procedure, animals were again anesthetized, and approximately 106 NF-expressing MSCs, or 106 control MSCs (expressing EGFP for cell tracking) suspended in saline were injected into the mid-body of the denervated TA of each animal via midline thyrotomy, while saline controls received saline alone. To inhibit reinnervation, half of the animals from each group received injections into the denervated PCA muscle with 4 µl of VNC (0.5µg/µl) aqueous solution to inhibit reinnervation of that muscle, while the other animals received saline alone (Table I). At two months or four months after the RLN transection procedure, six animals from each group were randomly selected to undergo anesthesia with videorecording of vocal fold motion and rating of motion in a blinded fashion. The vocal fold abduction was assessed during quiet respiration, and laryngeal adduction was assessed after inducing laryngospasm with a 20 gauge angiocatheter and during spontaneous glottic closure (with swallowing, coughing, etc). Laryngeal electromyography (LEMG) was performed on both the TA complex and PCA muscles to assess for the presence and pattern of reinnervation. For LEMG testing, a Nicolet Viking Quest electromyography machine was used with the amplitude set to 50 uV, with 10 ms sweep speeds. A 25 gauge bipolar concentric needle electrode was used for intramuscular testing, with a clamp placed on exposed lateral neck muscle for grounding. To ensure optimal needle insertion, the skin was incised and strap muscles were retracted to allow direct visualization of the larynx during the study. Each PCA was identified directly via a posterior approach while gently retracting the larynx contralaterally, and each TA was first identified and tested by insertion of the needle via the cricothyroid membrane, and then additional confirmation of correct needle placement (to ensure optimal signaling) was done by direct visualization through a midline laryngofissure. Recruitment of the TA was rated during laryngospasm, and recruitment for the PCA was rated during inspiration, with rating scales from 0–4 as previously described by Koufman et al.26 After individual muscle testing was done, the LEMG needle was p!aced in the left TA musc!e while a scalpel was used to transect the incoming neural supply to the larynx by sequentially transecting the left RLN, the left superior laryngeal nerve (SLN), the right RLN nerve, and the right SLN sources until the left TA LEMG activity was silenced. Animals were then euthanized, larynges harvested and frozen on liquid nitrogen. The tissue was sectioned as described by Nakagawa et al.14 Sections were analyzed under fluorescent microscopy to confirm EGFP expression from the TA in the MSC and NF-MSC treated groups. In addition, immunohistochemistry assessment of motor endplate to nerve contact was done using primary conjugated bungarotoxin antibody (Invitrogen, Eugene, OR) and beta-III tubulin antibody (Covance, Princeton, NJ) to demarcate the motor endplates and neurons, respectively. At four months, muscle samples were taken bilaterally from the TA complex and PCA for CNTF and other NF detection via RT-PCR. Student’s t-test and Fisher’s exact test were used for statistical analysis of outcomes, with significance set at p<0.05.
Table I.
Post-Injection Comparison Groups
| 1. Saline Injected TA + Saline Injected PCA: “Saline” (Ctl) Group (n=12) | 2 months (n=6) |
| 4 months (n=6) | |
| 2. MSC Injected TA + Saline Injected PCA: “EGFP” Group (n=12) | 2 months (n=6) |
| 4 months (n=6) | |
| 3. NF-MSC Injected TA + Saline Injected PCA: “CNTF” Group (n=12) | 2 months (n=6) |
| 4 months (n=6) | |
| 3. MSC Injected TA+ VNC Injected PCA: “EGFP-VNC” Group (n=12) | 2 months (n=6) |
| 4 months (n=6) | |
| 4. NF-MSC Injected TA+ VNC Injected PCA: “CNTF-VNC” Group (n=12) | 2 months (n=6) |
| 4 months (n=6) |
TA: thyroarytenoid muscle complex; PCA: posterior cricoarytenoid muscle; MSC: control muscle stem cells with tracking marker EGFP (enhanced green fluorescent protein); NF-MSC: neurotrophic factor-secreting muscle stem cells; Ctl: control. All animals undergo left RLN transection injury 3 weeks prior to the therapeutic injections. Follow-up time periods (right) are relative to the time of RLN Injury.
RESULTS
Results for Specific Aim 1A
Identification of an NF that Promotes MSC Survival
MSC Survival Assay
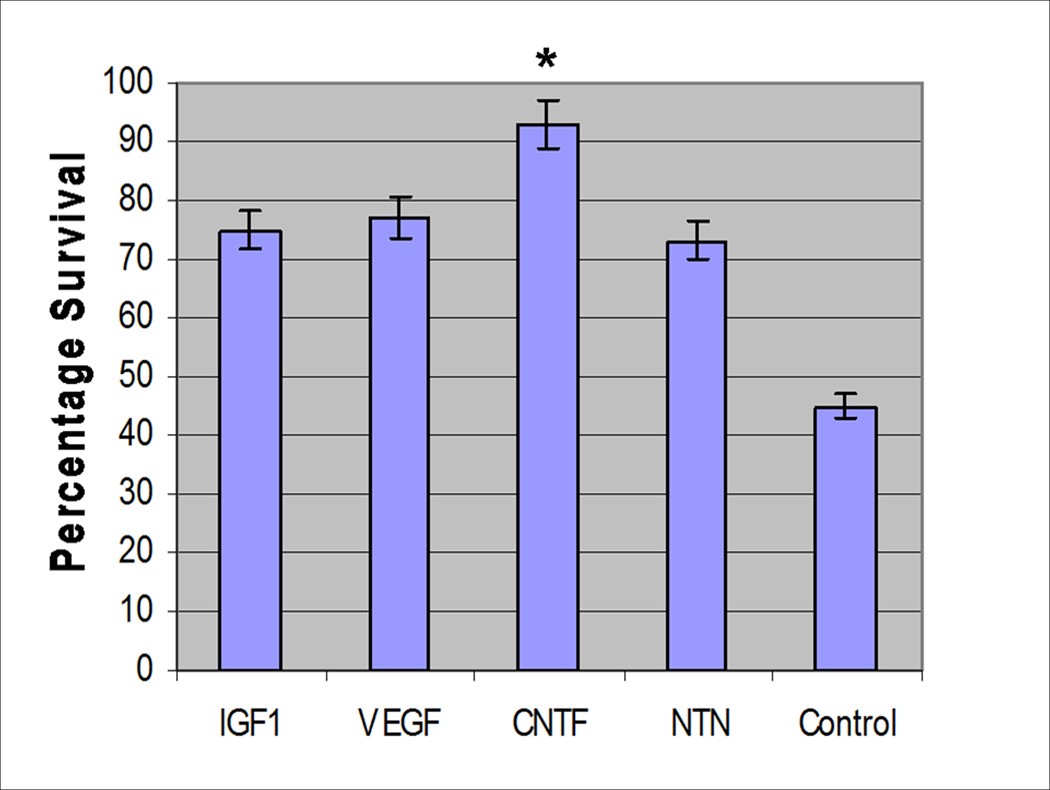
To characterize effects of NFs on MSC survival, the potentiation of IGF, VEGF, CNTF, and NTN on primary homogeneous cultures of MSCs was evaluated in an in vitro survival assay (see Methods). With three sets of primary MSC cultures, the CNTF treated cultures demonstrated the highest percentage of viable MSCs (p=0.02), suggesting that CNTF augments MSC survival in vitro (Figure 3). While the MSC cultures supplemented with other individual NFs (IGF-1, VEGF, and NTN) contained higher percentages of viable cells compared to saline control cultures, the difference was not significant.
Figure 3. Muscle Stem Cell Survival Based on the Neurotrophic Factor Additive.
The average of three survival assay experiments demonstrates a mean myoblast survival of 93% when MSCs were incubated with CNTF versus 45% in the controls (p=0.02). While the other factors also appeared to enhance survival, the difference was not significant (p>0.05).
Results for Specific Aim 1B
Determine the effect of CNTF on motoneuron outgrowth
Motoneuron Outgrowth Testing
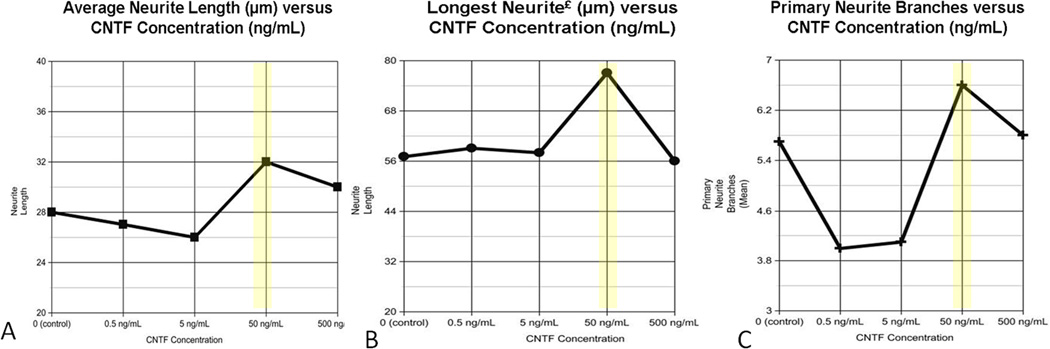
Because CNTF demonstrated the greatest impact on MSC survival, this series of experiments focused on CNTF at differing concentrations, to determine the effect of CNTF on CN X outgrowth in vitro. Recombinant rat CNTF was added at physiologic concentrations (0.5, 5, 50, or 500 ng/mL) every 24 hours for 3 days to the vagus motoneuron cultures, as described in the Methods. Results demonstrated that while CNTF at a concentration of 50 ng/mL resulted in the greatest mean motoneuron neurite length (Figure 4A), the difference did not reach significance. However, addition of CNTF at the same concentration (50 ng/mL) resulted in longer maximal neurite length than that of the other CNTF groups and the controls (p=0.001) (Figure 4B). In addition, a CNTF concentration of 50 ng/mL resulted in significantly more neurite branching with a mean of 6.6 branches per neurite compared to 5.7 branches in the controls (p=0.012) (Figure 4C). In addition to these CNTF investigations, our laboratory has also previously studied and reported the effects of other neurotrophic factors on CNX motoneuron outgrowth, with no factor identified that has greater outgrowth effect than that of CNTF.21
Figure 4. Neurite Outgrowth in Respose to CNTF.
