Abstract
Cardiomyopathy is a significant component in Duchenne muscular dystrophy. Although mdx mice are deficient in dystrophin, they only develop mild indicators of cardiomyopathy before 1 year-of-age, making therapeutic investigations using this model lengthy. In contrast, mdx mice also lacking utrophin (utrn−/−;mdx) show severely reduced cardiac contractile function and histological indicators of cardiomyopathy by 8–10 weeks-of-age. Here we demonstrate that utrn−/−;mdx mice show a similar pattern of cardiac damage to that in dystrophic patients. Matrix metalloproteinases required for ventricular remodeling during the evolution of heart failure are upregulated in utrn−/−;mdx mice concurrent with the onset of cardiac pathology by 10 weeks-of-age. Matrix metalloproteinase activity is further dysregulated due to reduced levels of endogenous tissue inhibitors and co-localizes with fibroblasts and collagen I-containing scars. utrn−/−;mdx mice are therefore a very useful model for investigating potential cardiac therapies.
Keywords: cardiomyopathy, heart failure, Duchenne muscular dystrophy, mdx, utrophin, matrix metalloproteinases, MMPs, tissue inhibitors of matrix metalloproteinases, TIMP, remodeling, MMP-9, TIMP-2
Introduction
Ninety-five percent of Duchenne muscular dystrophy (DMD) patients develop cardiomyopathy due to a lack of dystrophin protein in the heart, and greater than 25% die from heart failure [1–3]. As treatments to ameliorate the skeletal muscle pathology in DMD improve, it is expected that heart failure will account for an even higher proportion of DMD deaths [4, 5]. Although heart failure is an end-stage disease that can result from a wide variety of primary causes [6], a number of common features usually characterize heart failure progression, including remodeling of heart tissue, ventricular hypertrophy, cardiomegaly, and upregulation of matrix metalloproteinases (MMPs) [7–9]. Although the skeletal muscle pathogenesis associated with DMD has been extensively characterized, the cardiomyopathy and progression to heart failure has not been as well studied. Defining whether the pathogenesis of heart failure associated with muscular dystrophies shares features with heart failure from other causes will have direct implications for current treatment and in the future as new treatment options are developed.
A dystrophin-deficient mouse model of DMD, the mdx mouse, shows moderate cardiomyopathy and functional cardiac impairment, as measured by contractile response in isolated muscles, by 8–10 weeks of age [10, 11]. However, these mice do not demonstrate physiological indicators of heart failure early in life. Utrophin, a homolog of dystrophin, partially compensates for the lack of dystrophin in mdx mice and prevents extensive degeneration of cardiac and skeletal muscle. Mice deficient for both dystrophin and utrophin (utrn−/−;mdx or “dko”) have severe cardiomyopathy [11–13] and display the physiological indicators of end-stage heart failure by 8–10 weeks, including a reduction in force development and impairment of relaxation, a negative force-frequency relationship, and a severely blunted β-adrenergic response [10]. A thorough characterization of the cardiomyopathy in these mice will increase the usefulness of this animal model for research into treatments and diagnostics for muscular dystrophy-associated heart failure.
The study of matrix metalloproteinase (MMP) dysregulation in cardiovascular disease is an ever-growing field of research. Since the early 1990s, a large number of studies have shown that one or more members of this enzyme family are dysregulated (usually upregulated) in cardiovascular events leading to heart failure [14–18]. This dysregulation is observed in both patients and animal models. MMPs, especially the gelatinases MMP-2 and MMP-9, proteolyze the extracellular matrix (ECM) in the heart. The ECM-degrading activity of MMPs permits the remodeling process observed in heart failure evolution to occur [7]. Subsequent deposition of connective tissue leads to deleterious fibrosis, resulting in stiffening of the heart and reduced contractile capacity [19]. Studies have shown that MMP-2 or MMP-9 deficiency results in reduced cardiac damage [14, 19–22], except in the case of virally-induced cardiomyopathy [23].
MMPs are regulated on multiple levels: changes in gene expression of the proenzyme; activation of the enzyme by cleavage of the inhibitory amino-terminal peptide sequence; and regulation of expression of endogenous tissue inhibitors of MMPs (TIMPs), proteins that strongly inactivate MMPs [22, 24]. It is evident that MMP regulation is controlled through multiple mechanisms, which is in accordance with their critical roles in normal physiology and disease-related pathology. Although clinical treatments targeting MMPs have been elusive thus far, research to identify MMP-inhibitory drugs is ongoing, and MMPs may present practical targets in treating heart failure in the future [25].
MMPs have been shown to play an important role in skeletal muscle inflammation and fibrosis present in muscular dystrophy [26–33]. Genetic or pharmacological inhibition of MMP-2 or MMP-9 in mdx mice alters skeletal muscle regenerative capacity [27, 31]. Serum MMP-9 has also recently been demonstrated to be a biomarker of DMD disease progression in patients [34]. Recently, studies in old mdx mice have shown that increased levels of cardiac MMP-2 and MMP-9 correlates with increased cardiac pathology [35, 36]. Because of the clear importance of MMPs in general cardiovascular disease, in muscular dystrophy heart and skeletal muscle pathology, and their promise as drug targets for end-stage heart failure, analyzing MMP levels and activities in the more clinically relevant dko mouse model is necessary to better characterize its cardiac pathology, and indicate whether it could be useful for testing therapeutic strategies for DMD cardiac involvement. We hypothesized that increased MMP expression in the hearts of dko mice would correspond to their more severe cardiac pathology compared to mdx mice. We therefore analyzed the levels and localization of the gelatinases MMP-2 and MMP-9, and TIMP-1 and TIMP-2 levels in dko hearts compared to mdx and normal hearts.
