Abstract
Rapid development in the field of ultrasound triggered drug delivery has made it essential to study the real-time interaction between the membranes of live cells and the membranes of echogenic delivery vehicles under exposure to focused ultrasound. The objective of this work was to design an analysis system that combined fluorescent imagining, high speed videography, and definable pulse sequences of focused ultrasound to allow for real time observations of both cell and vehicle membranes. Documenting the behavior of the membranes themselves has not previously been possible due to limitations with existing optical systems used to understand the basic physics of microbubble/ultrasound interaction and the basic interaction between microbubbles and cells. The performance of this new system to monitor membrane behavior was demonstrated by documenting the modes of vehicle fragmentation at different ultrasound intensity levels. At 1.5 MPa the membranes were shown to completely fragment while at intensities below 1 MPa there is a popping and slow unfolding. The interaction between these vehicles and cell membranes was also documented by the removal of fluorescent particles from the surfaces of live cells out to 20 μm from the microbubble location. The fluid flow created by microstreaming around ensonated microbubbles was documented at video recording speeds from 60 to 18,000 frames per second. This information about membrane behavior allows the chemical and physical properties of the drug delivery vehicle to be designed along with the ultrasound pulse sequence to cause the most efficient drug delivery.
1. INTRODUCTION
Ultrasound triggered drug delivery promises to specifically deliver drugs to desired locations at desired times in the body [1, 2]. Ultrasound focused to the targeted tissue region interacts directly with systemically circulating echogenic drug delivery vehicles designed to release payload only in the ultrasound focal region. This can avoid unnecessary and potentially dangerous payload release in healthy tissue. Unfortunately, the development of new prototype echogenic delivery vehicles has been hampered due to the difficulty of imaging the real time interaction between the membranes of delivery vehicles and nearby cells during ensonation. Ultimately the success of a delivery vehicle design will depend on the interaction of its outer membrane with the membranes of live cells when exposed to focused ultrasound, especially its interaction with the membranes of vascular endothelial cells, which are the first cells to receive exposure.
The new instrumentation described here was developed to study the cellular interaction of the echogenic drug delivery vehicles with individual live cells allowing real time evaluation as well as long term monitoring. This makes it possible to rapidly evaluate many types of vehicle designs and materials.
The need for rapid evaluation of different delivery vehicle designs comes from a recent interest in the field of ultrasound triggered drug delivery to encapsulate microbubbles within drug delivery liposomes [3-8]. The basic design of the nested liposome vehicle is an outer lipid membrane that protects a payload of both gas microbubbles and therapeutic drugs [8]. These vehicles would be injected into the circulation system of the body passing through healthy tissue without allowing drug exposure. At the diseased region, the vehicles can be triggered to open and release their payloads upon ensonation with focused the ultrasound. Only the region of desired tissue located within the focal volume of the ultrasound would receive a sufficient intensity to trigger drug release [8]. Some of the released payload that is not taken up by the target tissue may be swept into systemic circulation, but will be diluted over the entire body reducing the concentration. These −6 dB focal volumes of ultrasound can be on the order of a few cubic millimeters in size [9] allowing for exquisite spatial resolution. The vehicles which are not within the focal volume of the ultrasound are left unaffected and will be eliminated from circulation through normal clearance mechanisms without exposing the healthy tissue to the drug payload. Reducing the exposure of healthy tissue to the active drug will reduce the systemic side effects experienced by many modern medications, especially those from chemotherapy. To achieve these delivery properties new vehicle designs using different materials need to be rapidly tested and evaluated. This requires a specialized imaging system to quickly evaluate the interaction of these vehicles with the membranes of live endothelial cells.
Previous imaging systems have been designed to understand the basic physics of microbubble interactions with ultrasound. These instruments have documented ultrasound driven microbubble oscillation at extremely high frame rates up to 6.2 Mfps [10, 11]. They have also documented acoustically induced lateral translations of microbubbles [12], changes to the internal structure of the microbubble during oscillation against a solid surface [13], escape and dissolution of gas from microbubbles undergoing oscillations, [14] and diameter changes of free floating microbubbles [15, 16]. Although these instruments are essential to understand the basic physics of microbubble interactions with ultrasound they are not designed to accommodate and image live cells.