When evaluating average neurite length (A), longest neurite (B), and the number of primary branches for each motoneuron (C), CNTF additive at a concentration of 50 ng/mL (yellow) consistently produced the greatest positive effect.
Plasmid Vector
These investigations aimed to determine the optimal vector for introducing CNTF-encoding genome into MSCs. While plasmid vectors lack immunogenicity, they are plagued by inefficient gene transfer. We hypothesized that an ex vivo gene delivery may circumvent the issue of low transduction rates, as primary MSCs proliferate rapidly in culture, allowing a therapeutic number of MSCs to be attained despite low gene transfer efficiency. To test this hypothesis, primary MSCs derived from 250 g male Fischer 344 rats were grown in culture to a cell count of 10^5. Cells were then transfected with EGFP expressing plasmid, and after 48 hours fluorescence activated cytometric analysis (FACS) (Becton Dickinson, San Jose, CA) was used to determine the percentage of cells with EGFP expression. This demonstrated tranfection efficiency with the plasmid to be 8%. After sorting the EGFP expressing MSCs, they were maintained in culture for 3 weeks. MSC fluorescence rapidly decreased over that time period, with no EGFP detectable at 3 weeks, suggesting plasmid extrusion. Thus, based on this rapid rate of plasmid extrusion, it was concluded that plasmid vector is suboptimal for these investigations.
Viral Vector
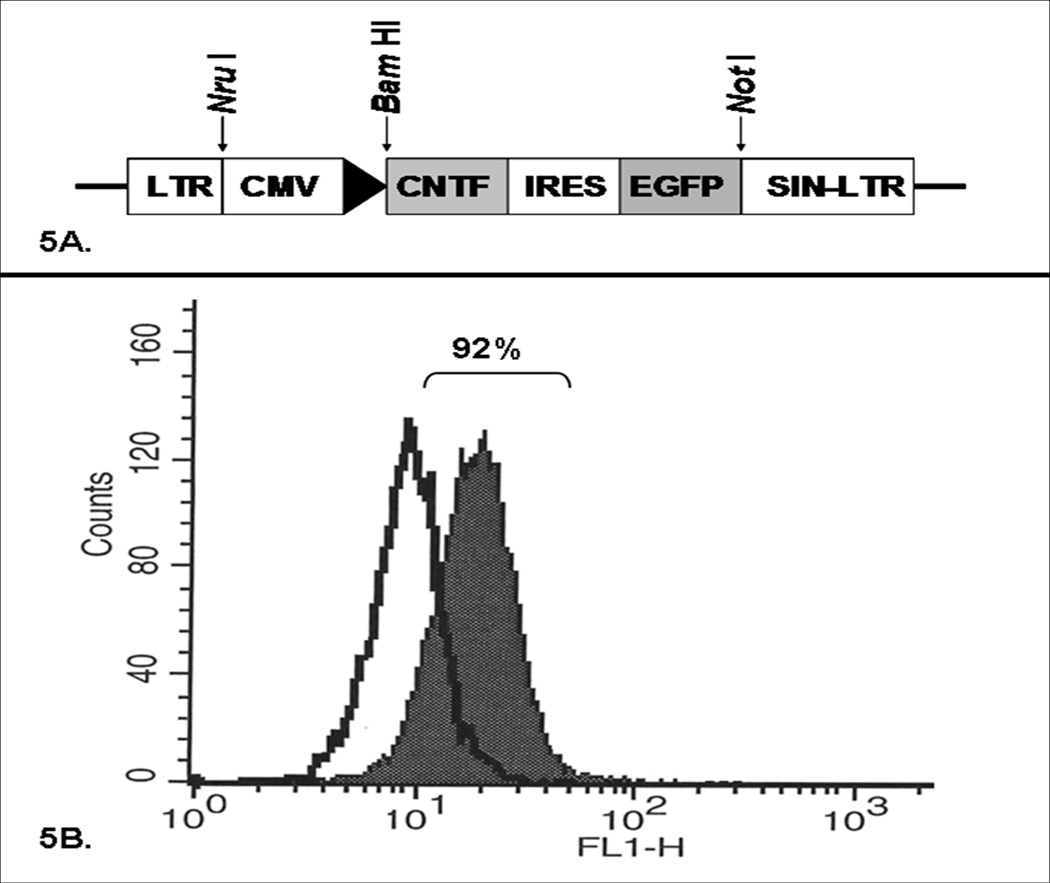
In previous in vivo MSC survival optimization studies, we demonstrated MSCs can be transduced with an EGFP-expressing retroviral vector.18 While the retroviral vector could be used for the experiments in this protocol, lentiviral vector is generally preferable for its high transduction efficiency and low risk of mutagenesis. Dr. Bas Blits generously donated a self-inactivating CMV-promoted CNTF-EGFP construct to our laboratory (Figure 5A).22 Dr. Kenneth Cornetta, the director of the Indiana University Vector Production Facility, kindly provided our laboratory with lentivirus of differing pseudotypes, with the lenti-adapted envelopes 4070A and RD114 are generated by Dr. Cornetta using plasmids kindly provided by Derek Person and Arthur Nienhuis of Memphis, TN.23 Transduction efficiencies with primary MSCs were then established with recombinant lentiviral vector pseudotyped with VSVG, pCAG-LPPP2, RD114, or amphotropic (pCAG 4 AMP) envelopes. The first three pseudotypes (VSVG, pCAG-LPPP2, and RD114) each demonstrated individual trial transduction efficiencies of <75%, while the lentiviral vector pseudotyped with amphotropic envelope transduced primary MSCs with over 90% efficiency in three consecutive trials (Figure 5B). ELISA confirmed that the MSCs transduced with CNTF-expressing amphotropic lentiviral vector reliably secreted CNTF in culture when compared to control MSCs. These findings supported the selection of the amphotropic pseudotyped recombinant lentiviral vector as a CNTF gene delivery vector for the Experiments to Address Specific Aim 3.
Figure 5. CNTF Lentiviral Vector Characterization.
Figure 5A. Lentiviral Vector Encoding CNTF. Schematic representation of the replication-deficient, self-inactivating lentiviral vector encoding for CNTF and EGFP under the control of a CMV promoter. Figure 5B. MSC Transduction Efficiency. Representative FACS analysis of MSCs transduced with EGFP-expressing lentiviral vector pseudotyped with an amphotropic envelope demonstrating a remarkably high (92%) transduction efficiency.