Materials and Methods
Animals
The protocol was approved by the Institutional Animal Care and Use Committee at The Ohio State University. utrn+/−;mdx mice were bred to produce utrn+/+;mdx (mdx) mice and utrn−/−;mdx (dko) littermates [37]. Wild-type C57BL/10 (C57) mice were maintained as a separate inbred colony. Both sexes of mice were used in equivalent numbers between groups. Breeding cages are maintained on a high-fat breeding diet and mice are fed a standard diet after weaning.
Tissue preparation
Hearts from male and female mice at 10 weeks were removed and segmented in 3 pieces from base to apex. The middle segment containing only ventricular tissue was further split into two pieces and each piece was homogenized in a different buffer. For immunoblotting, protein homogenates in Newcastle buffer (4 M urea, 75 mM Tris, pH 6.8, 3.8% SDS) were used. For zymography, homogenates in a non-denaturing buffer with protease inhibitors (100 mM Tris, pH 6.8, 200 mM NaCl, 100 mM CaCl2, 1% Triton X-100, 500 µM PMSF, 500 µM benzamidine, 250 µg/ml leupeptin, 0.1 U/ml aprotinin) were used. Total protein concentrations were quantified using the Dc Protein Assay (Bio-Rad, Hercules, CA).
The remainder of the heart was frozen in blocks in O.C.T. (Tissue-Tek, Torrance, CA) on liquid nitrogen-cooled isopentane. Cryosections (8 µm) were cut serially from blocks for in situ zymography, immunofluorescence, and histology.
Human cardiac magnetic resonance imaging (MRI)
The patient cardiac MRI was obtained with informed consent and approved by the Biomedical Sciences Institutional Review Board of The Ohio State University. In vivo human cardiac magnetic resonance imaging (MRI) was performed using the late gadolinium enhancement (LGE) technique [38] in a 15 year-old male with Duchenne muscular dystrophy as part of his clinical care, with intravenous administration of 0.2 mmol/kg gadolinium-DTPA, and T1-weighted inversion recovery imaging 10 minutes post-contrast injection.
Zymography
Zymography procedures were performed as previously described with minor modifications [23, 27, 39]. Samples (100 µg total protein) were loaded into zymography gels (10% SDS-polyacrylamide gels containing either 1 mg/ml gelatin [porcine type A, 175 bloom, Sigma, St. Louis, MO] or 1 mg/ml casein [Acros, Pittsburgh, PA]). Proteins were separated by electrophoresis in running buffer (2.5 mM Tris base, 19.2 mM glycine, 0.1% SDS), then re-natured in developing buffer (50 mM Tris, pH 8.8, 5 mM CaCl2, 0.02% NaN3) with 2.5% Triton X-100 for 2 × 30 min. MMPs were allowed to degrade proteins in developing buffer for 24–48 hours. Gels were stained with Coomassie Blue. Gelatinase bands were quantified by inverse densitometry using ImageJ software (NIH, Bethesda, MD). Individual data points were normalized to correct for gel background. MMP bands were identified as: gelatin zymography—MMP-2 proenzyme, 72 KDa, activated MMP-2, 68 KDa; MMP-9 proenzyme, 92 KDa, activated MMP-9, 86 and 80 KDa; casein zymography—MMP-3 proenzyme, 57 KDa. MMP bands were confirmed by comparison to control lanes using commercial MMP-2, MMP-3 and MMP-9 enzymes (Calbiochem, Gibbstown, NJ).
Immunoblotting
Samples (100 µg total protein) were loaded into 15% SDS-polyacrylamide gels. Proteins were separated by electrophoresis as above then transferred onto PVDF (Bio-Rad) at 80 V for 45 minutes in transfer buffer (2.5 mM Tris base, 19.2 mM glycine, 20% methanol, 0.1% SDS). Membranes were blocked for 45 minutes with 5% non-fat dry milk in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, 0.05% Tween-20) then probed for 2 hours with primary antibodies, diluted in TBST plus 1% normal goat serum (NGS), against TIMP-1 (Abcam ab86482, rat monoclonal, Cambridge, MA) at 1:250 dilution, TIMP-2 (Millipore MAB3310, mouse monoclonal) at 1:500, MMP-9 (Antibodies Inc., 75–100 mouse monoclonal) at 1:250, or alpha-sarcomeric actin (Sigma A2172, mouse monoclonal) at 1:6000. Blots were probed for 2 hours with secondary goat anti-IgG antibodies conjugated to horseradish peroxidase against mouse (Jackson Immuno Research 115-035-146, West Grove, PA) or rat (Invitrogen Molecular Probes A10549, Eugene, OR), diluted 1:10,000 in TBST plus 1% NGS. Blots were incubated with the ECL kit (GE Healthcare, Buckinghamshire, UK) for 1 minute and then exposed to autoradiographic film. Densitometric quantificiation of TIMP and MMP-9 bands were performed using Image J after normalizing to alpha-sarcomeric actin to control for cardiomyocyte content.
In situ zymography
Cryosections were fixed in acetone at −20 °C for 10 minutes, then incubated for 9 hours at 37 °C with a solution of gelatin-Oregon Green 488 (Invitrogen Molecular Probes) (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM CaCl2, 0.02% NaN3, 100 µg/ml gelatin-Oregon Green 488), then washed 2 × 5 minutes with 10 mM EDTA in PBS (without Ca2+ or Mg2+). Vectashield (Vector Labs, Burlingame, CA) containing 2 µg/ml DAPI (Sigma) was added to each slide. Fluorescence was viewed with a Nikon Eclipse 800 microscope (Nikon Corporation, Tokyo, Japan) with a SPOT-RTslider digital camera and SPOT software (Diagnostic Instruments, Inc., Sterling Heights, MI).