Typically, the cellular effects of ultrasound triggered drug delivery vehicles have been documented by imaging a cell population before and after ensonation [17-19]. Though fluorescent tags can aid in tracking destruction and delivery, researchers are left to infer the exact occurrences during the application of ultrasound. Electron microscopy and flow cytometry have also been used to study the affect of microbubbles on cell membranes [20] as well as atomic force microscopy [21, 22], but these studies were also conducted post ultrasound exposure and were not capable of giving real-time information. Acquiring real-time information about ultrasound-particle interactions is necessary to provide a complete story.
Instruments have been designed to document the interaction of microbubbles adjacent to cells using white light illumination [23] and the interaction of microbubbles internalized by free floating cells with ultrasound [15], but detailed studies of membrane interaction were not possible due to the difficulty of white light to visualize the edges of the membranes. Fluorescent systems have been designed to monitor the influx of cell membrane impermeant dyes into cell interiors after nearby microbubbles were ensonated with ultrasound [24, 25]. However, only white light was used to visualize the cell membrane itself so the extent of the influence over the entire cell surface from the microbubble was not measurable.
Fluorescent imaging has been used to monitor the influx of calcium ions into cells that contain the calcium sensitive Fura2 fluorescent probe [26]. The documented increase in fluorescence inside the cells showed that calcium transport across the membrane into the cells was facilitated by the sonoporation effect from nearby microbubbles, but the instrumentation was not able to visualize the high-speed interactions between the microbubble membrane and the cell membrane that lead to the observed increase in permeability.
The release of fluorescent dye from within liposomes due to nearby ultrasound driven microbubble oscillation has been shown [6] but the system was not able to visualize the actual membranes.
The facilitation of fluorescent nanoparticle delivery into cells by sonoporation has been documented, but the effects on the microbubble membrane and the whole cell effect on individual cell membranes was not monitored [27-29]. Microbubble interactions with empty liposomes has been documented using fluorescence [30], but the system did not monitor live cells.
Although useful for understanding basic microbubble physics and basic cellular interactions, the specialized instruments described above are not designed to study the high speed interactions of the delivery vehicles and their membranes with the entire membranes of live adherent endothelial cells. These adherent cells are most like the endothelial cells found lining the blood vessels that these drug delivery vehicles will first interact with. The lipid composition of the vehicle membranes, including the cholesterol content, can significantly change the flexibility resulting in different cellular and ultrasound interactions. These interactions ultimately determine if the vehicle being tested will have useful drug delivery properties.
The instrument described here has been designed to combine fluorescent imaging, white light illumination, focused ultrasound, and high speed videography to monitor the membrane interaction between echogenic drug delivery vehicles and live cells. This allows the fragmentation of the membranes to be studied along with their real time cellular interaction. The system also allows the fluid motion that is created by microstreaming around oscillating microbubbles and cavitation shockwave events to be monitored. Using these techniques, the extent of microbubble and echogenic drug delivery vehicle influence on the surface of cells can be monitored.
2. MATERIALS
L-α-phosphatidylcholine (EPC) from chicken eggs, distearoyl phosphatidylcholine (DSPC), distearoyl phosphatidylethanolamine-methyl poly(ethylene glycol) MW5000 (mPEG-DSPE), and cholesterol were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). 1,2-propanediol, glycerol, ethanol, and perfluorohexane were purchased from Sigma-Aldrich. All water was purified using the Milli-Q Plus System (Millipore Corporation, Bedford, USA). DiO was purchased from Biotium, Inc. CA. The PBS was purchased from Hyclone Laboratories Inc. (Logan, UT). Quantum dots were purchased from Life Technologies (California, USA).
3. INSTRUMENT DESIGN
3.1. Water Tank
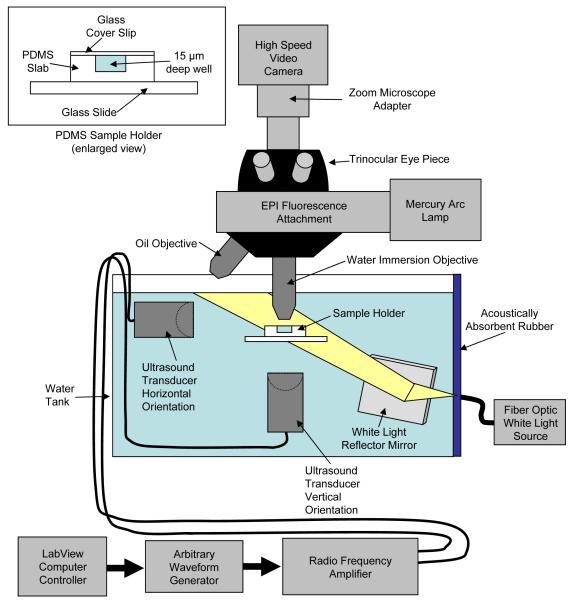
A 10 gallon water tank was used to allow coupling between the ultrasound transducer and the submerged sample. The size of the tank helped attenuate reflections of ultrasound from the sides of the tank and from the air/water interface. To further reduce reflections a 1-cm thick block of acoustically absorbent rubber (Aptflex F28, Precision Acoustics Ltd., Dorchester, UK) was installed at the opposite end of the tank directly in line with the longitudinal axis of the transducer as shown in Fig. 1.