Preliminary Studies for Experiments to Address Specific Aim 3
VNC Effects In Vivo
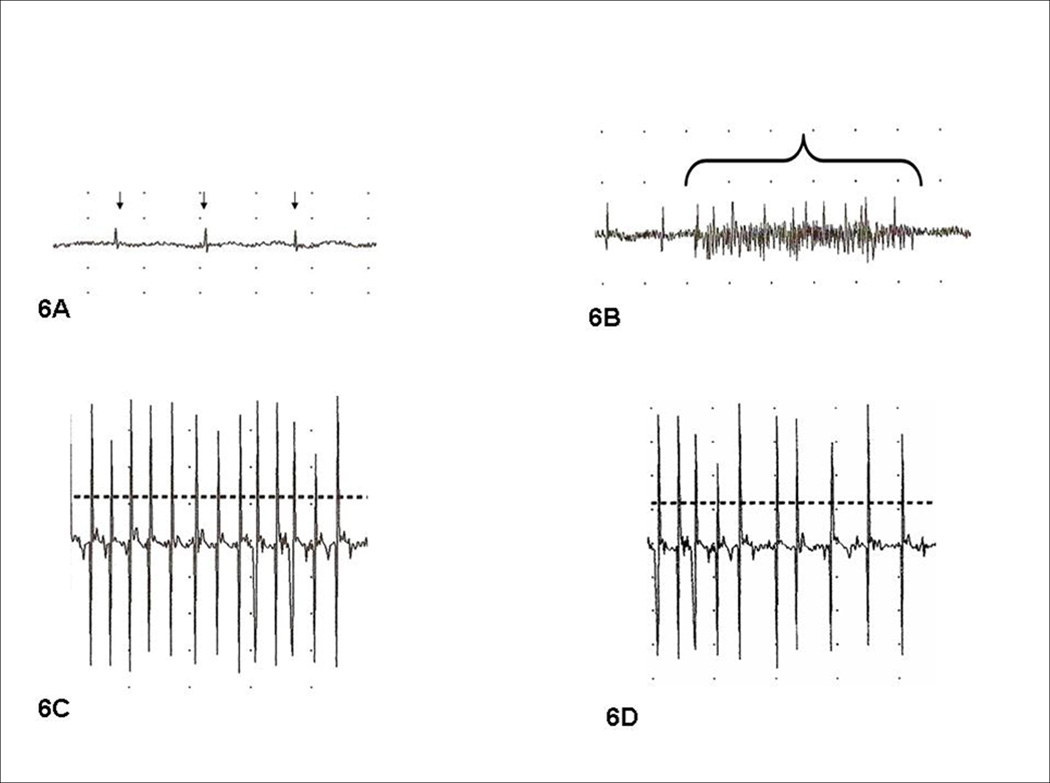
Before initiating the proposed experiments to address specific aim 3, an optimal concentration of VNC was identified that would not negatively affect the spontaneous reinnervation and MSC survival in adjacent laryngeal muscles. In preliminary studies on RLN injury, it was noted that RLN reinnervation to the TA typically preceded PCA reinnervation and waveforms demonstrated larger amplitude and intensity at various time points. Thus, we first investigated VNC for the inhibition of TA reinnervation; the results of this investigation have recently been published.19 Directly pertinent to the Experiments to Address Specific Aim 3, the first goal of the investigation was to determine if low-dose (0.5µg/µl) VNC could selectively inhibit spontaneous PCA reinnervation without impairing reinnervation to the ipsilateral TA muscle complex. In addition, a second goal of the experiments was to determine if VNC injections into the PCA would adversely affect MSC survival when MSCs were injected into the ipsilateral TA. Thus, these preliminary studies were done to ensure that VNC effects on the ipsilateral TA muscle complex would not pose an obstacle to proceeding with the Experiments to Address Specific Aim 3. This investigation involved 20 male Fischer 344 rats. For this pilot investigation, all animals underwent left RLN transection and SCM biopsies under anesthesia with ketamine/xylazine. From the SCM biopsies, MSC cultures were derived and MSCs were transduced with EGFP lentiviral vectors. Three weeks after RLN injury, the denervated TA of each animal was injected MSCs (n=10) for the study group or saline (n=10) for the controls; for each group, half the animals (n=5) simultaneous received injections into the ipsilateral PCA with 4 µl of VNC (0.5µg/µl), while saline was injected into the PCA in the remaining animals (n=5) in each group. One month later, animals were evaluated in a blinded manner with direct laryngoscopy and LEMG under general anesthesia, followed by euthanasia. Laryngeal specimens were harvested, fixed and sectioned. Results demonstrated that VNC was effective in preventing reinnervation to the PCA, with three animals having fibrillations/fasciculations only (no recruitment) (Figure 6A) and one animal with 1+ recruitment. The saline treated animals had PCA motor units that were smaller in amplitude than the TA motor units, but all had evidence of reinnervation (Figure 6B). Reinnervation of the TA was similar among all animals, regardless of whether vincristine (VNC) or saline was injected into the ipsilateral PCA (Figure 6C and Figure 6D), suggesting VNC had not influenced reinnervation of the ipsilateral TA. Regarding MSC survival, viable MSCs were detected throughout all the MSC injected TA. The TA complexes demonstrated no difference in fluorescent intensity (EGFP expression) under fluorescent microscopy (data not shown), indicating that ipsilateral VNC PCA injection did not adversely impact MSC viability.
Figure 6. LEMG Findings after RLN injury & VNC PCA injection.
Figure 6A. Denervated, VNC-treated PCA 8 weeks after RLN injury shows severe denervation with fibrillations and fasciculations (small arrows), suggesting the VNC injection had effectively impaired reinnervation. Figure 6B. Denervated PCA with saline injection demonstrates spontaneous reinnervation based on inspiratory recruitment of small motor units. Figure 6C. Denervated TA with spontaneous reinnervation after the ipsilateral PCA had been treated with VNC. Note the reinnervation of C is similar to the TA reinnervation of 6D (control), suggesting that the VNC treatment did not affect TA reinnervation. Figure 6D. Control denervated TA with spontaneous reinnervation after ipsilateral PCA received saline alone.
Results for Specific Aim 3
Investigating CNTF-expressing MSCs with VNC in an animal model of RLN Injury
Due to a standard attrition rate (5–10%) associated with multiple survival surgeries in the rat model, the final number of animals in each group varied slightly, with groups at the time of final assessment ranging from 4–6 animals per treatment condition. Vocal fold motion evaluation, LEMG testing, and immunohistochemistry analysis were completed with the principal investigator blinded to each animal’s treatment group. As anticipated, the TA demonstrated strong fluorescent intensity at both time points, with minimal change in intensity demonstrated over time (Figure 7) suggesting excellent MSC post-injection survival.
Figure 7. TA myofibers in the CNTF-MSC injected group at 2 months and 4 months.
Fluorescent microscopy at 20X demonstrates that representative sections of CNTF-MSC injection TA at 2 months (7A) and 4 months (7B) have similar levels of green fluorescent intensity, confirming survival of the CNTF-MSCs. The asterisk (*) overlies the laryngeal cartilage (dark), showing the EGFP tracking marker is specific to the myofibers. The bottom microphotograph (7C) demonstrates a representative control (saline) injected TA with no fluorescence identified within the myofibers.
