Abstract
The aim of the present study was to determine the effects of fat- and sugar-rich diets in utero and during the pre-weaning period on bodyweight and responses to drugs of abuse. In Exp. 1, dams were fed a balanced control diet or high-fat diet (HFD), and female offspring were cross-fostered to dams consuming the balanced diet. The HFD-exposed offspring, compared to controls, were heavier in body weight, had increased circulating triglyceride levels, and consumed more alcohol and HFD in adulthood. In Exp. 2, dams were fed standard chow alone or standard chow plus a 16% high-fructose corn syrup (HFCS) or 10% sucrose solution. Sets of offspring from each group were cross-fostered to dams in the other groups, allowing for the effects of HFCS or sucrose exposure during the gestational period or pre-weaning period to be determined. The offspring (both female and male) exposed to HFCS or sucrose in utero had higher body weights in adulthood and exhibited increased alcohol intake as shown in female offspring and increased amphetamine-induced locomotor activity as shown in males. Exposure to HFCS or sucrose only during the pre-weaning period had a similar effect of increasing amphetamine-induced locomotor activity in males, but produced no change in circulating triglycerides or alcohol intake. Collectively, these data suggest that prenatal as well as pre-weaning exposure to fat- and sugar-rich diets, in addition to increasing body weight, can affect responses to drugs of abuse.
Keywords: Prenatal diet, perinatal nutrition, obesity, drug use, palatable diet, rat
1. Introduction
Studies have focused on the effects of maternal diets on the body weight of the offspring, uncovering potential epigenetic obesity factors [1–3]. Over- and undernutrition in utero are found to have similar effects, with studies in rats and humans showing undernutrition during select periods of prenatal development to increase body weight of the offspring [4–6] and perinatal overnutrition to increase both food intake and body weight [7–14]. Further studies have identified brain mechanisms that might, in part, explain these effects, with maternal obesity or excessive consumption of a highly-palatable diet being linked to changes in hypothalamic neuropeptides and circulating levels of triglycerides that affect food intake in the offspring [10, 15–18].
Although prenatal nutrition studies have focused mainly on aberrant metabolic outcomes in the offspring, recent studies have begun to assess the influence that specific diets in utero can have on their appetitive behavior. This extension of focus from metabolic to reward mechanisms has been, in part, spurred by studies showing that prenatal diets can affect the offspring’s preference for palatable foods [10, 11, 19, 20], while producing concomitant aberrations in reward pathway-related neurochemicals and expression of the associated genes [19, 21, 22]. Given this suggestion that highly-palatable diets in utero can sensitize several reward-related brain systems, the present study aimed to assess drug reactivity in animals exposed prenatally to palatable diets.
Further, previous studies have shown that the effects of high-energy diets during gestation differ from those that are seen when such diets are given during the pre-weaning stage [23–25]. This suggests that exposure to palatable foods might differentially affect behavior in the offspring depending on whether the animals are exposed in utero or during nursing. Thus, the present study was designed to isolate the possible effects of palatable-diet exposure during the gestational versus lactation periods, in order to see their independent effects on drug reactivity.
Lastly, obesity and being overweight are pre-existing conditions in 40% of women who are initiating pregnancy [26]. This increased body weight is most likely due to excess intake of highly-caloric, palatable foods, which often contain both fat and sugar and are craved by women during pregnancy [27]. Also, these different nutrients, fats vs. sugars, have been shown to differentially affect brain reward systems [28]. Therefore, in the present study, we tested different palatable foods that are either predominantly fat or predominantly carbohydrate (sugar). We also compared two different sweeteners, sucrose vs. high-fructose corn syrup (HFCS), since some studies suggest that there may be differences in their metabolic effects [29, 30].
2. Materials and Methods
Animals
Pregnant Sprague-Dawley rats (220–240 g) from Charles River Breeding Laboratories (Hartford, CT) were delivered to the animal facility on embryonic day 5 (E5). The dams were individually housed in flat-bottom plastic cages, in a fully AAALAC-accredited facility (room temperature: 22°C, with a 12:12 h light-dark cycle with lights off at 0600 h), according to protocols as specified in the NIH Guide to the Care and Use of Animals and also with approval of the Princeton University Animal Care and Use Committee.