Histology
Cryosections were fixed in 100% ethanol for 30 s, then stained with hematoxylin and eosin using standard procedures.
Immunofluorescence
Unfixed cryosections were equilibrated in KPBS (16.4 mM K2HPO4, 3.6 mM KH2PO4, 160 mM NaCl) for 5 minutes then blocked with KPBS + 1% gelatin for 15 minutes. Slides were washed with KPBS + 0.2% gelatin (KPBSG), then probed for 2 hours with primary antibodies, diluted with KPBSG + 1% NGS, against collagen I (Abcam, ab292 rabbit polyclonal) at 1:100, collagen III (Abcam, ab7778 rabbit polyclonal) at 1:100, ERT-R7 (Abcam, ab51824 rat monoclonal) at 1:100, or CD45 (BD Biosciences, 550539, Franklin Lakes, NJ) at 1:50. Slides were washed 3 × 5 minutes and probed for 1 hour with secondary goat anti-IgG antibodies conjugated to Cy3 against rabbit (Jackson Immuno Research, 111-165-144) or rat (Jackson Immuno Research, 712-165-153), diluted 1:100 in KPBSG + 1% NGS. Slides were washed 3 × 5 minutes, followed by addition of Vectashield containing 2 µg/ml DAPI. Fluorescence was determined as described above.
Statistics
Genotypic comparisons of enzymatic bands in zymography gels and protein bands on western blots were made using ANOVA followed by post-hoc Bonferroni t-test analysis (where applicable). Two-tailed p values of less than 0.05 were considered significant. For zymography, an average value of all MMP-2 or MMP-9 bands was calculated by inverse densitometry. Bands for each sample were divided by the average value for that gel to give a relative value of activity per sample in the gel. Bands with less than average intensity have values below 1.0 while those with greater than average intensity have values above 1.0. These relative values were then compared and ANOVA was performed as described.
Results
The pattern of cardiac tissue injury is similar between dko mice and DMD patients
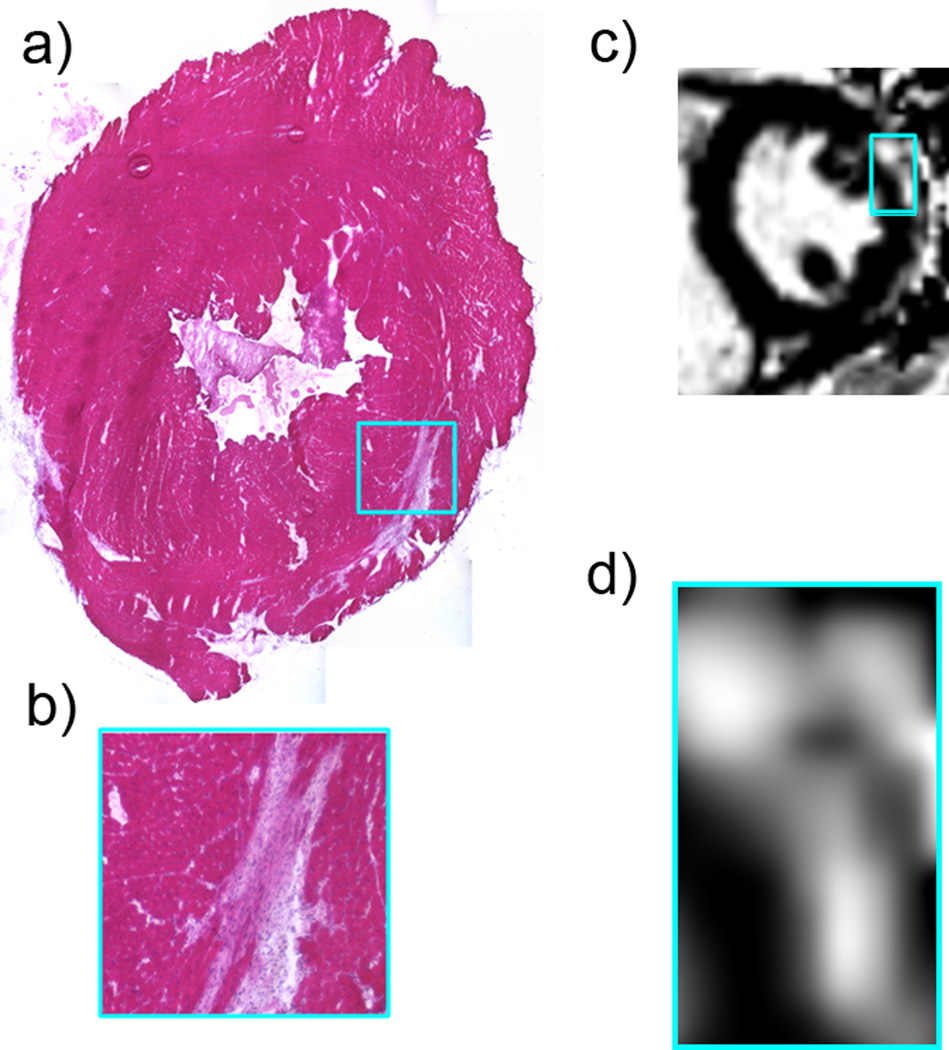
Although we have previously shown that hearts from all dko mice show substantial cardiac damage by 10 weeks-of-age, current human cardiac magnetic resonance imaging (MRI) technology allows for side-by-side comparison of the pattern of damage between DMD patients and this mouse model [11, 38]. Histological analysis of a transverse section through the left ventricle of a representative 10-week-old dko heart (Figure 1a–b) shows a crescent-shaped region of fibrosis in the left ventricular epicardium. This pattern of damage is consistent with what is typically observed in human cardiac MRI of DMD patients using the late gadolinium enhancement (LGE) technique [38, 40]. The short axis image (Figure 1c–d) shows a region of hyperenhancement (bright region relative to dark myocardium elsewhere) consistent with midwall to epicardial fibrosis in a heart from a DMD patient with a preserved normal ejection fraction.