Two Month Outcomes
Videolaryngoscopy Findings
On videolaryngoscopy assessment, none of the animals had vocal fold abduction during respiration. Of the saline controls, none had detectable vocal fold adduction. One animal in the CNTF group had weak adduction during laryngospasm, with complete glottic closure, but no motion during quiet respiration. None of the remaining animals in the CNTF group or other groups (EGFP, EGFP-VNC, CNTF-VNC) had detectable motion.
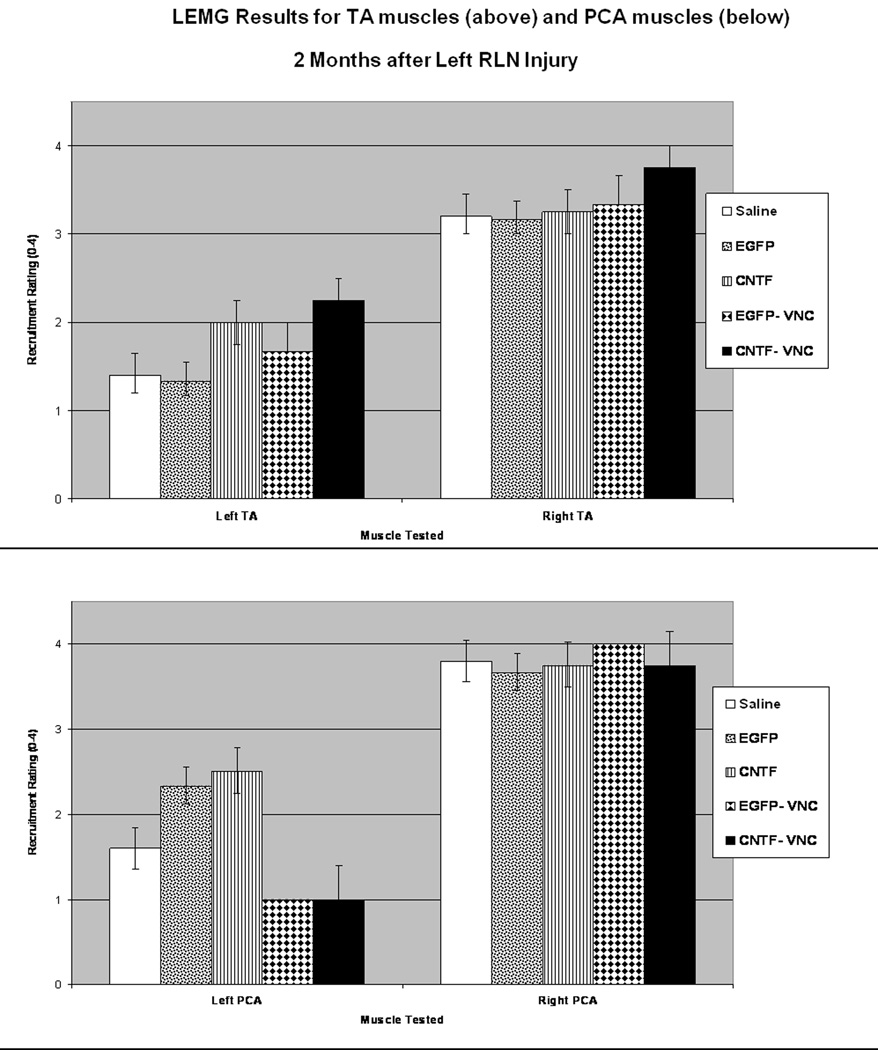
LEMG Results
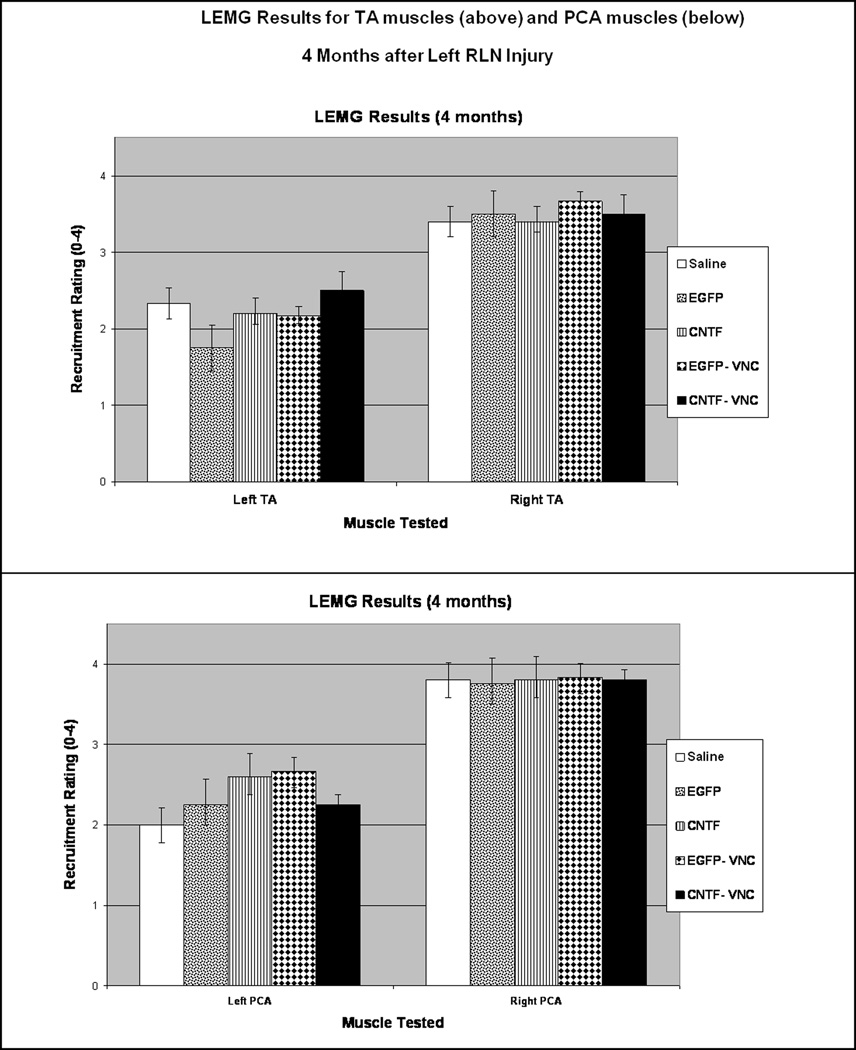
LEMG assessment of recruitment and waveform morphology of the TA was graded based on active firing during laryngospasm, while the PCA activity was tested during quiet respiration. The TA and PCA muscle LEMG recruitment ratings for each group are summarized in Figure 8. In brief, the CNTF group had greater recruitment in the TA muscle complex and the PCA muscle than the saline controls (p= 0.02 and 0.04, respectively). The CNTF-VNC group also had greater recruitment in the TA muscle complex than the saline controls (p=0.04). As was anticipated, there was minimal recruitment (0–1+) in the PCA muscles of the animals that had received VNC injections into the PCA, but when compared to the saline controls the difference did not reach significance (p=0.05 and 0.07). When looking at waveform size based on treatment groups, 40% (2/5) of the saline controls demonstrated giant motor units in the TAs, 75% (6/8) of the CNTF-MSC injected TAs demonstrated giant units, and 50% (5/10) of the EGFP-MSC injected TAs had giant motor units. Regarding the animals that received VNC injections to the PCA muscles, only 11% (1/9) of VNC-injected PCAs demonstrated giant units, while 57% (8/14) of the saline-injected PCAs demonstrated giant units (p=0.04). Upon intra-operative evaluation of the laryngeal anatomy prior to euthanasia, all animals were noted to have a tract of regenerated tissue extending from the transected RLN stump to the larynx, suggestive of a regenerated RLN segment. Immediately prior to euthanasia, while monitoring the left TA with continuous LEMG, transecting this regenerated ipsilateral RLN silenced the TA in 15 animals, while 7 animals required transection of the ipsilateral SLN before the left TA activity silenced. One animal died prior to obtaining useful data on the innervating source.
Figure 8. LEMG Results at 2 Months.
LEMG recruitment ratings were on a scale from 0–4+, with all ratings performed with the investigator blinded to the treatment that the animal had received (see Methods for further details). Average recruitment scores are demonstrated for the TA muscles bilaterally (above) and the PCA muscles bilaterally (below). There was no significant difference in recruitment on the right (normally innervated) side represented on the right side of the bar graphs, however the left TA and PCA groups showed recruitment patterns that varied in relation to the type of injection given to the muscle (* indicates p<0.05).
Motor Endplate to Nerve Contact
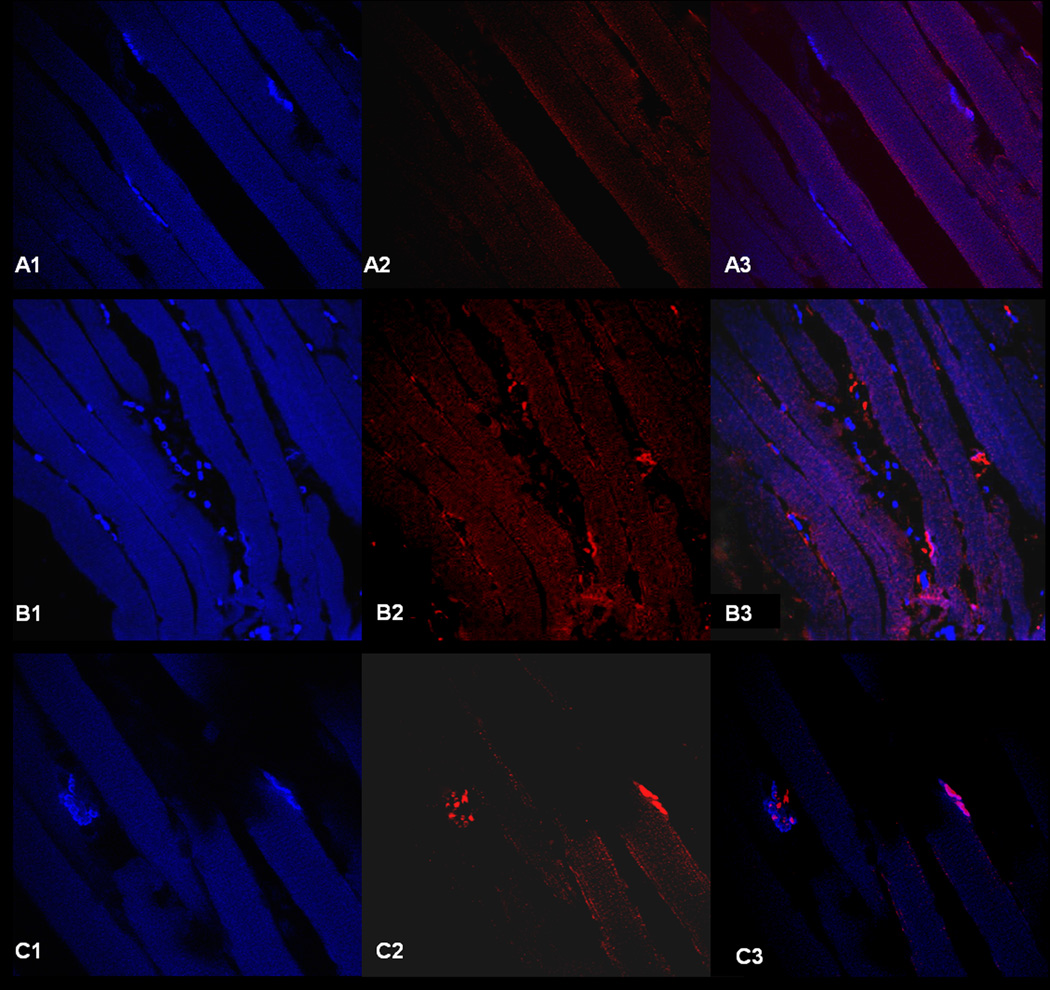
Motor endplate to nerve contact was assessed with immunohistochemistry using bungarotoxin for motor endplate identification and beta III tubulin for nerve staining. Six mid TA axial sections were reviewed for each treatment group, and maximum motor endplate to nerve contact noted within those sections was classified as denervated (less than 33% of motor endplates with nerve contact), partially reinnervated (approximately 33-66% of motor endplates with nerve contact), or strongly reinnervated (greater than 66% of motor endplates with nerve contact) (Figure 9). At two months, all of the stem cell treated groups (EGFP, EGFP-VNC, CNTF, CNTF-VNC) demonstrated partial reinnervation. Four out of five of the control left TA specimens demonstrated partial reinnervation, while one was classified as denervated.
Figure 9. Motor Endplate to Nerve Contact.
Representative high power (60X) confocal microphotographs of immunohistochemistry slides with bungarotoxin (blue) demarcating the motor endplates along the myofibers (left), and the middle figures demonstrating staining with the neuronal specific marker, beta III tubulin (red). On the right, the two images are merged; those sites with colocalization of the bungarotoxin (blue) and beta III tubulin (red) represent motor endplates with nerve contact (reinnervation), with the areas of bungarotoxin alone (blue) representing denervated motor endplates. Figures A1–A3 demonstrates a section series classified as denervated (<33% of motor endplates with nerve contact), while B1-3 demonstrates a partially reinnervated section (33–66% of motor endplates with nerve contact), and C1-C3 demonstrates a strongly reinnervated section (>66% of motor endplates with nerve contact). Note the high variability in the number of motor endplates between sections, with series B1-B3 having the greatest number of motor endplates within the section.