2.1 Experiment 1: The effects of high-fat diet vs. control diet exposure in utero on triglyceride levels and ingestive behavior
Dams were maintained with ad libitum access, from E6 onwards, to either a high-fat diet (5.15 kcal/g; HFD), or a control, balanced diet (4.29 kcal/g; CD). Both diets were nutritionally complete and prepared in the laboratory [10]. Diet composition was calculated as percent of total energy, with the HFD containing 50% fat, 25% carbohydrate, and 25% protein and the CD containing 25% fat, 50% carbohydrate, and 25% protein. In this study, a CD was used, rather than a low-fat diet, in order to mimic those levels of fat recommended for human consumption. Standard lab chow (Purina Rodent Chow 5001) was also available for 3 days (until E9), while the dams adapted to the mixed diet. Over the course of the experiment, dams’ food intake were measured three times per week, and body weights were recorded weekly. On postnatal day 1 (P1), litters were culled (n=10/litter), and pups were cross-fostered to dams on a CD. Pups born to CD dams were cross-fostered to different CD dams. On P22, female pups were weaned and maintained on ad libitum rodent chow (n=12–13/group). The male offspring were used in a different study.
Alcohol training
Beginning on P25, rats were trained to drink unsweetened alcohol [31], which was offered for 12-h daily (starting 4-h into the dark period); every 4–8 days, the concentration of ethanol was increased in the following manner: 1%, 2%, 4%, 7%, 9%, 10%, 12% (v/v). Standard chow and water were available ad libitum. Alcohol was measured daily at the 9–12% concentrations, and chow intake was measured every 4 days, which allowed for chow at each concentration of alcohol to be analyzed.
Triglyceride analysis
On day P69, blood samples were obtained 1 h after alcohol access (5 h into the dark cycle) using methods previously published [32], to be tested for serum triglyceride levels (Triglyceride Assay kit; Sigma-Aldrich Co., St. Louis, MO). This was done because increased triglyceride levels have been associated with alcohol [33] as well as HFD intake [34], and have been noted in animals exposed to HFD in utero [7, 35].
HFD intake
Following alcohol tests, intake of HFD was measured using the same HFD diet that was given to designated dams during pregnancy. On P69 (after the blood sample collection described above), chow was removed, and rats were given 1 h access to the HFD, starting 5 h into the dark cycle (no standard chow was available during the tests, but water was available). This daily 1-h access to HFD was given for 5 consecutive days, and intake was averaged across the final 3 days of access.
2.2 Experiment 2: The effects of sucrose vs. HFCS exposure in utero and pre-weaning on alcohol intake (females) and amphetamine-induced locomotor activity (males)
As in Exp. 1, pregnant rats were delivered on E5 from Charles River Breeding Facility. Upon delivery, the experimental groups were given ad libitum access to rodent chow (Purina, 3.02 kcal/g, 13.5% fat, 58% carbohydrate, and 28.5% protein), water and either a 10% sucrose solution (0.4 kcal/mL; w/v) or 16% HFCS solution (0.48 kcal/mL; Nature’s Flavors; v/v), which were selected because of similar caloric value and palatability. The control group was fed standard chow and water only. Dams’ sugar intake was measured daily, and food intake and body weight were measured every 3 days.
On P1, litters were culled (n=8/litter), and pups were cross-fostered such that rats born to HFCS or sucrose-consuming dams were nursed by chow-fed dams, and likewise, in order to assess the effects of exposure to these diets during the pre-weaning period, rats born to chow dams were nursed by HFCS or sucrose-consuming dams (Fig. 1). Pups born to dams in the control group were cross-fostered to different chow-fed dams. On P22, all pups were weaned, individually housed and maintained on ad libitum rodent chow (n=12 males/group, n=12 females/group). Body weights and food intake were recorded weekly for the duration of the experiment, with selected time points used to analyze chow intake (P45 and P85).
Fig 1.