Figure 1.
a. Hematoxylin and eosin-stained transverse section through the left ventricle of a dko heart shows a large, crescent-shaped region of tissue damage near the epicardium. b. Higher magnification of the region boxed in (a). c. Cardiac MRI from a patient with DMD, showing a similar pattern of tissue damage as observed in the dko mouse model. d. Higher magnification of the region boxed in (c).
MMP-2 and MMP-9 levels and activation are increased in dko hearts
To determine MMP levels and activation in dko hearts, we performed gelatinase (MMP-2 and MMP-9) zymography (Figure 2a) using heart homogenates containing only ventricular tissue. We quantified the levels of the MMPs in hearts from dko, mdx and isogenic wild-type C57 control mice using inverse densitometry (Figure 2b). We observed that dko hearts had the highest levels of both MMP-2 and MMP-9 proenzymes and activated MMP-9. ANOVA analysis indicated genotype-based dependence of both MMP-2 and MMP-9 levels (p < 0.05 for each). Post-hoc t-test analysis indicated statistical differences between dko versus C57 and dko versus mdx levels of MMP-9 proenzyme (p < 0.05), while the post-hoc analysis of MMP-2 proenzyme levels indicated a borderline statistical significance (dko versus C57, p = 0.055). In summary, the gelatin zymography data show upregulation of MMP-2 and MMP-9 levels in dko hearts relative to wild type C57 hearts, and further upregulation of MMP-9 levels in dko compared with mdx hearts.
Figure 2.
a. Gelatin zymogram showing representative samples of C57, mdx and dko heart homogenates. Larger regions of gelatin degradation are present at the molecular weight corresponding to activated and proenzyme forms of MMP-9 (M9a and M9p, respectively) in dko than in C57 and mdx samples. Gelatin degradation is observed in mdx and dko hearts for the MMP-2 proenzyme (M2p), but appears highest in dko samples. Activated MMP-2 (M2a) is present in all samples but appears highest in dko hearts. MMP-2 and MMP-9 standards are shown as controls. b. Densitometry of numerous zymograms of independent samples show a significant elevation (*) of cardiac MMP-9 proenzyme levels in dko compared to mdx and C57 (p < 0.05), and a significant genotype-specific effect (*) of dko on MMP-2 proenzyme levels compared to C57 (ANOVA p < 0.05, post-hoc t-test indicated C57 versus dko p = 0.055). Error bars represent the standard error measurements of the data. (C57 n = 4, mdx n = 6, dko n = 9.)
MMP-3 has also been implicated in heart failure, and MMP-3 levels can be easily analyzed using casein zymography [15, 24, 41]. Therefore, we next tested whether MMP-3 is also upregulated in dko hearts. We could identify the proenzyme form of MMP-3 using a commercially available MMP-3 standard (Figure 3). However, there was no apparent MMP-3 activity in any of the hearts from the three genotypes, showing that there is not a generalized increase in MMP levels in dko hearts.
Figure 3.
Casein zymogram showing representative samples of C57, mdx and dko heart protein homogenates. The MMP-3 control enzyme sample demonstrates the degradation of casein at the correct molecular weight for MMP-3 (right lane, white band). However, no casein degradation is observed in samples from any of the three genotypes, despite the presence of large amounts of total protein (dark bands).
We further verified the increase in cardiac MMP-9 observed in dko hearts by zymography using immunoblotting (Figure 4). Western blots showed dko MMP-9 levels were 3.7-fold higher than in C57 hearts (p < 0.01) and 2.3-fold higher than in mdx hearts (p < 0.05). A small increase in MMP-9 levels was also detected in mdx versus C57 hearts (p < 0.05).
Figure 4.
a. MMP-9 western blot on representative samples of C57, mdx and dko heart total protein homogenates. Arrows show the predicted molecular weights for MMP-9 proenzyme (p) and active (a) forms. Actin bands were used as a normalization control for cardiomyocyte content. b. Quantification of MMP-9 band intensities (both proenzyme and activated enzyme bands together) using densitometric measurement. Actin was used as a normalization control for cardiomyocyte content. * Mdx or dko versus C57 (p < 0.05 and p < 0.01, respectively). ** Dko versus mdx (p < 0.05).
MMP-2 and MMP-9 are further dysregulated in dko hearts by reduced TIMP-2 levels
MMP-2 and MMP-9 activities are inhibited by expression of TIMPs, including TIMP-1 and TIMP-2. TIMPs bind to MMPs to regulate their activity in a 1:1 stoichiometry. Thus, if TIMP levels are equal to or higher than MMP levels, this suggests that proteolytic activity of the MMPs is completely inhibited. TIMP expression has been shown to be reduced in other forms of cardiomyopathy and heart failure[9]. Therefore, we determined the levels of TIMP-1 and TIMP-2 in dko hearts in comparison to mdx and C57 by immunoblotting. TIMP-1 levels were consistently low and were not significantly different between the three genotypes (Figure 5a,c). However, we observed that both dko and mdx hearts had significantly reduced TIMP-2 levels compared to C57 (Figure 5b,c). The mean level of TIMP-2 in dko hearts (0.34 ± 0.08 arbitrary units (a.u.), n = 10) was significantly lower than in mdx (1.49 ± 0.70 a.u., n = 9, p < 0.05) and C57 (5.19 ± 1.52 a.u., n = 9, p < 0.01). Therefore, higher than normal MMP-2 and MMP-9 levels observed in dko hearts are not balanced by correspondingly higher levels of TIMP-2. These data suggest a mild dysregulation of low levels of MMP-9 activity in mdx hearts and severe dysregulation of activity of both MMP-2 and MMP-9 in dko hearts.