FIG. 1.
Schematic of the optical, electrical and acoustic components of the system.
3.2. Focused Ultrasound Generation
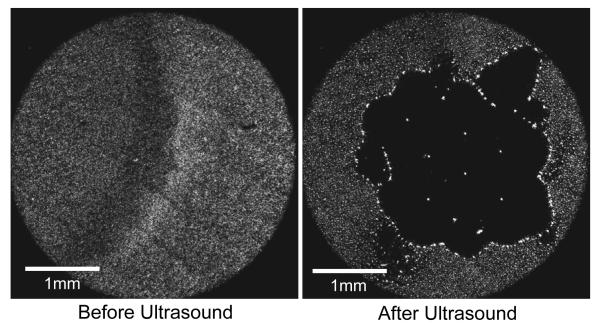
The ultrasound pulse sequence used to ensonify the sample was generated using a National Instruments PCI 5412 arbitrary waveform generator board run by a custom designed automation program using LabVIEW 8.2. The arbitrary waveform generator allowed custom ultrasound pulse sequences to be designed to create different interactions between the microbubbles and the membranes. The ultrasound pulse sequence was sent to a Panametrics 2.25 MHz transducer (V305-SU) with a 2.54 cm spherical-focus. The −6dB focal zone of the ultrasound was 1 mm3. Localization of the microbubble/ultrasound interaction to the focal zone is shown in Fig 2. Here a 5X objective was able to monitor an entire population of microbubbles which appear as individual white dots before ultrasound exposure. Exposure to the focused ultrasound causes aggregation of the microbubbles only in the focal zone clearing a 2.5 mm diameter space in the middle of the field.
FIG 2.
Localization of microbubble/ultrasound interaction to the focal zone of the ultrasound. In the before ultrasound frame the monolayer of microbubbles trapped between a glass slide and a cover slip appear as white dots with the use of a 5X objective. In the after ultrasound frame the microbubbles interact with the focused ultrasound and aggregate clearing a 2.5 mm space in the center of the frame. The major effects were seen only in the focal zone.
A Panametrics BCU −58 - 6W waterproof connector cable was used to fully submerge the transducer under water. A 300 W radio frequency amplifier from Vox Technologies (VTC2057574) was used to amplify the signals sent to the transducer, creating acoustic intensities in the focal region of up to 1.6 MPa.
3.3. Sound Field Quantification
The sound field of the transducer within the tank was mapped using a submersible broadband needle hydrophone (HNP-0400) from Onda Corporation in connection with their AH - 2020-100 hydrophone pre amp (50kHz - 100 MHz, 0 +20 db). The directionality and 1 mm3 spatial volume allowed the needle hydrophone to easily find the actual −6dB focal zone of the transducer so the samples could be directly aligned in that volume.
3.4. Optical System
The microscope system was designed with two types of objectives. The first was the Nikon MRF07620 CFI W Flour 60X water dipping objective. This could be submerged 5 cm under the surface of the water to observe ultrasound interactions in the bulk fluid. This objective had a 2 mm working distance which was large enough to allow the lens to stay out of the ultrasound focal region while imaging the sample.