Four Month Outcomes
Videolaryngoscopy Findings
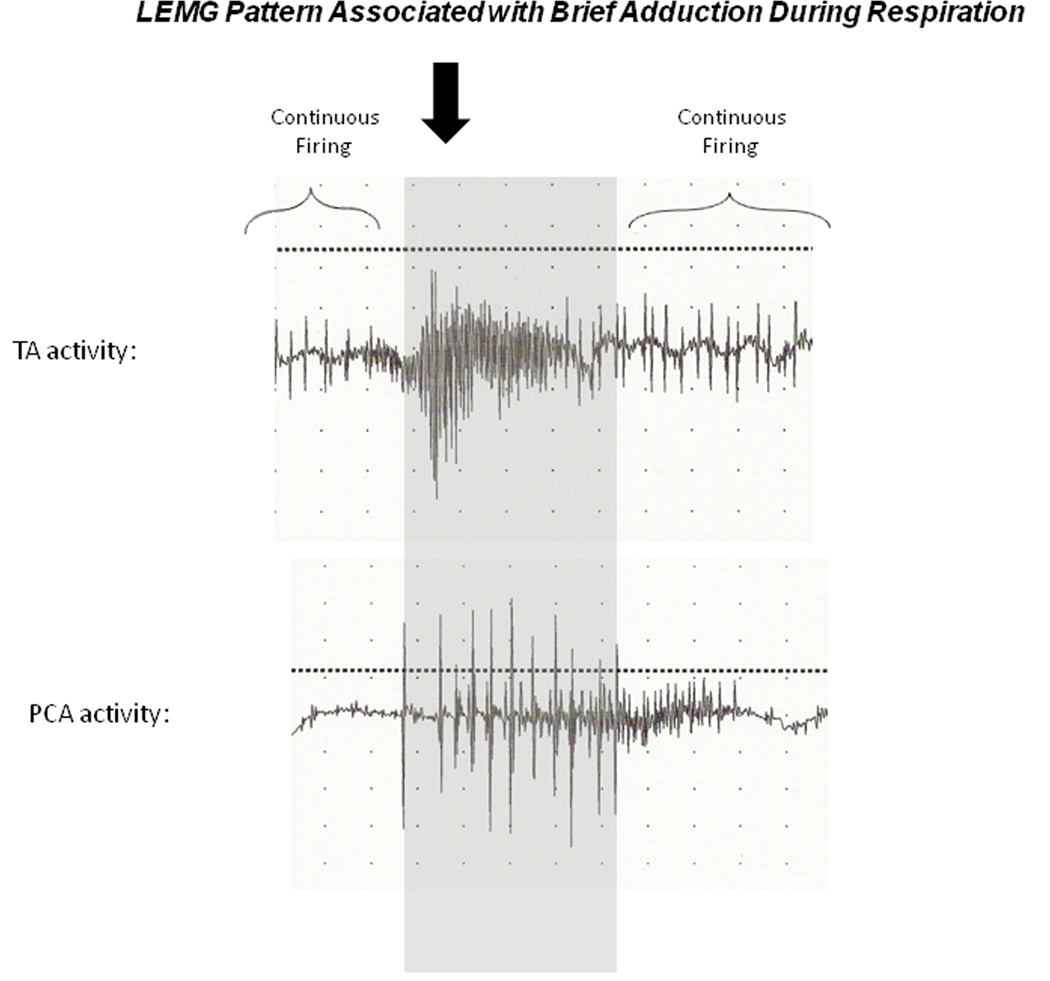
On videolaryngoscopy assessment, none of the animals had vocal fold abduction on the paralyzed side. Six of the twenty animals in stem cell treated groups (1/4 in EGFP group, 1/5 in CNTF group, 2/6 in EGFP-VNC group, and 2/5 in CNTF-VNC group), and one animal (1/5) in the saline control group appeared to have weak adductor activity during laryngospasm. In seven stem cell-treated animals (1/4 EGFP, 2/5 in CNTF, 2/6 in EGFP-VNC group, and 2/5 in CNTF-VNC group), there was a brief adductor “twitch” during respiration which was not present in the control animals (p<0.01). In all of these animals, LEMG findings demonstrated firing in the TA complex that corresponded with the adduction. While the adductor firing varied in timing, there was minimal ipsilateral oppositional PCA firing simultaneously in the animals with adductor motion, suggesting unopposed TA firing was responsible for the motion in these animals. Figure 10 is a representative figure demonstrating the timing of the adductor motion relative to the firing of the denervated TA and PCA muscles on LEMG.
Figure 10. LEMG Patterns Associated with Brief Adduction During Respiration.
Characteristic LEMG firing patterns at 4 months that correlated with videolaryngoscopy findings of an adductor twitch during respiration. Gray shaded area demarcates the inspiratory PCA recruitment with giant motor units (3+), while the solid arrow denotes a burst of increased firing activity in the TA with relatively little antagonistic firing from the PCA, leading to brief adduction during inspiration. The PCA was characteristically silent between inspiratory firing, while the TA demonstrated continuous firing at baseline.
LEMG Results
As with the two month assessments, LEMG rating of recruitment and waveform morphology of the TA was done during active firing on laryngospasm, while the PCA activity was tested during quiet respiration, and the investigator was blinded to the treatment group of the animal being tested. Bilateral TA and PCA muscle LEMG recruitment ratings for each group are summarized in Figure 11. In brief, all the left TA and PCA muscles demonstrated greater recruitment than that at two months (Figure 8). However, there was no significant difference between the recruitment in the saline groups and the stem cell-treated groups for either the TA (Figure 11, above) or the PCA (Figure 11, below). The morphology of the waveforms also varied between the two month and four month animals, with more of the four month TA and PCA muscles demonstrating giant units, often with polyphasic potentials. When looking specifically at treatment groups, 60% (3/5) of the saline controls demonstrated giant motor units in the TA, while 80% (8/10) of the CNTF-MSC injected TAs demonstrated giant TA units and 60% (6/10) of the EGFP-MSC injected TAs had giant TA motor units. Regarding the animals that received VNC injections to the PCA muscles, 50% (5/10) of VNC-injected PCAs demonstrated giant units, compared to 53% (8/15) of the saline-injected PCAs demonstrating giant units. Similar to the two month group, all animals demonstrated gross evidence of RLN regeneration based on a thin white tract extending from the area of the transected RLN to the larynx. Immediately prior to euthanasia, while monitoring the left TA with continuous LEMG, transecting the regenerated ipsilateral RLN silenced the TA in 14 animals, while 9 animals required transection of the ipsilateral SLN before the left TA activity silenced. Two animals died intraoperatively before the innervation source could be determined. Two of the animals with SLN reinnervation sources were saline controls, while seven were stem cell-treated. No relationship was found between the SLN reinnervation and treatment with the CNTF-MSCs.
Figure 11. LEMG Results at 4 Months.
Average recruitment scores are demonstrated for the TA muscles bilaterally (above) and the PCA muscles bilaterally (below).
Motor Endplate to Nerve Contact
As with the two month TA specimens, motor endplate to nerve contact was assessed with immunohistochemistry using bungarotoxin for motor endplate identification and beta III tubulin for nerve staining, with sections classified as denervated (less than 33% of motor endplates with nerve contact), partially reinnervated (approximately 33–66% of motor endplates with nerve contact), or strongly reinnervated (greater than 66% of motor endplates with nerve contact) (Figure 9). At four months, eight of the stem cell treated animals were classified as strongly reinnervated (3/5 in CNTF, 2/4 EGFP, 3/5 in CNTF-VNC group), with the remaining specimens and all of the saline controls (5/5) partially reinnervated. Thus, 6/10 of the CNTF-MSC injected TAs demonstrated strong reinnervation based on immunohistochemistry (p=0.02). Within the CNTF treated animals, high variability was noted in the percentage of motor endplates with nerve contact within individual TA specimens.