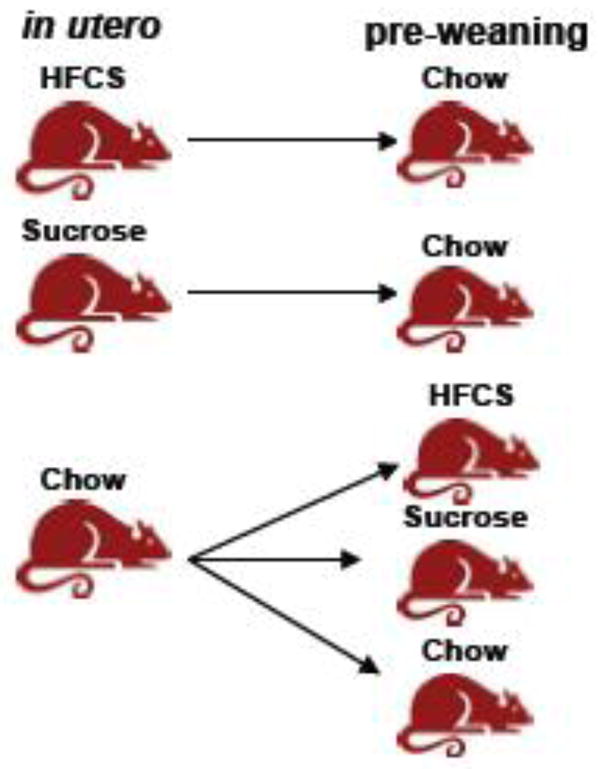
Diagram of the cross-fostering procedures used in Exp. 2. Rats born to chow-fed, HFCS-fed or sucrose-fed dams were cross-fostered to allow for the independent assessment of diet during gestation or pre-weaning on behavior, body weight and triglyceride levels.
Alcohol training
Beginning on P25, female rats were trained to drink unsweetened alcohol as described in Exp. 1. This was done to see if the effects of a high fat, palatable-diet in utero on alcohol intake (Exp. 1) extended to other palatable foods, such as sugars, and also to assess the effects of exposure to such diets during the nursing period on future alcohol consumption.
Triglyceride analysis
On P60 blood was obtained from the female rats’ tails, as done in Exp. 1, and serum was analyzed for triglyceride levels (Cayman Chemicals, kit #10010303). Serum was also obtained at the end of the study (P180) to again analyze triglyceride levels. Blood was also collected on P58 and P180 for the male rats for assays.
Amphetamine-induced locomotor activity testing
Beginning on P170, male rats were tested for amphetamine-induced locomotor activity. Locomotor activity was measured in a computerized 43.2 × 43.2 cm, open-field activity chamber with 30.5 cm high acrylic sidewalls and 16 infrared photocells on each of the three axes (MED Associates, Georgia, VT). Testing occurred 5–10 h into the dark cycle. Rats were allowed to habituate to the activity chamber for 15 min. Then, they were administered an i.p. injection of saline and locomotor activity was measured for 30 min, in order to establish a baseline activity level that controls for the handling and injection procedures. Locomotor counts were quantified as the number of infrared beam breaks. Each rat was then administered 0.5 mg/kg, i.p., amphetamine sulfate (Supreme Pharmaceutical Co., New York, NY, USA) dissolved in saline. Animals were placed back in the activity chamber for 15 min for the drug to take effect, and then locomotor activity was again measured for 30 min. Data were analyzed individually for each rat, comparing the change in locomotor activity in response to saline vs. amphetamine.
2.3 Statistical Analysis
Body weight (g) and alcohol intake data (g of alcohol/kg rat body weight) were analyzed with repeated measures ANOVA, with post hoc Student’s t-test, Tukey test, or one-way ANOVA, as appropriate. Triglyceride levels, HFD intake, and locomotion were analyzed using a one-way ANOVA (with post hoc t-tests) or independent group t-tests, as appropriate. To control for group differences in baseline activity, the activity levels following amphetamine injection were calculated by normalizing the data to the individual rats’ saline-injection baseline.
3. Results
3.1 Experiment 1: HFD in utero increases body weight, HFD intake, alcohol intake and circulating triglyceride levels in female offspring
Nutrition of the dams
During pregnancy, there was no difference between groups in body weight (F(1,13)=1.95, p=n.s.; E21: CD= 328.4 ± 12.2 g, HFD= 342.9 ± 16.1 g) or average daily kcal intake (F(1,12)= 3.54, p=n.s.; mean daily intake E10–E21: CD= 64.4 ± 4.6 kcal, HFD= 78.7 ± 6.0 kcal). There was also no difference in number of pups per litter between groups (F(1,11)=0.68, p=n.s.; CD=12.1 ± 0.6 pups, HFD=13.0 ± 1.0 pups).