A Nikon 100X oil immersion objective with a greater numerical aperture was also used because the oil allowed the fluorescent light to be collected more efficiently. This allowed 100X magnification videos to be collected while maintaining the desired frame rate of at least 60 fps. Keeping the oil in place between the objective and cover slip was difficult when submerging the entire sample holder underwater to achieve the ultrasound coupling. The oil tended to float on the water breaking the optical coupling between the objective and the cover slip. This prevented the total submersion of the sample holder into the water tank. The orientation of the sample holder was changed to be located at the air/water interface with the cover slip slightly above the water surface to allow for oil coupling to the objective and the PDMS submerged as much as possible into the water to proved coupling to the focused ultrasound. The ultrasound transducer orientation was changed to aim the focal zone directly at the sample in the vertical orientation. This orientation could potentially create interference patterns in the region of the sample due to reflections from the air/water interface, but acoustic energy was deposited in that region which had significant interaction with the samples. Placing the samples at the air/water interface is also a model for monitoring interaction between ultrasound and the delivery particles near the internal surface of the lungs.
3.5. White Light Illumination
The sample could be illuminated with white light using a Ram Optical Instrumentation 150 Illuminator fiber optic light source which generated the light from outside the tank and directed it through the tank wall to a mirror submerged in the water. The mirror was adjusted to reflect the light up into the objective. The white light allowed the cells to be observed without the need for fluorescence and was very useful to find microbubbles which were not visible when using just the fluorescence. Independent control over the two light sources was important for viewing cell and liposome interactions with ultrasound simultaneously.
3.6. Experimental Sample Retention
Three different sample holders were designed to analyze ultrasound interaction under different conditions. Blocks of agar tissue phantoms were added to these sample holders to either encase them, or sit between them and the ultrasound transducer to better simulate acoustic conditions that would be experienced in vivo.
The first sample holder was a custom fabricated 15 μm-deep microwell chamber molded in a 0.5 cm thick slab of polydimethylsiloxane (PDMS) and covered on top by a glass cover slip. The PDMS is an optically clear, flexible, rubber like material that attenuates ultrasound but allowed a significant amount of energy through a 0.5 cm thickness to reach the sample contained within the well. The microwell was used both at the air/water interface for use with the oil immersion objective and completely submerged underwater for use with the water immersion objective. When used at the air/water interface the cover slip was positioned above the water surface with the PDMS slab halfway submerged in the water so as to conduct the ultrasound from the underwater transducer into the sample well.
The second sample holder design consisted of two pieces of thin clear plastic sheet placed on top of each other and held taught within a circular hoop support structure. The plastic sheets were optically clear and did not significantly attenuate the ultrasound energy. Holding the sheets tightly together allowed a 15 μL of sample fluid to spread out between the sheets thin enough to reduce background fluorescence from out of focus liposomes. This holder was used completely submerged underwater with the water dipping objective.
The third sample holder was a 200 μm diameter micro-cellulose dialysis hollow fiber manufactured by Spectrum. These thin tubes were nearly both optically and acoustically transparent and were used completely submerged with the water immersion objective. The cellulose tubes were connected to syringes to inject fluid containing the delivery vehicles allowing them to flow through the tubes to study ultrasound interaction in a simulated blood vessel environment and under conditions of flow. The samples needed to be diluted in these situations because the background fluorescence from the out of focus liposomes in the tube created unfavorable signal to noise ratios under dense vehicle concentrations.
These different sample holders were designed to be embedded in agar tissue phantoms to help better simulate the distortions and scattering that focused ultrasound would experience in vivo. This allowed the robustness of these ultrasound activations to be studied for better understanding of how these particles would interact in the vivo ultrasound sound field.
3.7. Alignment
The sample holder, the transducer, and the needle hydrophone were mounted to manual XYZ directional microstages so their alignment could be precisely controlled. The sample holder, transducer focal volume, and focal zone of the microscope objective were all precisely aligned to make sure sufficient ultrasound energy was deposited into the observable region of the sample holder.
3.8. Fluorescent Imaging
Florescence imaging was essential to detect the fluorescently labeled liposome membranes. This was achieved by incorporating a Nikon J-FL EPI-Fluorescence attachment with the use of a Nikon GFP-3035B-NTE GFP Brightline filter cube. A Nikon 100W Hg arc lamp with a CHIU Technical Corp M-100T power supply provided the excitation light. A Nikon trinocular body tube was mounted to the top of the EPI-Fluorescence attachment which allowed the bionocular eye pieces to be installed for visual observation, as well as a Nikon 0.9x to 2.25x zoom CCTV/microscope adapter which allowed the field to be scanned at low magnification power and then zoom in on important details.