Figure 5.
a. TIMP-1 immunoblot showing representative samples of C57, mdx and dko total protein heart homogenates. b. TIMP-2 immunoblot showing representative samples of C57, mdx and dko total protein heart homogenates. Actin is shown as the normalization control for (a) and (b). c. Quantification of TIMP-1 and TIMP-2 band intensities using densitometric measurement. Actin was used as a normalization control for cardiomyocyte content. * Mdx versus C57, p < 0.05. ** dko versus C57, p < 0.01. Error bars show the standard error measurements of the data. (TIMP-1: C57 n = 7, mdx n = 4, dko n = 4; TIMP-2: C57 n = 9, mdx n = 9, dko n = 10.)
Gelatinase activity is localized to areas of cardiac damage
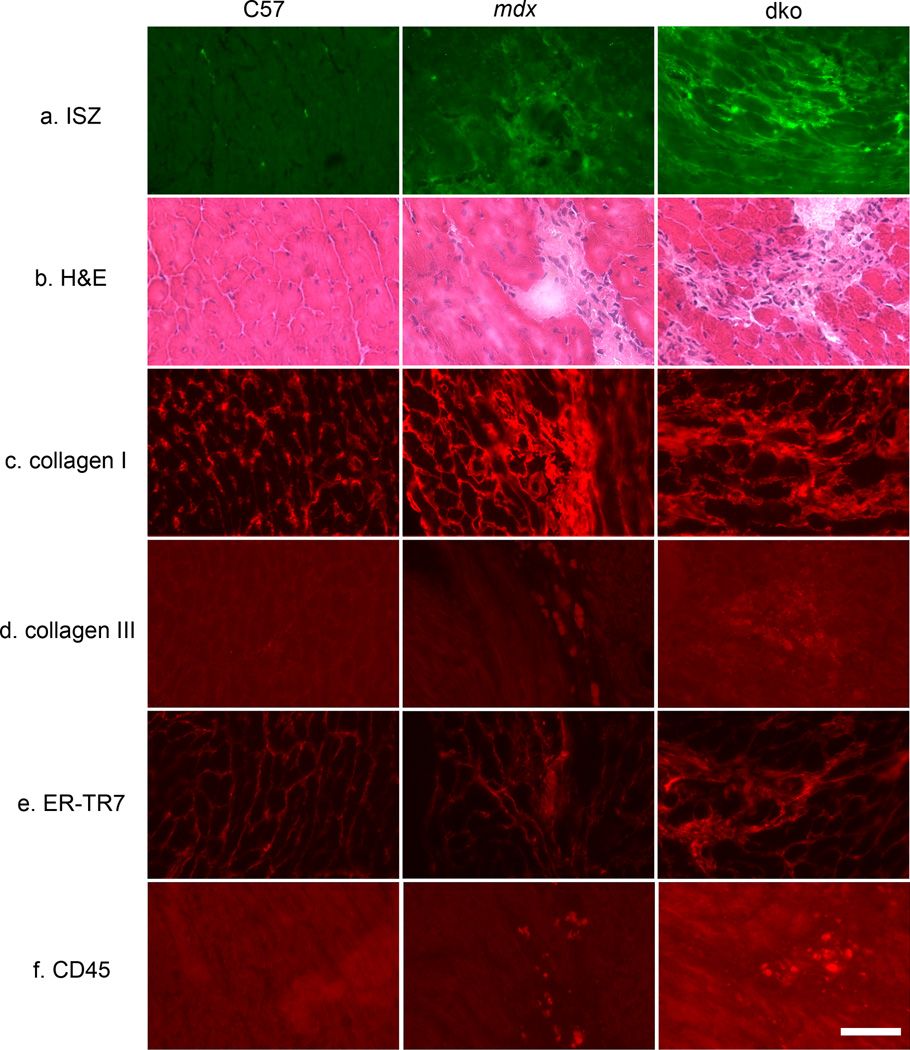
To further confirm the dysregulation of MMP activity and identify its localization in dko hearts we assessed endogenous gelatinase (MMP-2 and/or MMP-9) activity by in situ zymography using a gelatin substrate fluorescently labeled with Oregon Green 488. Regions of high gelatinase activity become highly fluorescent due to cleavage of the substrate. Both dko and mdx heart sections showed gelatinase activity but the activity in dko hearts was overall much brighter and more widespread. In contrast, C57 hearts had negligible gelatinase activity (Figure 6a).
Figure 6.