HFD in utero is associated with increased body weight in adulthood
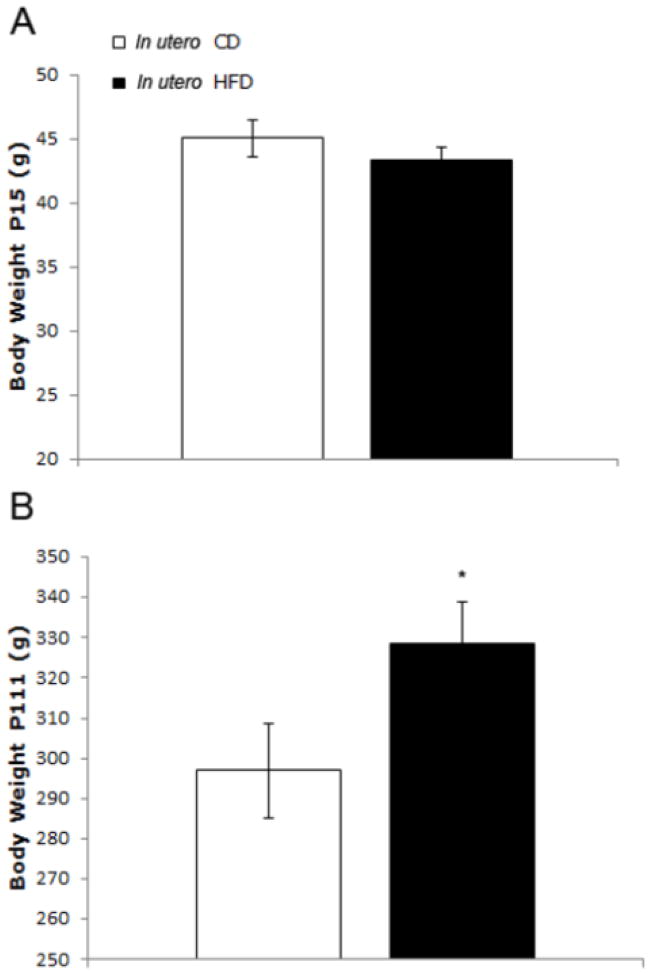
Before weaning (P15), there was no difference in body weight between rats exposed to CD vs. HFD in utero (t(24)=0.95, p=n.s., Fig 2A). However, post weaning (P22 and onward), there was a main effect of time (F(12,240)=760.47, p<0.0001) and an interaction between time and group (F(12,240)=2.47, p<0.01; Fig. 2A). By the end of the study (P111), those rats with in utero exposure to HFD had increased body weights compared to rats born to CD dams (t(22)=2.08, p=0.05).
Fig 2.
Body weight of female offspring from Exp. 1 fed either a control diet (CD) or a high-fat diet (HFD) in utero. While there was no significant difference in body weight at P15 (A), by P111 (B) the offspring of the HFD-fed group weighed significantly more than the CD-fed control offspring. *p<0.05, error is SEM.