3.9. High Speed Videography
A Photron FASTCAM 1024 PCI high speed video camera acquired the image sequences. The camera was fitted with a C mount adapter to interface with the top of the zoom CCTV/microscope adapter on the trinocular body tube. Movies of the fluorescent imaging could be captured at speeds of 60 – 18,000 frames per second. The use of quantum dots created much brighter fluorescence than the traditional fluorescent dyes allowing the higher frame rates to be achieved.
The lighting used to collect these images was a combination of both the fluorescent and white light sources. This allowed both the microbubble as well as the fluorescently labels membranes to simultaneously be visible.
4. SYSTEM PERFORMANCE
4.1. Documentation of Microbubble Interaction with Fluorescent Liposome Membrane
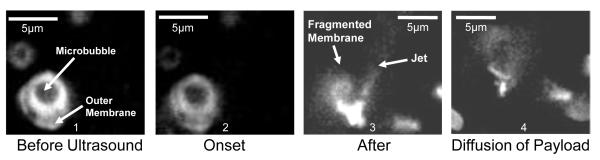
With the fluorescent imaging it was possible to visualize the interaction of just the echogenic drug delivery vehicles themselves with the focused ultrasound as shown in Fig. 3. The manufacturing of the vehicles as well as the fluorescent liposomes followed previously established protocols [8]. The vehicle shown by the arrow consisted of a microbubble on the inside surrounded by a water space and a fluorescently labeled outer membrane. A thorough characterization of these particles is described in Ibsen et al. 2011 [8]. Here the 1.5 MPa ultrasound pulse sequence was designed using the arbitrary waveform generator to cause inertial cavitation of the encapsulated microbubble as seen in frame 2. Inertial cavitation of microbubbles has been shown to occur with ultrasound intensity levels as low as 0.6 MPa [31]. Frame 3 shows the fragmentation of that fluorescent membrane creating a jet of debris that is characteristic of cavitation [19]. The lipid debris cloud is seen diffusing in frame 4.
FIG. 3.
Fluorescent image sequence showing the interaction of focused ultrasound with the microbubble inside the echogenic drug delivery vehicle and subsequent rupture of the outer membrane leaving a debris field of fluorescent lipid particles.
4.2. Documentation of Echogenic Drug Delivery Vehicle Interaction with Nearby Artificial Cell Membranes
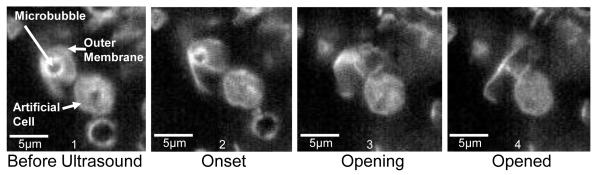
Artificial cell membranes were created to test membrane interactions under less violent conditions. A 1 MPa ultrasound pulse sequence was designed to pop the microbubble rather than cavitate it. This was conducted while the echogenic vehicle was near the membrane surface of an artificial cell as seem in frame 1 of Fig. 4. These artificial cells were also labeled with fluorescent dye to determine their exact edges. In frames 2-4 the outer membrane of the vehicle was popped open and slowly unfolded near the artificial cell causing no visible damage to its outer membrane.
FIG. 4.
Fluorescent image sequence of the interaction between an echogenic drug delivery vehicle and an artificial cell membrane. The ultrasound pulse sequence was designed to induce a single rupture and subsequent unfolding of the vehicle’s outer membrane.
4.3. Documentation of Echogenic Delivery Vehicle Interaction with Cell Membranes
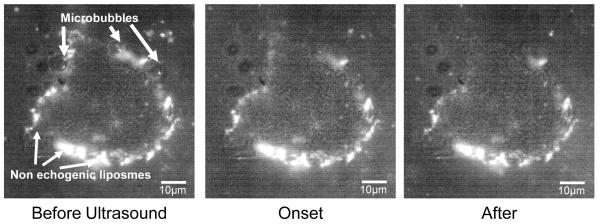
The interaction of the echogenic drug delivery vehicles with cell membranes of adherent endothelial cells was also documented as shown in Fig 5. Here the surface of the cell is covered in fluorescent liposomes which are non-echogenic and do not respond to ultrasound. Only three are pointed out in the figure but the rest of the surface is covered with them as shown. These liposomes are biochemically targeted to integrins expressed on the cell surface and mark the exact edges of the cell membrane. When exposed to the focused ultrasound the three microbubbles cavitated sending out a shockwave which affected the surface of the cell by removing the attached liposomes. The attachment of the liposomes to the surface of the cell is particularly important to understand the extent of the affect from the microbubble. This allows the entire surface of the cell to be monitored to understand more than just whether the membrane has been permeabilized. This image sequence shows disturbance to the cell membrane only in the upper third of the cell.