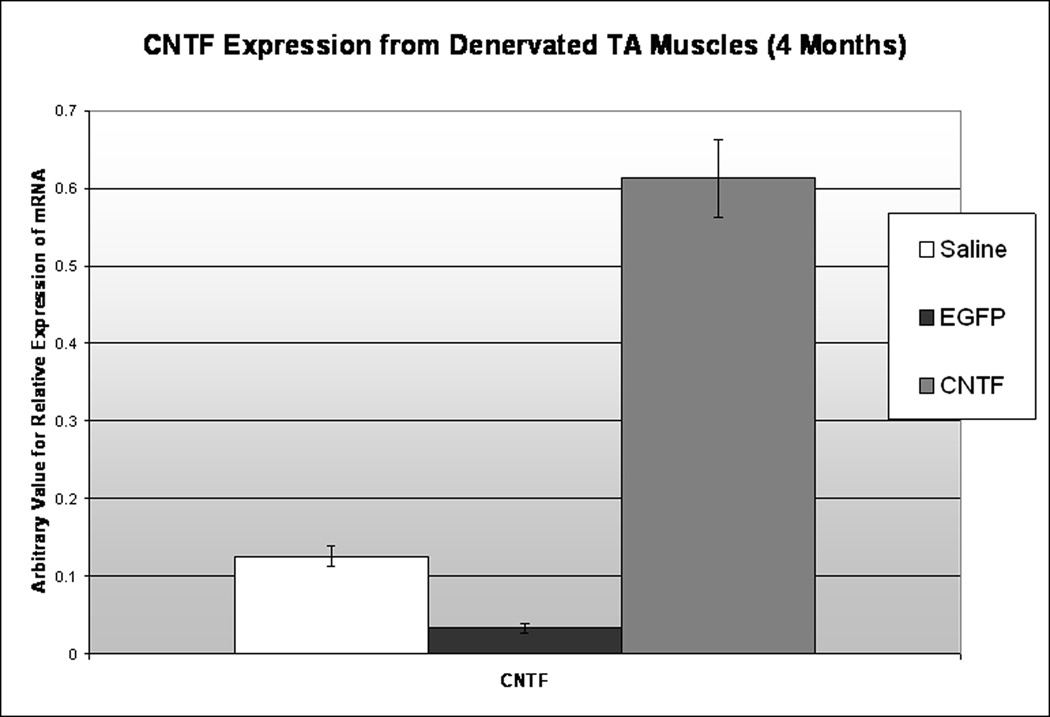
RT-PCR
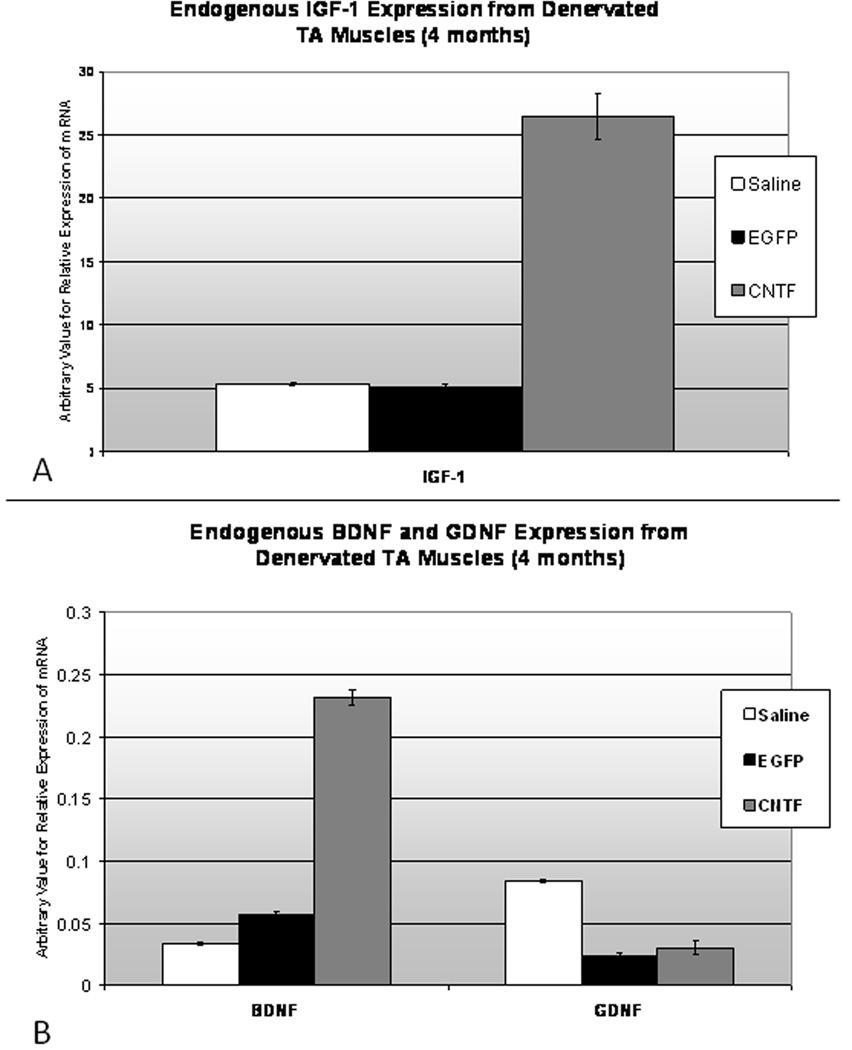
At four months, to determine if CNTF expression from the muscle was still enhanced in the CNTF-MSC injected TAs, a small sample of denervated (left) TA muscle from each TA treatment group (saline, EGFP, CNTF) was attained. Two samples were pooled from each treatment group. The RT-PCR trials were run in triplicate, with GAPDH as a standard internal control. Results confirmed highly upregulated CNTF expression from the CNTF-MSC injected denervated TA at four months after RLN injury (Figure 12). There was no significant difference in CNTF expression between the control MSC (EGFP) injected group and the saline control. Because brief adductor motion was detected in seven of the stem cell-treated animals, including both the EGFP and CNTF-MSC injected groups, additional RT-PCR testing was completed to determine if NF expression was altered in the EGFP-MSC injected TA muscles. No difference was detected in IGF-1, BDNF, or GDNF expression between the EGFP-MSC and saline control groups. However, the CNTF-MSC injected TA specimens demonstrated increased expression of IGF-1 and BDNF (Figure 13A and 13B, respectively) when compared to the saline controls and EGFP-MSC groups.
Figure 12. CNTF Expression from Denervated TA Specimens.
At 4 months, RT-PCR demonstrates persistently upregulated CNTF expression (right) from the denervated TA in the animals who had received CNTF-MSC injections. In comparison, the animals treated with MSCs alone (middle) and those animals injected with saline (left) demonstrated no upregulation of CNTF. The arbitrary values of mRNA expression were determined based on GAPDH as a standard internal control.
Figure 13. Endogenous NF Expression from Denervated TA Specimens.
Figure 13A. Because there was a several fold difference in IGF-1 expression compared to BDNF and GDNF, IGF-1 is represented in the above (14A) figure with its unique y axis. At 4 months, RT-PCR demonstrates increased IGF-1 expression (above) from the denervated TA in the animals who had received CNTF-MSC injections (* indicates p<0.05). In comparison, the animals treated with MSCs alone (middle) and those animals injected with saline (left) demonstrated similar levels of IGF-1. The arbitrary values of mRNA expression for all NFs were determined based on GAPDH as a standard internal control. Figure 13B. At 4 months, RT-PCR also demonstrated increased BDNF expression from the denervated TA in the animals who had received CNTF-MSC injections, while the GDNF expression showed no difference between groups (* indicates p<0.05). For the denervated TA muscles treated with MSCs alone (EGFP) and saline, no significant differences were noted between BDNF and GDNF expression levels.
Discussion
The current investigations started with using in vitro assays for screening neurotrophic factors that my have therapeutic applications for management of RLN injury. The muscle stem cell survival assay is a model in which muscle stem cells are placed in culture in a state of serum deprivation (which slowly induces cell death) to determine which additive factors have the greatest positive effect on survival. These in vitro investigations demonstrated CNTF to have greater survival-promoting effects on the MSCs than any of the other factors tested, which is consistent with our previous in vivo study that demonstrated addition of CNTF at the time of MSC injection in a denervated rat hemilarynx (TA) led to greater MSC survival when compared to other trophic factors.18 No previous studies have investigated therapeutic CNTF delivery for RLN injury, although multiple investigations have studied the effects of other neurotrophic factors such as IGF-1 and NT-3 on RLN regeneration.12–15,27 Because CNTF had not been previously studied for RLN injury, the motoneuron assays described in the Experiments to Address Specific Aim 1B were essential to determine if CNTF at physiologic concentrations would have a positive effect on CNX motoneuron outgrowth. In this model, CNTF was shown to promote motoneuron elongation (longest neurite) and branching. Furthermore, additional studies within our laboratory have recently found CNTF to consistently have greater positive effect on CNX motoneuron outgrowth than IGF-1 or other classic neurotrophins.21 As is commonly found with neurotrophic factors, the CNTF concentration that promoted motoneuron outgrowth was very specific (50 ng/mL), and positive outgrowth effects were absent at lower and higher concentrations (although the differences between tested concentrations was ten fold). From a translational standpoint, these findings suggest that in vivo models may face a major challenge to develop a genetic model for introducing CNTF with a resultant CNTF concentration and intramuscular distribution that is optimal for promoting reinnervation throughout a laryngeal muscle. In our animal experiments, we suspect that the high variability in motor endplates with nerve contact within individual TA specimens in the CNTF-treated groups was directly related to variability in CNTF concentrations throughout the TA muscles.
In designing a vector model to engineer the MSCs to secrete CNTF, many options were contemplated and several were tested within the Experiments to Address Specific Aim 2. While plasmid has strong safety features, our experiments demonstrated poor transfection efficiency and unstable gene expression in MSCs in vitro limiting the utility of plasmid for the in vivo investigations. Regarding viral vectors, replication-defective adenoviral vector is plagued by safety issues, low transduction rates, and immunogenic reaction.28,29 Adeno-associated viral vector has stronger safety profile, but many humans carry neutralizing antibodies that inactivate the vector.30,31 Replication-defective retroviruses were initially attractive tools for gene therapy because of their ability to integrate into genomic DNA, with the risk for insertional mutagenesis thought to be low. Our laboratory previously used retroviral vector to program MSCs to secrete EGFP for cellular tracking purposes, supporting the feasibility of effectively using this vector with primary MSCs.18 However, clinical trials involving ex vivo, retrovirally mediated transfer of the IL-2 receptor gamma chain gene into CD34+ cells demonstrated several patients developed leukemia thought to be due to vector insertional activation of the LMO2 proto-oncogene,32,33 leading to serious concerns about standard retroviral vector safety. Within the retroviral vector class, the lentiviral vector remains a promising candidate for gene delivery, as certain modifications of lentiviral vector can result in enhanced safety profiles. For example, deletion of promoter and enhancer elements from the U3 region of the long terminal repeat (LTR) produces a self-inactivating lentivector that is less likely to result in insertional mutagenesis. Non-integrating forms of lentiviral vector can also be constructed via selected mutations of the lentiviral integrase protein, thereby preventing integration and risk of insertional mutagenesis, while still permitting effective transgene expression. Lentiviral vectors can also be pseudotyped with envelope proteins or hybrid combinations of envelope proteins from murine and non-murine viruses, facilitating tissue-specific targeting.34 While vesicular stomatitis virus glycoprotein (VSVG) is most widely used, it is not optimal for many applications. For example, in our preliminary investigations with primary MSCs we found transduction efficiency with VSVG pseudotyped lentivirus to be approximately 50% while transduction efficiency was consistently over 90% with amphotropic (pCAG 4 AMP) pseudotyped lentivirus. This high transduction efficiency with the MSCs was critical, as it allowed for a therapeutic number of NF-secreting MSCs to rapidly be obtained in culture (less than 3 weeks), which will ultimately be extremely important for clinical translation of such a model.