Localization of indicators of tissue damage and ventricular remodeling in serial sections of C57, mdx and dko hearts. a. In-situ zymography (ISZ) shows activity of the gelatinases MMP-2 and MMP-9 at low levels in mdx and at higher levels in dko hearts that correspond to regions of tissue injury (b). No gelatinase activity is observed in C57 control samples. b. Hematoxylin and eosin (H&E) staining showing overall histology of heart sections. Regions of injury and fibrosis are observed in mdx and dko mice, but not in C57 controls. (c–f) Immunostaining of serial sections of all three genotypes corresponding to the regions of MMP activity and tissue injury in mdx and dko samples (panels a and b) with markers of fibrosis (c,d), fibroblasts (e), and immune cells (f). c. Collagen I normally present interstitially (C57) has been laid down in the injured areas in mdx and dko hearts demonstrating the well characterized process of early fibrotic scar formation. d. Collagen III is observed at only extremely low levels in dko fibrotic regions. Brightness of fluorescence was enhanced to show localization. e. ER-TR7 demonstrates that the majority of cells infiltrating the injured area are fibroblasts (with reticular fibers). f. Only a few cells from a hematopoietic lineage, positive for CD-45, are present in the injured region suggesting that by 10 weeks-of-age in mdx and dko mice, the immune response in early cardiac damage has already subsided. Brightness of fluorescence was enhanced to show localization.
Hematoxylin and eosin staining (H&E; Figure 6b) showed that regions of high gelatinase activity correspond to regions with a substantial loss of cardiomyocytes and replacement by fibrotic tissue in dko and mdx hearts. Collagen levels are known to be upregulated with elevated MMP-9 expression, and collagens I and III make up 80% and 10%, respectively, of the ECM in the normal heart [42]. Thus, to determine whether the fibrosis observed by histopathology contained collagen, we performed immunofluorescence using anti-collagen I and III antibodies. We observed that collagen I localization is predominant in dko and mdx hearts in areas of fibrotic scarring (Figure 6c). Collagen III localization (Figure 6d) was much less predominant than collagen I overall, but showed some localization to fibrotic scars in dko sections only. We did not observe either fibrotic scars or collagen I or collagen III replacement of cardiomyocytes in C57 hearts.
To determine whether gelatinase activity colocalized with immune cells and/or increased presence of cardiac fibroblasts, we performed cell-type specific immunofluorescence. To detect fibroblasts, we used an anti-ER-TR7 antibody (which also detects reticular fibers) (Figure 6e). To detect hematopoietic-lineage (immune) cells, we used an anti-CD-45 antibody (Figure 6f). We observed that localization of fibroblasts and reticular fibers was greatest in regions of damage in dko hearts, and, to a lesser extent, mdx hearts. Only a very small proportion of the mononuclear cell infiltrate in dko hearts was immune cells and these were rare or absent within mdx and C57 myocardial tissue. Therefore, the gelatinase activity colocalizes with fibrotic scars containing collagen I along with fibroblasts and a small number of immune cells.
Discussion
High activity of the gelatinases MMP-2 and MMP-9 are present in 10-week-old dko hearts in addition to previously demonstrated cardiac contractile functional deficits [10] and histopathology [11]. The dko cardiac and skeletal pathology in younger-aged mice more closely models the clinical presentation of DMD than the genotypic mdx model [12]. Indeed, we now show that the pattern of cardiac fibrosis in dko mice is similar to that of DMD patients. This pattern is observed years prior to reduced whole heart function in DMD[40], and supports that the dko model is relevant for investigating the evolution of DMD-related cardiomyopathy and testing potential therapeutic prevention strategies.
In addition to increased levels of MMP-2 and MMP-9, the dysregulation of their activity in dko hearts is further exacerbated due to significantly reduced levels of TIMP-2, a strong inhibitor of these MMPs. As a result, the balances between TIMP-2 and MMP-2 and MMP-9 are lower in dko than in age-matched young mdx hearts. Interestingly, TIMP-2 gene expression was recently reported to be increased along with increased MMP-9 levels in old mdx mice [36]. In contrast, the high MMP-2 and MMP-9 protein levels in conjunction with low TIMP-2 protein levels in dko hearts closely mimics the observations in most human heart failure. Increased MMPs and reduced TIMPs are often concurrent in human cardiac pathology, and reduced TIMP levels correlate with worsened cardiac outcomes [8, 14, 43, 44]. Genetic ablation of TIMPs also augments heart failure in animal models [19, 21, 22, 45].
In this study, we focused specifically on TIMP-1, with known inhibitory effects against pro-MMP-9 and TIMP-2 with known inhibitory effects against pro-MMP-2 and nearly all MMPs since these TIMPs are the most commonly investigated with MMP-2 and MMP-9 in human heart failure [14, 24]. It should be noted that this study does not preclude the possibility that TIMP-3 and TIMP-4 may be upregulated concurrent with TIMP-2 downregulation, but to our knowledge, there are no documented cases where this type of compensatory mechanism occurs [19].
Upregulation of MMP-2 and MMP-9 described recently in the hearts of mdx mice is only observed consistently after 10 to 12 months of life [35, 36]. On the other hand, we have observed these effects by 10 weeks-of-age in dko hearts. This situation parallels the severe and mild cardiac functional impairment observed in dko and mdx mice, respectively [10].
MMPs are capable of becoming activated in the full-length proenzyme form through oxidation by S-glutathione, reactive oxygen species, and phosphorylation [20, 22, 46, 47]. These modes of activation suggest that even the proenzyme forms of MMP-2 and MMP-9 in dko hearts could be active in the absence of equimolar levels of TIMPs, and is supported by the high level of gelatinase activity observed by in situ zymography in dko heart sections.
The regions of high gelatinase activity in dko and mdx hearts correspond tightly to regions of fibrosis by histology and collagen I deposition. In many forms of human heart failure, collagen I deposition is the major component of the remodeled matrix [9]. We have shown that the regions with high MMP activity and fibrotic scar tissue in dko hearts have mononuclear cellular infiltrates with proportionally more fibroblasts than immune cells. This supports that the immune response is very limited once fibrotic scarring develops in dko hearts. Other studies have also shown that immune cells, particularly macrophages, play a role in early cardiac damage, but that fibroblasts predominate to form the fibrotic scar tissue composed mostly of collagen I in the later stages of fibrosis. Fibroblasts are known to be major producers of MMPs [14, 19, 20, 42].