HFD in utero is associated with increased alcohol intake, but does not affect chow intake
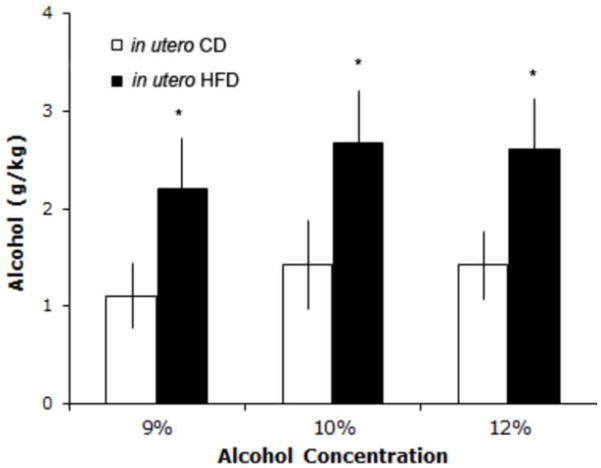
There was a main effect of prenatal dietary fat content on the offspring’s alcohol intake (F(2,42)=3.24, p=0.05) but no interaction between alcohol concentration and group (F(2,42)=0.01, p=n.s.). However, a significant group difference was noted (F(1,21)=4.58, p<0.05), with post hoc Student’s t-tests at each alcohol concentration showing the HFD-exposed rats to consume significantly more unsweetened alcohol at the 9% (t(21)=2.08, p=0.05), 10% (t(21)=2.09, p<0.05) and 12% (t(21)=2.12, p<0.05) concentrations as compared to the CD-exposed offspring (Fig. 3). There were no group differences in their average daily water intake (e.g., at 9% alcohol: t(24)=0.03, p=n.s., CD=17.2 ± 1.7 mL, HFD=17.2 ± 1.6 mL), average daily chow intake (e.g., at 9% alcohol: t(24)=0.48, p=n.s., CD= 59.4 ± 2.1 kcal, HFD= 61.4 ± 3.8 kcal), or total daily caloric intake (alcohol kcal + chow kcal; e.g., at 9% alcohol, t(23)= 1.29, p=n.s.; CD= 59.7 ± 2.1 kcal, HFD= 64.4 ± 3.0 kcal).
Fig 3.
Alcohol intake of rats from Exp.1. Female offspring of high fat diet (HFD)-fed dams consumed more unsweetened alcohol at 9, 10, and 12% during the 12-h access period, compared to offspring born to control dams (CD). *p<0.05, error is SEM.
HFD in utero is associated with increased circulating triglyceride levels in adulthood
Measurements of circulating triglycerides at P69 showed significantly higher levels in the offspring with in utero exposure to HFD compared to those with CD exposure (t(24)=2.7, p<0.05; 59.0 ± 3.5 mg/dL vs. 42.5 ± 16.5 mg/dL, respectively).
HFD in utero is associated with increased intake of HFD in adulthood
When measured at P71–P73, rats with in utero exposure to HFD showed greater intake of HFD in the 1-h access period over the 3-day test, compared to those animals with in utero exposure to CD (t(23)=2.3, p<0.05, mean 1-h intake: 27.5 ± 0.6 kcal vs. 24.8 ± 1.0 kcal, respectively).
3.2 Experiment 2: The effects of sucrose vs. HFCS exposure in utero and pre-weaning on alcohol intake and amphetamine-induced locomotor activity
Nutrition of the dams
Dams were given access to chow alone or chow together with a HFCS or sucrose solution during pregnancy. There was no difference in body weight between groups during pregnancy (F(2,14)= 0.73, p=n.s.; E21: chow= 383.3 ± 8.7 g, HFCS= 365.7 ± 4.9 g, sucrose= 376.4 ± 6.0 g) or while nursing (F(2,14)=1.03, p=n.s.; P8: chow= 323.1 ± 5.5 g, HFCS= 308.3 ± 4.0 g, sucrose= 319.3 ± 9.6 g). There was, however, a difference in average total daily kcal consumed (F(2,14)=6.22, p<0.05), with the sucrose-consuming dams ingesting significantly more kcal (84.9 ± 4.0) than the chow- (73.3 ± 2.4 kcal, p<0.001) or HFCS-consuming dams (65.2 ± 4.6 kcal, p<0.05). Both the sucrose and HFCS groups had lower daily chow intake relative to the chow control group (Chow: 73.3 ± 2.4 kcal, Sucrose: 47.1 ± 5.2 kcal, HFCS: 43.7 ± 4.0 kcal).