In reviewing the results of the animal study, the CNTF-MSC treatment appeared to significantly promote reinnervation based on LEMG and immunohistochemistry findings. First, at two months, the CNTF group and CNTF-VNC groups both had had greater recruitment in the TA muscle complex than the saline controls (p= 0.02 and 0.04, respectively). Second, by four months 3/5 animals in the CNTF group and 3/5 animals in the CNTF-VNC groups had TA sections that were classified as strongly reinnervated (greater than 66% of motor endplates with nerve contact), which was significantly greater than that found in the saline and EGFP-MSC control sections. It likely that the CNTF treated TAs no longer showed appreciable differences on LEMG at four months because with all denervated TAs having partial to strong reinnervation the four-point LEMG rating scale was not sensitive enough to detect subtle differences.26 Based on our previous experience with LEMG in this animal model, waveform size has often been noted to reflect the timing of reinnervation, with nascent or small motor units detected early in the course of reinnervation, and large (giant) motor units demonstrated as reinnervation matures. In the current study, giant units were more often detected in the denervated TA than the denervated PCA at both time points, potentially suggesting the TA reinnervation preceded that of the PCA. Most notably, the CNTF-MSC group demonstrated more giant units in the TA muscles at both time points when compared to the TA muscles of the other treatment groups (EGFP or saline), suggesting preferential early reinnervation in the CNTF-MSC treated TA muscles. In contrast, the VNC-treated PCA muscles demonstrated much smaller units, with only one animal (11%) with giant units at 2 months and minimal recruitment on LEMG at two months (Figure 8). However, by four months 45% of TA muscles demonstrated giant units and differences in recruitment were no longer appreciable with LEMG (Figure 12). Taken together, these findings suggest that VNC treatment at the administered dose had delayed but not completely inhibited reinnervation of the left PCA.
We initially hypothesized that vocal fold adductor activity may be restored if we were to selectively enhance reinnervation to selected laryngeal muscles with NF-secreting MSCs after RLN injury while simultaneously inhibiting antagonistic reinnervation (synkinesis). While normal motion was not restored in any of the animals, one unexpected finding on videolaryngoscopy was the presence of a brief adductor “twitch” in some animals during respiration. We were initially concerned that this could represent Bernoulli’s principle; however, the animals with adductor “twitches” did have LEMGs demonstrating corresponding simultaneous bursts of activity in the adductor complex, thereby supporting active muscular contraction rather than passive motion. In all of the animals, to help delineate the source of EMG activity, we transected the sources of incoming neural supply to the larynx while continuously monitoring the left TA muscles. This approach revealed that many of the animals had reinnervation through the pathway of the ipsilateral RLN; however, nearly half of the animals had at least some persistent continuous LEMG firing of the left TA complex after ipsilateral RLN transection which did not silence until cutting the pathway of the ipsilateral superior laryngeal nerve (SLN). Since the LEMG was not quantitative, the methodology did not exclude the possibility of both the RLN and SLN contributing to TA reinnervation in these cases. Just as the investigation of Hydman and colleagues suggested that the SLN contributes to reinnervation of the PCA by intramuscular sprouting after RLN injury,9 our findings suggest that an analogous phenomenon may occur within the TA complex after RLN injury. Certainly, reinnervation by more than one source is highly feasible based on the multiple communicating branches that exist between the SLN and RLN;35 in addition, variable ratios of reinnervation from the SLN and RLN could potentially explain the strong reinnervation and inherent variability of firing patterns that characterizes LEMG findings after RLN injury.
Considering this animal model is preliminary in nature and unrefined from a standpoint of optimizing CNTF concentration and distribution, finding significant reinnervation enhancement is promising, and warrants further investigations to optimize effectiveness. For example, while CNTF expression from the CNTF-MSC injected TA muscles at four months (over 3 months after the CNTF-MSC injection) was substantially elevated (Figure 12), future studies will be important to determine how this increase in CNTF expression correlates with the CNTF protein concentration throughout the TA muscle complex, as the response to CNTF was highly concentration specific (Figure 4). The findings from the RT-PCR studies also revealed that injection of CNTF-MSCs leads to increased expression of other neurotrophic factors including IGF-1 and BDNF up to four months after treatment. While the mechanism of this is unclear, the extracellular signal-regulated protein kinase (ERK) pathway (a member of mitogen-activated protein kinase (MAPK) family) may be directly or indirectly activated by the CNTF-expressing MSCs, as ERK activation results in increased expression of factors such as BDNF that are essential to Schwann cell and neuronal regeneration.36,37 A future goal will be to identify the optimal concentration of CNTF for enhancement of reinnervation in vivo, which can then be targeted by titrating the number of CNTF-expressing MSCs injected. For example, while we selected 106 cells to inject into the TA based on previous studies in our laboratory using non-modified MSCs,17,18 the number of cells could be readily increased or decreased to optimize the CNTF concentration in the tissues and the cells could be more evenly distributed with multi-injector techniques to improve the effectiveness and consistency of this model.
While this investigation targeted reinnervation of the adductor muscle group after unilateral RLN injury as proof of concept, the model ultimately may be most clinically useful for enhancing PCA reinnervation in cases of bilateral true vocal fold paralysis. In addition, as reinnervation procedures such as Marie’s phrenic nerve grafting techniques have now evolved to the point of achieving dynamic abduction in many cases,11 the microenvironmental addition of trophic factors via stem cell vectors may be a means of consistently enhancing and directing neuronal regeneration at the time of reinnervation surgery. An analogous approach could be applicable to directing reinnervation after laryngeal transplantation.38 Thus, incorporation of stem cell vectors for microenvironmental guidance of reinnervation has a wide range of potential future neurolaryngologic roles.
Stem cell therapies and cellular vectors for gene delivery will inevitably be part of our future therapeutic armamentarium, but we need to translate from bench to bedside which involves extensive experimental trouble-shooting, and ultimately overcoming a tremendous number of regulatory hurdles. While the current studies involved laryngeal delivery of therapeutic substance(s) via autologous MSCs for the treatment of vocal fold paralysis, the model of delivering therapeutic proteins via cellular vectors may ultimately be applicable to other areas of laryngology such as controlling respiratory papillomatosis recurrence, or providing adjuvant therapy for laryngeal squamous cell carcinoma. The model is readily clinically translatable based on the ease of procurement of large quantities of autologous MSCs, and the technical ease of delivery via laryngeal injection. In fact, if this model is applied to humans, surgeries would be limited to a small skeletal muscle biopsy which can be derived under local anesthetic in the office, and a laryngeal injection. The autologous nature of the MSCs also obviates risk of adverse reaction or rejection, and MSCs are ideal for gene delivery because they rapidly proliferate in culture and have innate features that protect against tumorigenesis when injected in vivo. Thus, this model is highly feasible and holds great therapeutic potential for a wide range of applications.
Conclusion
The current investigations demonstrated that CNTF is a strong promoter of MSC survival and CN X outgrowth in vitro, and CNTF-secreting muscle stem cells can be used therapeutically to enhance reinnervation to select muscles after RLN injury in vivo. While this model focused on enhancing TA reinnervation as proof of concept, it may ultimately lead to a minimally invasive approach for enhancing reinnervation of selected laryngeal muscles, potentially providing an adjuvant therapy for reinnervation procedures. The basic model of safe and effective therapeutic gene delivery via MSCs (cellular vectors) may also be applicable to a wide variety of other otolaryngologic disorders.
Supplementary Material
Acknowledgements
These projects were supported by Indiana University School of Medicine Otolaryngology-Head and Neck Surgery Department funds, and was by Award Number K08DC009583 from the National Institute On Deafness And Other Communication Disorders (NIDCD) within the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or NIH.
Many thanks to my primary mentor, Dr. D. Wade Clapp, for his extensive counseling and advice on these investigations designed for an NIH Mentored Clinician-Scientist Award. In addition, thanks to co-mentors on the award, Dr. Gayle Woodson and Dr. Kenneth Cornetta who provided important guidance pertaining to the animal experiments and genetic transfer methodology, respectively. Also, thanks to Dr. Anthony Atala for his advice and support, and Dr. John Kincaid who generously provided the EMG equipment for the experiments and assisted with LEMG interpretation. Sincere thanks and appreciation to Dr. P. Ashley Wackym and Dr. Richard Miyamoto for their ongoing support and encouragement, and to former laryngology mentors Dr. Greg Postma, Dr. Jamie Koufman, Dr. Al Merati, and Dr. Robert Toohill. Finally, very special thanks to my husband, Dr. Ramon Halum, for his incredible understanding and support.