Dko mice would be useful for testing anti-MMP compounds as heart failure treatments. Their strong MMP-cardiomyopathy correlation can also be used as an outcome measure when testing other heart failure therapeutic strategies. Indeed, we have used utrophin-haploinsufficient mice (utrn+/−;mdx), which show more severe cardiac pathology than mdx mice, but live longer than dko mice (which succumb to their skeletal muscle pathology after 10 weeks) [48], to test heart failure drugs. We have found that along with reduced physiological indicators of heart failure and a reduction in cardiac fibrosis, MMP activity was also decreased in drug-treated utrn+/−;mdx mice [49].
In summary, we have shown that the dko mouse displays an important biochemical hallmark of ventricular remodeling: dysregulation of MMP-2 and MMP-9 in addition to previously characterized histopathological and physiological indicators of heart failure. Therefore, these mice continue to be a useful model of muscular dystrophy-based cardiomyopathy and can be used for further investigations of MMP-targeted potential therapeutics. Importantly, these investigations can be performed in a matter of weeks in these mice rather than the one year required for disease progression in mdx mice.
Acknowledgements
We would like to thank Federica Montanaro for helpful discussions, and Cameron Black for technical assistance. This work was supported by a Research Grant from the Muscular Dystrophy Association (to JRF), OSU internal funding, and by NIH 1T32 HL098039 (support to DAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 2.Markham LW, Spicer RL, Cripe LH. The heart in muscular dystrophy. Pediatric Annals. 2005;34:531–535. doi: 10.3928/0090-4481-20050701-10. [DOI] [PubMed] [Google Scholar]
- 3.Zheng QS, Guo WG, Lu ZF, Shi XQ, Su FF, Li H. Dystrophin: from non-ischemic cardiomyopathy to ischemic cardiomyopathy. Medical Hypotheses. 2008;71:434–438. doi: 10.1016/j.mehy.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Molecular Therapy. 2008;16:832–835. doi: 10.1038/mt.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Human Molecular Genetics. 2006;15(Spec No 2):R253–R261. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miniño AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. National Vital Statistics Reports. 2007;55:1–119. [PubMed] [Google Scholar]
- 7.Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure: from molecules to man (Part I) Cardiovascular Pathology. 2005;14:1–11. doi: 10.1016/j.carpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. Journal of Clinical Investigation. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polyakova V, Loeffler I, Hein S, et al. Fibrosis in endstage human heart failure: Severe changes in collagen metabolism and MMP/TIMP profiles. International Journal of Cardiology. 2010;151:18–33. doi: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 10.Janssen PM, Hiranandani N, Mays TA, Rafael-Fortney JA. Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. American journal of Physiology Heart and Circulatory Physiology. 2005;289:H2373–H2378. doi: 10.1152/ajpheart.00448.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hainsey TA, Senapati S, Kuhn DE, Rafael JA. Cardiomyopathic features associated with muscular dystrophy are independent of dystrophin absence in cardiovasculature. Neuromuscular Disorders. 2003;13:294–302. doi: 10.1016/s0960-8966(02)00286-9. [DOI] [PubMed] [Google Scholar]
- 12.Deconinck AE, Rafael JA, Skinner JA, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 13.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 14.Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure From molecules to man (Part II) Cardiovascular Pathology. 2005;14:49–60. doi: 10.1016/j.carpath.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Buralli S, Dini FL, Ballo P, et al. Circulating matrix metalloproteinase-3 and metalloproteinase-9 and tissue Doppler measures of diastolic dysfunction to risk stratify patients with systolic heart failure. American Journal of Cardiology. 2010;105:853–856. doi: 10.1016/j.amjcard.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Kitaoka H, Kubo T, Okawa M, et al. Impact of metalloproteinases on left ventricular remodeling and heart failure events in patients with hypertrophic cardiomyopathy. Circulation Journal. 2010;74:1191–1196. doi: 10.1253/circj.cj-09-1013. [DOI] [PubMed] [Google Scholar]
- 17.Muller AL, Dhalla NS. Role of various proteases in cardiac remodeling and progression of heart failure. Heart Failure Reviews. 2011 doi: 10.1007/s10741-011-9269-8. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovascular Research. 2006;69:666–676. doi: 10.1016/j.cardiores.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher GL, Jackson CJ, Hunyor SN. Myocardial extracellular matrix remodeling in ischemic heart failure. Frontiers in Bioscience. 2007;12:1410–1419. doi: 10.2741/2157. [DOI] [PubMed] [Google Scholar]
- 20.Graham HK, Horn M, Trafford AW. Extracellular matrix profiles in the progression to heart failure. European Young Physiologists Symposium Keynote Lecture-Bratislava 2007. Acta Physiologica. 2008;194:3–21. doi: 10.1111/j.1748-1716.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 21.Janssens S, Lijnen HR. What has been learned about the cardiovascular effects of matrix metalloproteinases from mouse models? Cardiovascular Research. 2006;69:585–94. doi: 10.1016/j.cardiores.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular Research. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Cheung C, Marchant D, Walker EK, et al. Ablation of matrix metalloproteinase-9 increases severity of viral myocarditis in mice. Circulation. 2008;117:1574–1582. doi: 10.1161/CIRCULATIONAHA.107.733238. [DOI] [PubMed] [Google Scholar]