FIG. 5.
Fluorescent and white light image sequence showing the interaction of microbubbles with the labeled surface of an adherent endothelial cell. Non-echogenic liposomes have been biochemically targeted to attach to the surface of the cell outlining the exact edges of the cell in fluorescence in the “Before Ultrasound” frame. In the “Onset” frame the ultrasound interaction shows the cavitation of the microbubbles and subsequent removal of adherent fluorescent liposomes from the cell surface due to interaction caused by the cavitation shockwave. In the “After” frame the fluorescent liposomes are torn from the cell membrane only in the upper third portion of the cell.
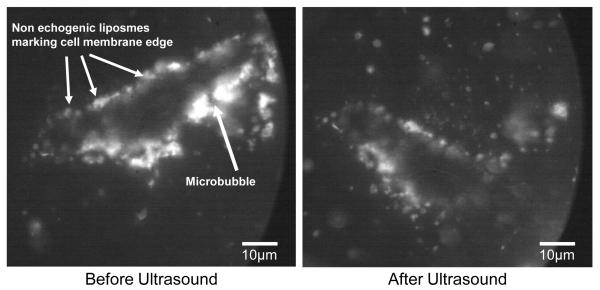
The effect of a single microbubble on the surface of an adherent cell is shown in Fig. 6. Here the surface of the cell is covered with targeted non-echogenic liposomes which show the outline of the cell. A single microbubble is pointed out toward the right. The cavitation event created upon ensonation with ultrasound was so violent that the cell was partially broken from the substrate causing a rotational translation of the cell. The cavitation also affected the surface of the cell membrane removing the non-echogenic liposomes from nearly half of the cell surface.
FIG. 6.
Fluorescent image sequence of the interaction between a single microbubble and an adherent endothelial cell. The cell is outlined by non-echogenic liposomes which have been biochemically targeted to integrin proteins present on the surface of the cell. The shockwave produced by the cavitation event provided enough energy to partially detach the cell from the glass and cause a 45 degree clockwise rotational translation as well as remove liposomes from the surface of nearly half the surface of the cell.
4.4. Documentation of Microstreaming and Fluid Flow around Microbubbles
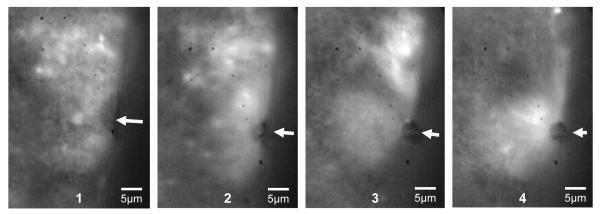
The fluid motion effects of a microbubble exposed to a long duration pulse of ultrasound are shown in Fig. 7. The microbubble is shown as the large dark circle in the center of the image in frame 1. It is surrounded by non-echogenic fluorescent liposomes which follow streamlines of fluid motion created around the oscillating microbubble when exposed to ultrasound. The particle shown by the arrow in frame 1 is tracked through frames 2-4 showing a circular flow pattern that drags particles along the edge of the bubble and sends them down towards the bottom of the frame away from the microbubble. These types of microstreaming interactions with cell surfaces are of great interest when considering ultrasound pulse sequence and vehicle design.
FIG. 7.
Fluorescent image sequence of particles caught in streamlines of fluid motion created around the microbubble during ensonation with ultrasound. The microbubble is shown as the dark circle in the middle of the frame. A single fluorescent particle is tracked by the white arrows from frame 1-4. The entire sequence is taken during the ensonation period of the ultrasound.
The region over which the microbubble microstreaming occurs can be much larger than the microbubble itself as shown in Fig. 8. The arrow points to a single microbubble which is ensonated with focused ultrasound creating a flow pattern that has a diameter 6 times larger than the diameter of the microbubble itself. A large cluster of fluorescent lipids shown in the upper portion of frame 3 are pulled into the vortex motion of the fluid around the microbubble as seen in frame 4.
FIG. 8.