Body weight of offspring
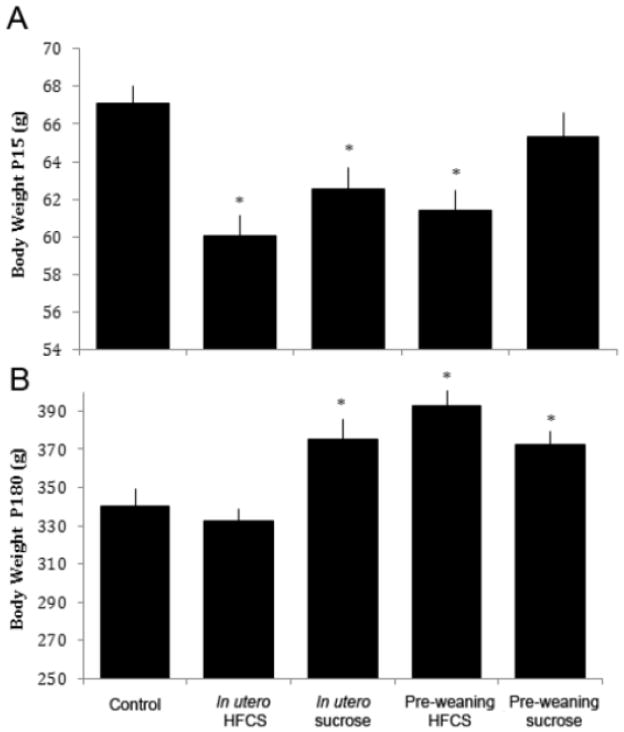
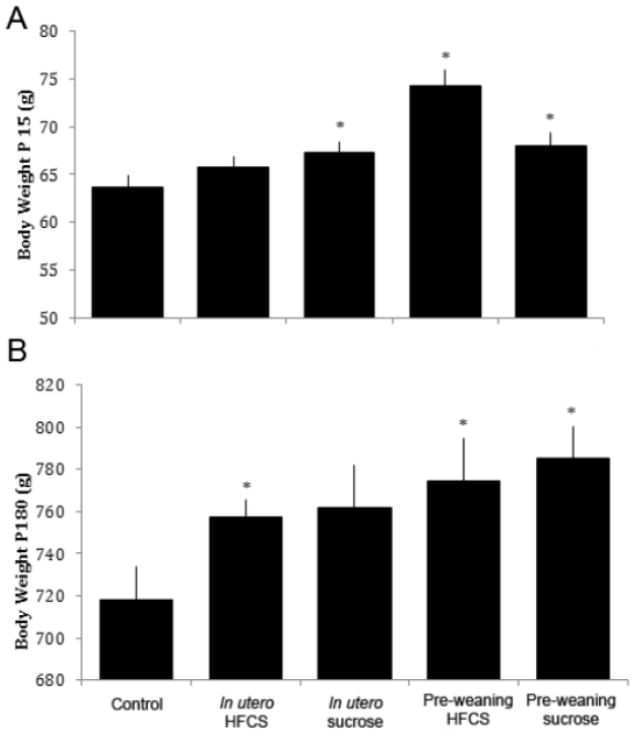
Cross-fostering of the offspring resulted in five groups (Fig.1) (1) HFCS in utero/chow weaning, (2) sucrose in utero/chow weaning, (3) chow in utero/HFCS weaning, (4) chow in utero/sucrose weaning, and (5) chow in utero/chow weaning. There was a significant difference between the groups in terms of pre-weaning body weight (P15; females: F(4,58)=6.62, p<0.001; Fig. 4A; males: F(4,59)=9.31, p<0.001; Fig. 5A). While females born to dams fed HFCS (p<0.01) or sucrose (p<0.01), or nursed by HFCS-fed dams (p<0.01) had decreased body weight compared to offspring born to chow-fed dams, the opposite change was seen in the males, with those born to dams fed sucrose (p<0.05) or nursed by sucrose- (p<0.05) or HFCS-fed (p<0.001) dams weighing significantly more compared with those born to chow-fed dams.
Fig 4.
Body weight of female rats from Exp. 2. (A) At P15, rats exposed to HFCS or sucrose in utero, or HFCS during the pre-weaning phase, had decreased body weight relative to chow-fed controls. (B) At P180, those rats that were born to dams given sucrose during gestation, or fed HFCS or sucrose during the pre-weaning stage, were heavier than controls. *p<0.05, error is SEM.
Fig 5.
Body weight of male rats from Exp. 2. (A) At P15, rats exposed to sucrose in utero, or HFCS or sucrose during the pre-weaning phase, had increased body weight relative to chow-fed controls. (B) At P180, rats born to dams given HFCS during gestation, or fed HFCS or sucrose during the pre-weaning stage, were heavier than controls. *p<0.05, error is SEM.