- 24.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochimica et Biophysica Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorman G, Kocsis-Szommer K, Spadoni C, Ferdinandy P. MMP inhibitors in cardiac diseases: an update. Recent Patents in Cardiovascular Drug Discovery. 2007;2:186–194. doi: 10.2174/157489007782418964. [DOI] [PubMed] [Google Scholar]
- 26.Bani C, Lagrota-Candido J, Pinheiro DF, et al. Pattern of metalloprotease activity and myofiber regeneration in skeletal muscles of mdx mice. Muscle and Nerve. 2008;37:583–592. doi: 10.1002/mus.20970. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Mittal A, Makonchuk DY, Bhatnagar S, Kumar A. Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Human Molecular Genetics. 2009;18:2584–2598. doi: 10.1093/hmg/ddp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukushima K, Nakamura A, Ueda H, et al. Activation and localization of matrix metalloproteinase-2 and-9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ) BMC Musculoskeletal Disorders. 2007;8:54. doi: 10.1186/1471-2474-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanotti S, Gibertini S, Mora M. Altered production of extra-cellular matrix components by muscle-derived Duchenne muscular dystrophy fibroblasts before and after TGF-beta1 treatment. Cell Tissue Res. 2010;339:397–410. doi: 10.1007/s00441-009-0889-4. [DOI] [PubMed] [Google Scholar]
- 30.Leite PE, Lagrota-Candido J, Moraes L, et al. Nicotinic acetylcholine receptor activation reduces skeletal muscle inflammation of mdx mice. J Neuroimmunol. 2010;227:44–51. doi: 10.1016/j.jneuroim.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S. Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum Mol Genet. 2011;20:1787–1799. doi: 10.1093/hmg/ddr062. [DOI] [PubMed] [Google Scholar]
- 32.Nevo Y, Aga-Mizrachi S, Elmakayes E, et al. The Ras antagonist, farnesylthiosalicylic acid (FTS), decreases fibrosis and improves muscle strength in dy/dy mouse model of muscular dystrophy. PLoS One. 2011;6:e18049. doi: 10.1371/journal.pone.0018049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahiya S, Bhatnagar S, Hindi SM, et al. Elevated levels of active matrix metalloproteinase-9 cause hypertrophy in skeletal muscle of normal and dystrophin-deficient mdx mice. Hum Mol Genet. 2011;20:4345–4359. doi: 10.1093/hmg/ddr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadarajah VD, van Putten M, Chaouch A, et al. Serum matrix metalloproteinase-9 (MMP-9) as a biomarker for monitoring disease progression in Duchenne muscular dystrophy (DMD) Neuromuscul Disord. 2011;21:569–578. doi: 10.1016/j.nmd.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Au CG, Butler TL, Sherwood MC, Egan JR, North KN, Winlaw DS. Increased connective tissue growth factor associated with cardiac fibrosis in the mdx mouse model of dystrophic cardiomyopathy. International Journal of Experimental Pathology. 2011;92:57–65. doi: 10.1111/j.1365-2613.2010.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahiya S, Givvimani S, Bhatnagar S, Qipshidze N, Tyagi SC, Kumar A. Osteopontin-Stimulated Expression of Matrix Metalloproteinase-9 Causes Cardiomyopathy in the mdx Model of Duchenne Muscular Dystrophy. Journal of Immunology. 2011;187:2723–2731. doi: 10.4049/jimmunol.1101342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanford JL, Edwards JD, Mays TA, Gong B, Merriam AP, Rafael-Fortney JA. Claudin-5 localizes to the lateral membranes of cardiomyocytes and is altered in utrophin/dystrophin-deficient cardiomyopathic mice. Journal of molecular and cellular cardiology. 2005;38:323–332. doi: 10.1016/j.yjmcc.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Puchalski MD, Williams RV, Askovich B, et al. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? International Journal of Cardiovascular Imaging. 2009;25:57–63. doi: 10.1007/s10554-008-9352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawkes SP, Li H, Taniguchi GT. Zymography and reverse zymography for detecting MMPs and TIMPs. Methods in Molecular Biology: Matrix Metalloproteinase Protocols. 2010;622:257–269. doi: 10.1007/978-1-60327-299-5_16. [DOI] [PubMed] [Google Scholar]
- 40.Verhaert D, Richards K, Rafael-Fortney JA, Raman SV. Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations. Circulation Cardiovascular Imaging. 2011;4:67–76. doi: 10.1161/CIRCIMAGING.110.960740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bujak M, Dobaczewski M, Chatila K, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. American Journal of Pathology. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacology and Therapeutics. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Schwartzkopff B, Fassbach M, Pelzer B, Brehm M, Strauer BE. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. European Journal of Heart Failure. 2002;4:439–434. doi: 10.1016/s1388-9842(02)00092-2. [DOI] [PubMed] [Google Scholar]
- 44.Creemers EE, Davis JN, Parkhurst AM, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. American Journal of Physiology Heart and Circulatory Physiology. 2003;284:H364–H371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 45.Kandalam V, Basu R, Abraham T, et al. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circulation research. 2010;106:796–808. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 46.Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Frontiers in Bioscience. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 47.Ishihara H, Kubota H, Lindberg RL, et al. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. Journal of Neuropatholy and Experimental Neurology? 2008;67:435–448. doi: 10.1097/NEN.0b013e31816fd622. [DOI] [PubMed] [Google Scholar]
- 48.Rafael JA, Tinsley JM, Potter AC, Deconinck AE, Davies KE. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nature Genetics. 1998;19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- 49.Rafael-Fortney JA, Chimanji NS, Schill KE, et al. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in duchenne muscular dystrophy mice. Circulation. 2011;124:582–588. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]