Fluorescent image sequence showing microstreaming around a dark circular microbubble pointed out by the arrows in each frame. Frame 1 shows the field of non-echogenic liposomes before ultrasound application. Frame 2 shows the microstreaming flow field starting at the beginning of ensonation. The diameter of the flow field is 6 times larger than the diameter of the microbubble itself and can pull in larger clusters of fluorescent liposomes as seen in frames 3 and 4.
5. DISCUSSION
The instrument described here for the first time combines fluorescent imaging, focused ultrasound, and high speed videography to study interaction of an emerging class of echogenic drug delivery vehicles with adherent cell membranes. Tracking the motion and fragmentation of lipid membranes with only white light illumination is very difficult due to insufficient contrast between the membrane and the surrounding aqueous fluid. The use of lipophilic fluorescent dyes, such as DiO, highlights the liposome membrane creating excellent contrast with the background. We have shown here that fluorescent membrane fragments below 1 μm in diameter can be effectively tracked over time after being created by inertial cavitation of internal and nearby microbubbles. The use of fluorescent liposomes also allows the liposome membranes to be differentiated from nearby cell membranes allowing the interaction between cells and liposomes to be monitored. This includes the removal of biochemically targeted liposomes from the surface of the cells due to cavitation events, and also the documentation of lipid fragments that are left behind on the surface resulting in delivery of membrane material to the cell surface.
The use of fluorescent particles also allows the fluid flow created by ultrasound induced microstreaming around the microbubbles to be effectively visualized. This allows the debris field resulting from cavitation to be quantified as well as monitoring the extent of fluid flow from microstreaming.
This information about fluid flow and membrane behavior is invaluable when designing the properties of the drug delivery vehicles. The membrane composition including the types of lipids and cholesterol content can significantly change the flexibility and stability of the membrane. The degree of flexibility affects the entire structure’s interaction with ultrasound driven microbubble behavior. The use of this analysis system allows the user to screen different compositions to achieve the desired interaction of these vehicles with individual live cells in real-time
The ultrasound pulse sequence and intensity can also have a significant effect on the behavior of these delivery vehicles. The arbitrary waveform generator allows the user to create a set of different pulse sequences that can be screened for the desired results. For example the 1.5 MPa ultrasound pulse sequence shown here resulted in complete fragmentation of the delivery vehicle, while lower intensities below 1 MPA resulted in a rupture and slow unfolding of the outer membrane.
Highlights.
We have developed a new instrument to monitor microbubble interactions with cells
Combines high speed camera and fluorescence microscopy with focused ultrasound
Use of fluorescent dyes in membranes to track debris formation
Allows many different phenomena and their interaction with live cells to be studied
ACKNOWLEDGMENTS
The authors are grateful for the insightful discussions with Eugene Cho while designing this system. The study was supported by the NCI Grant No. 5U54CA119335-05, and by the UCSD Cancer Center Specialized Support Grant P30 CA23100.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gao AM, et al. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2007;48(4):260–270. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery - a general review. Expert Opin. Drug Delivery. 2004;1(1):37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang S-L. Liposomes in ultrasonic drug and gene delivery☆. Advanced Drug Delivery Reviews. 2008;60:1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki R, et al. Effective gene delivery with novel liposomal bubbles and ultrasonic destruction technology. International Journal of Pharmaceutics. 2008;354:49–55. doi: 10.1016/j.ijpharm.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki R, et al. Tumor specific ultrasound enhanced gene transfer in vivo with novel liposomal bubbles. Journal of Controlled Release. 2008;125:137–144. doi: 10.1016/j.jconrel.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Wrenn S, et al. Controlling Cavitation for Controlled Release. Proc. IEEE Int. Ultrason. Symp. 2009:104–107. [Google Scholar]