After weaning, there was a main effect of time (female: F(10,510)=2954.51, p<0.001, male: F(10,540)=6359.31, p<0.001) and group (female: F(4,51)=9.89, p<0.001, male: F(4,54)=4.77, p<0.005), as well as an interaction between time and group (female: F(40,510)=4.46, p<0.001, male: (F(40,540)=1.88, p=0.001). Post hoc tests revealed that, at the end of the study (P180), males and females nursed by HFCS- or sucrose-fed dams had increased body weights compared to chow-fed controls (p<0.05, Fig. 5B; p<0.001, Fig. 4B respectively). Further, females born to sucrose-fed dams and males born to HFCS-fed dams weighed significantly more than those animals born to the chow-fed controls.
Serum triglyceride levels were different in offspring
For the male offspring, at P58 there was a statistically significant difference among the groups, with males nursed by HFCS dams having elevated serum triglyceride levels (F(4,59)=4.64, p=0.003; Chow= 140.4 ± 11.1 mg/dL, pre-weaning HFCS= 204.5 ± 10.9 mg/dL, pre-weaning sucrose= 150.2 ± 12.2 mg/dL, in utero sucrose= 128.6 ± 14.6 mg/dL, in utero HFCS= 163.3 ± 17.3 mg/dl). This difference, however, was no longer apparent when blood was collected again on P180 (F(4,59)= 2.27, p=n.s.). Also, there was no difference in serum triglyceride levels among the female offspring, either on P55 (F(4,58)=0.84, p=n.s.) or at the end of the study (P180; (F(4,57)=1.66, p=n.s.).
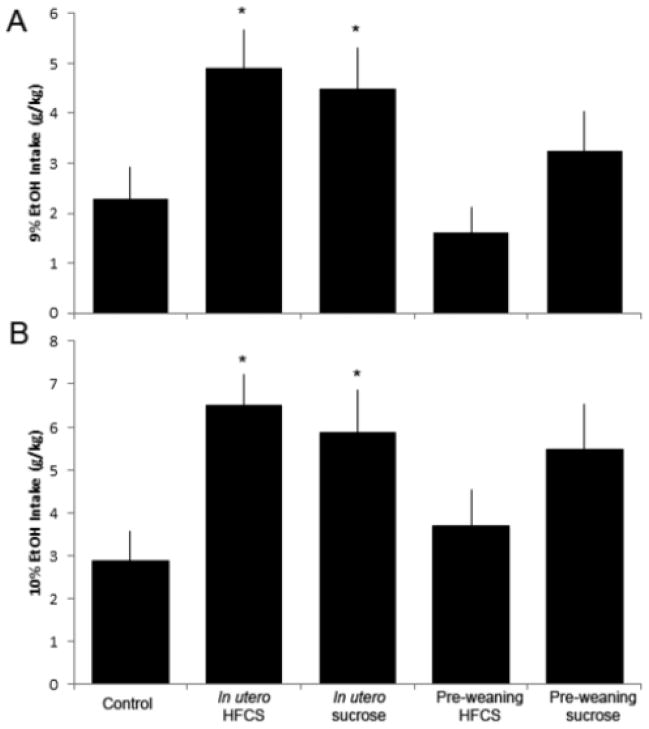
Sucrose or HFCS in utero is associated with increased alcohol intake in female rats
With these tests performed in the female rats, there was a significant difference between groups in terms of alcohol intake at the 9% and 10% concentrations (F(4,57)=3.7, p<0.01, F(4,56)=3.0, p<0.03, respectively; Fig. 6). Pair-wise comparisons indicated increased alcohol intake only in the rats with sucrose or HFCS exposure in utero compared to controls (p<0.05) There were no differences in water intake between groups (e.g., at 9% alcohol, water: (F(4,57)=1.49, p=n.s., Chow= 32.7 ± 3.5 mL, pre-weaning HFCS= 38.2 ± 3.8mL, pre-weaning sucrose= 34.3 ± 3.5 mL, in utero sucrose= 26.5 ± 5.4 mL, in utero HFCS= 18.6 ± 5.4 mL).
Fig 6.
Alcohol intake of rats from Exp. 2. Female rats exposed to sucrose or HFCS in utero, compared to controls, consumed more alcohol during the 12-h access period at the 9%, 10% and 12% concentrations. *p<0.05, error is SEM.