Footnotes
This manuscript was Stacey Halum’s Triological Thesis, presented at the national Triological Society meeting at COSM in Chicago, IL (April, 2011).
- These projects were supported by Indiana University School of Medicine Otolaryngology-Head and Neck Surgery Department funds, and was by Award Number K08DC009583 from the National Institute On Deafness And Other Communication Disorders (NIDCD) within the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or NIH
- The authors have no financial interests in any companies or other entities that have an interest in the information in this manuscript, and no other financial disclosures
Conflict of Interest- None
Literature Cited
- 1.Koufman JA, Postma GN, Cummins MM, et al. Vocal fold paresis. Otolaryngol Head Neck Surg. 2000;122(4):537–541. doi: 10.1067/mhn.2000.102574. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Kotz T, Shapiro J. Dysphagia and aspiration with unilateral vocal fold immobility: incidence, characterization, and response to surgical treatment. Ann Otol Rhinol Laryngol. 2002;111(8):672–679. doi: 10.1177/000348940211100803. [DOI] [PubMed] [Google Scholar]
- 3.Crumley RL. Laryngeal synkinesis revisited. Ann Otol Rhinol Laryngol. 2000;109(4):365–371. doi: 10.1177/000348940010900405. [DOI] [PubMed] [Google Scholar]
- 4.Woodson GE. Spontaneous laryngeal reinnervation after recurrent laryngeal nerve or vagus nerve injury. Ann Otol Rhinol Laryngol. 2007;116(1):57–65. doi: 10.1177/000348940711600110. [DOI] [PubMed] [Google Scholar]
- 5.Vega-Cordova X, Cosenza NM, Helfert RH, Woodson GE. Neurotrophin expression of laryngeal muscles in response to recurrent laryngeal nerve transection. Laryngoscope. 2010;120(8):1591–1596. doi: 10.1002/lary.21026. [DOI] [PubMed] [Google Scholar]
- 6.Nomoto M, Yoshihara T, Kanda T, Kaneko T. Synapse formation by autonomic nerves in the previously denervated neuromuscular junctions of the feline intrinsic laryngeal muscles. Brain Research. 1991;539:276–280. doi: 10.1016/0006-8993(91)91632-b. [DOI] [PubMed] [Google Scholar]
- 7.Nomoto M, Yoshihara T, Kanda T. Persistent Adrenergic Reinnervation of Previously Denervated Muscle in Cat. Electron Microsc. 1993;42:236–239. [PubMed] [Google Scholar]
- 8.Hydman J, Mattsson P. Collateral reinnervation by the superior laryngeal nerve after recurrent laryngeal nerve injury. Muscle Nerve. 2008;38(4):1280–1289. doi: 10.1002/mus.21124. [DOI] [PubMed] [Google Scholar]
- 9.van Lith-Bijl JT, Mahieu HF, Stolk RJ, Tonnaer JA, Groenhout C, Konings PN. Laryngeal abductor function after recurrent laryngeal nerve injury in cats. Arch Otolaryngol Head Neck Surg. 1996;122(4):393–396. doi: 10.1001/archotol.1996.01890160035007. [DOI] [PubMed] [Google Scholar]
- 10.Crumley RL. Selective reinnervation of vocal cord adductors in unilateral vocal cord paralysis. Ann Otol Rhinol Laryngol. 1984;93(4 Pt 1):351–356. doi: 10.1177/000348948409300414. [DOI] [PubMed] [Google Scholar]
- 11.Marie JP. Human bilateral laryngeal reinnervation: implications for transplantation. State of the Art Lecture for American Laryngological Association Combined Otolaryngologic Sections Meeting (COSM); May 28–29, 2009; Phoenix, AZ. [Google Scholar]
- 12.Flint PW, Shiotani A, O'Malley BW., Jr IGF-1 gene transfer into denervated rat laryngeal muscle. Arch Otolaryngol Head Neck Surg. 1999;125(3):274–279. doi: 10.1001/archotol.125.3.274. [DOI] [PubMed] [Google Scholar]
- 13.Shiotani A, O'Malley BW, Jr, Coleman ME, et al. Reinnervation of motor endplates and increased muscle fiber size after human insulin-like growth factor I gene transfer into the paralyzed larynx. Hum Gene Ther. 1998;9(14):2039–2047. doi: 10.1089/hum.1998.9.14-2039. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa H, Shiotani A, O’Malley BW, et al. Timing of Human Insulin-Like Growth Factor-1 Gene Transfer in Reinnervating Laryngeal Muscle. Laryngoscope. 2004;114:726–732. doi: 10.1097/00005537-200404000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Shiotani A, O'Malley BW, Jr, Coleman ME, et al. Reinnervation of motor endplates and increased muscle fiber size after human insulin-like growth factor I gene transfer into the paralyzed larynx. Hum Gene Ther. 1998;9(14):2039–2047. doi: 10.1089/hum.1998.9.14-2039. [DOI] [PubMed] [Google Scholar]
- 16.Paydarfar JA, Paniello RC. Functional study of four neurotoxins as inhibitors of post-traumatic nerve regeneration. Laryngoscope. 2001;111:844–850. doi: 10.1097/00005537-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Halum SL, Naidu M, Delo DM, et al. Injection of autologous muscle stem cells (myoblasts) for the treatment of vocal fold paralysis: a pilot study. Laryngoscope. 2007;117(5):917–922. doi: 10.1097/MLG.0b013e31803e8c8d. [DOI] [PubMed] [Google Scholar]
- 18.Halum SL, Hiatt KK, Naidu M, et al. Optimization of autologous muscle stem cell survival in the denervated hemilarynx. Laryngoscope. 2008;118(7):1308–1312. doi: 10.1097/MLG.0b013e31816c438e. [DOI] [PubMed] [Google Scholar]
- 19.McRae BR, Kincaid JC, Illing EA, et al. Local neurotoxins for prevention of laryngeal synkinesis after recurrent laryngeal nerve injury. Ann Otol Rhinol Laryngol. 2009;118(12):887–893. doi: 10.1177/000348940911801210. [DOI] [PubMed] [Google Scholar]
- 20.Kivell BM, McDonald FJ, Miller JH. Method for serum-free culture of late fetal and early postnatal rat brainstem neurons. Brain Res Brain Res Protoc. 2001;6(3):91–99. doi: 10.1016/s1385-299x(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 21.McRae BR, Aaron GP, Patel RG, Halum SL. Dissociated cultures and trophic factor effects on cranial nerve X motoneurons in vitro. Triological Combined Sections Meeting (podium presentation); February, 2010; Orlando, FL. [Google Scholar]
- 22.Blits B, Kitay BM, Farahvar A, et al. Lentiviral vector-mediated transduction of neural progenitor cells before implantation into injured spinal cord and brain to detect their migration, deliver neurotrophic factors and repair tissue. Restor Neurol Neurosci. 2005;23:313–324. [PubMed] [Google Scholar]
- 23.Hanawa H, Kelly PF, Nathwani AC, et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hemaopoietic cells from human blood. Molecular Therapy. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 24.Sastry L, Xu Y, Johnson T, Desai K, et al. Certification assays for HIV-1-based vectors: frequent passage of gag sequences without evidence of replication-competent viruses. Mol Ther. 2003;8(5):830–839. doi: 10.1016/j.ymthe.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Leaver SG, Plant GW, et al. Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Molecular Therapy. 2005;11:906–915. doi: 10.1016/j.ymthe.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Koufman JA, Walker FO. Laryngeal electromyography in clinical practice: indications, techniques, and interpretation. Phonoscope. 1998;1:57–70. [Google Scholar]
- 27.Kingham PJ, Hughes A, Mitchard L, Burt R, Murison P, Jones A, Terenghi G, Birchall MA. Effect of neurotrophin-3 on reinnervation of the larynx using the phrenic nerve transfer technique. Eur J Neurosci. 2007;25(2):331–340. doi: 10.1111/j.1460-9568.2007.05310.x. [DOI] [PubMed] [Google Scholar]
- 28.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in an ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Raper SE, Yudkoff M, Chirmule N, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- 30.Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses — normal integration does not require viral gene-expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chirmule N, Propert KJ, Magosin SA, et al. Immune responses to adenovirus and adenoassociated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 32.Hacein-Bey-Abina S, Le Deist F, Carlier, et al. Sustained correction of X-linked severe combined immunodeficiency byex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 33.Hacein-Bey-Abina S, von Kalle C, Schmidt, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 34.Goncalves MA, Holkers M, Cudre-Mauroux C, et al. Transduction of myogenic cells by retargeted dual high-capacity hybrid viral vectors: robust dystrophin synthesis in Duchenne muscular dystrophy muscle cells. Mol Ther. 2006;13:976–986. doi: 10.1016/j.ymthe.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Sanudo JR, Maranillo E, Leon X, et al. An anatomical study of anastomoses between the laryngeal nerves. Laryngoscope. 1999;109:983–987. doi: 10.1097/00005537-199906000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166:392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 37.Heumann R, Lindholm D, Bandtlow C, et al. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A. 1987;84(23):8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenz RR, Hicks DM, Shields RW, Jr, et al. Laryngeal nerve function after total laryngeal transplantation. Otolaryngol Head Neck Surg. 2004;131(6):1016–1018. doi: 10.1016/j.otohns.2004.02.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.