- 7.Huang S, MacDonald R. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochemica et Biophysica Acta. 2004;1665:134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Ibsen S, et al. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. Journal of Controlled Release. 2011;155(3):358–366. doi: 10.1016/j.jconrel.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanelli CI, et al. Beamforming for Therapy with High Intensity Focused Ultrasound (HIFU) Using Quantitative Schlieren. IEEE Ultrasonics Symposium. 1993:1233–1238. [Google Scholar]
- 10.Chin CT, et al. Brandaris 128: A digital 25 million frames per second camera with 128 highly sensitive frames. Review of Scientific Instruments. 2003;74(12):5026–5034. [Google Scholar]
- 11.Garbin V, et al. Changes in microbubble dynamics near a boundary revealed by combined optical micromanipulation and high-speed imaging. Applied Physics Letters. 2007;90(11) [Google Scholar]
- 12.Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J. Acoust. Soc. Am. 2002;112(5):2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 13.Crum LA. Surface Oscillations and Jet Development in Pulsating Bubbles. Journal De Physique. 1979;40(11):285–288. [Google Scholar]
- 14.Bouakaz A, Versluis M, Jong N.d. High-speed optical observations of contrast agent destruction. Ultrasound in Medicine & Biology. 2005;31(3):391–399. doi: 10.1016/j.ultrasmedbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Dayton PA, et al. Optical and Acoustical Dynamics of Microbubble Contrast Agents inside Neutrophils. Biophysical Journa. 2001;80:1547–1556. doi: 10.1016/S0006-3495(01)76127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomas JE, et al. Threshold of fragmentation for ultrasonic contrast agents. Journal of Biomedical Optics. 2001;6(2):141–150. doi: 10.1117/1.1352752. [DOI] [PubMed] [Google Scholar]
- 17.Ward M, Wu J, Chiu J-F. Experimental study of the effects of optison® concentration on sonoporationin vitro. Ultrasound in Medicine & Biology. 2000;26(7):1169–1175. doi: 10.1016/s0301-5629(00)00260-x. [DOI] [PubMed] [Google Scholar]
- 18.Ward M, Wu J, Chiu J-F. Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents. J. Acoust. Soc. Am. 1999;105(5):2951–2957. doi: 10.1121/1.426908. [DOI] [PubMed] [Google Scholar]
- 19.Miller MW, Miller DL, Brayman AA. A Review of In Vitro Bioeffects of Inertial Ultrasonic Cavitation from a Mechanistic Perspective. Ultrasound in Med. & Biol. 1996;22(9):1131–1154. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- 20.Karshafian R, et al. Ultrasound-Induced Uptake of Different Size Markers in Mammalian Cells. IEEE Ultrasonics Symposium. 2005:13–16. [Google Scholar]
- 21.Ross JP, et al. Optical and atomic force microscopic studies on sonoporation. The Journal of the Acoustical Society of America. 2002;111(3):1161–1164. doi: 10.1121/1.1448340. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y-Z, et al. Phospholipids-based microbubbles sonoporation pore size and reseal of cell membrane cultured in vitro. Journal of Drug Targeting. 2008;16(1):18–25. doi: 10.1080/10611860701637792. [DOI] [PubMed] [Google Scholar]
- 23.Wolfrum B, et al. Observations of pressure-wave-excited contrast agent bubble in the vicinity of cells. Applied Physics Letters. 2002;81(26):5060–5062. [Google Scholar]
- 24.Kudo N, et al. Study on Mechanism of Cell Damage Caused by Microbubbles Exposed to Ultrasound. IEEE Ultrasonics Symposium. 2002:1383–1386. [Google Scholar]
- 25.Okada K, et al. A basic study on sonoporation with microbubbles exposed to pulsed ultrasound. J Med Ultrasonics. 2005;32:3–11. doi: 10.1007/s10396-005-0031-5. [DOI] [PubMed] [Google Scholar]
- 26.Sabens D, et al. Calcium Imaging of Sonoporation of Mammalian Cells AIP Conf. Proc. 2005;829:533–537. [Google Scholar]
- 27.Miller DL, Quddus J. Sonoporation of Monolayer Cells by Diagnostic Ultrasound Activation of Contrast-Agent Gas Bodies. Ultrasound in Med. & Biol. 2000;26(4):661–667. doi: 10.1016/s0301-5629(99)00170-2. [DOI] [PubMed] [Google Scholar]
- 28.Han YW, et al. Sonoporation Is an Efficient Tool for Intracellular Fluorescent Dextran Delivery and One-Step Double-Crossover Mutant Construction in Fusobacterium nucleatum. Appl. Environ. Microbiol. 2007;73(11):3677–3683. doi: 10.1128/AEM.00428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wamel A.v., et al. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. Journal of Controlled Release. 2006;112:149–155. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423:153–156. doi: 10.1038/nature01613. [DOI] [PubMed] [Google Scholar]
- 31.Miller DL, Thomas RM. Ultrasound Contrast Agents Nucleate Inertial Cavitation In Vitro. Ultrasound in Med. & Biol. 1995;21(8):1059–1065. doi: 10.1016/0301-5629(95)93252-u. [DOI] [PubMed] [Google Scholar]