Figure 7.
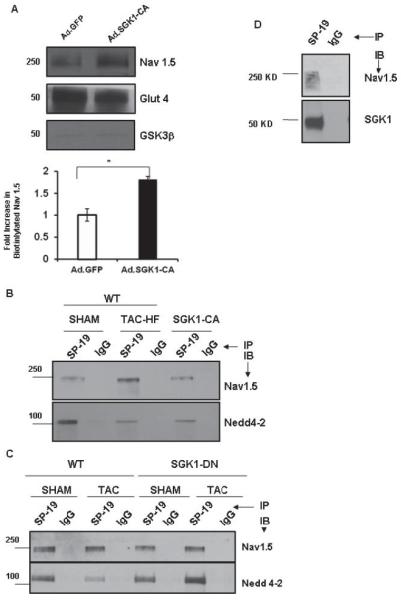
SGK1 activation is necessary and sufficient for alterations in cardiac sodium channel seen in failing hearts. (A) Cardiomyocytes were infected with either Ad.SGK1-CA or Ad.GFP, and biotin labeled to tag surface (e.g. Nav1.5, Glut4) but not cytoplasmic proteins (e.g. GSK3β). Biotin-labeled proteins were captured using an avidin column and subjected to immunoblotting. SGK1-CA expression increased surface expression of Nav1.5, *p<0.05, n=3 independent experiments. (B) Total heart lysates from WT (sham-operated), TAC-HF, or unoperated SGK1-CA mice were immunoprecipitated with a pan-Na+-channel antibody (SP-19). Immunoblotting with antibodies to Nav1.5 or Nedd4-2 showed a decrease in Nedd4-2 binding in TAC-HF and SGK1-CA hearts. (C) Cardiac lysates from SGK1-DN transgenic or WT mice subjected to TAC or sham-operation were immunoprecipitated with SP-19 and immunoblotted with the antibody specific Nav1.5 or Nedd4-2 (bottom panel). The decrease in Nedd4-2 binding to Nav1.5 seen in failing WT hearts is completely prevented in SGK1-DN hearts after TAC. (D) SGK1 directly associates with Nav1.5: Sodium channel was immunoprecipitated from heart lysates using SP-19 or control IgG antibody-coupled columns and subjected to immunoblotting as labeled. Figures are representative of 3 independent experiments.