Chow intake differences were noted in female rats
Differences in chow intake were also noted in the female rats (P45: (F(4,58)=4.23, p<0.005) and P85:(F(4,58)=5.9, p<0.001)), with pair-wise comparisons indicating that those rats born to the HFCS-consuming dams ingested less chow than those born to chow-fed controls (p<0.001; Table 1). There was no difference in chow intake between the male groups (P45: (F(4,55)=1.14, p=n.s.), P85: (F(4,55)=1.92, p=n.s.)).
Table 1.
Standard chow intake of offspring from Experiment 2
| Chow Intake (kcal) | ||||
|---|---|---|---|---|
| P45 | P85 | |||
| Group | Females | Males | Females | Males |
| Control | 58.7 ± 0.9 | 80.9 ± 1.2 | 61.9 ± 0.9 | 96.6 ± 2.7 |
| In utero HFCS | 53.2 ± 0.9* | 80.6 ± 1.5 | 55.3 ± 1.8* | 103.9 ± 3.3 |
| In utero Sucrose | 59.2 ± 1.5 | 83.7 ± 1.5 | 62.8 ± 0.6 | 103.6 ± 1.2 |
| Pre-weaning HFCS | 58.5 ± 1.2 | 83.7 ± 1.2 | 60.1 ± 1.2 | 106.3 ± 2.7 |
| Pre-weaning Sucrose | 58.8 ± 1.2 | 81.2 ± 1.8 | 59.5 ± 1.2 | 106.0 ± 2.4 |
Significantly different than the Control group (p<0.05).
Rats born to or nursed by sucrose or HFCS dams, compared to those born to chow-fed controls, were hyperactive in response to amphetamine challenge
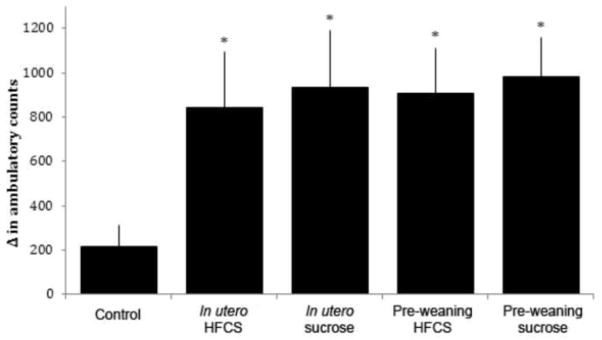
Additional analysis examining amphetamine-induced locomotor activity showed differences in baseline locomotor activity between groups (F(4,59)=3.87, p<0.01), with post hoc Tukey tests indicating that the rats born to sucrose-consuming dams were hypoactive compared with all the other groups (p=0.05). After normalizing the activity counts to the individual rat’s baseline, there was a significant difference between groups in terms of amphetamine-induced locomotor activity (F(4,57)=2.67, p<0.05, Fig. 7). Post hoc Student’s t-tests indicated that rats born to or nursed by sucrose and HFCS dams were hyperactive compared to those born to chow-fed controls (pre-weaning HFCS: p<0.01; pre-weaning sucrose: p<0.02; in utero HFCS: p<0.05; in utero sucrose: p<0.05).
Fig 7.
Locomotor activity in response to an amphetamine challenge in male offspring. Rats exposed to HFCS or sucrose in utero or during the pre-weaning period were hyperactive following a 0.5 mg/kg dose of amphetamine, relative to controls. *p<0.05, error is SEM.
4. Discussion
Despite the fact that critical brain development is known to occur during the perinatal period, we understand relatively little about the effects that nutrition during this time can have on behavior later in life. The present study adds to the growing literature suggesting that the type of diet offered to dams during pregnancy or while nursing can have long-lasting effects on the body weight and behaviors exhibited by the offspring. In particular, the findings from this study suggest that rats exposed to a HFD in utero, compared to a balanced diet, weigh more as adults, have higher levels of triglycerides, and drink more alcohol. Further, rats exposed prenatally to sucrose or HFCS also weigh more but have normal levels of circulating lipids, and they exhibit an increase in both alcohol intake and amphetamine-induced locomotor activity. This stimulatory effect on body weight and responsiveness to amphetamine is also seen after exposure to HFCS or sucrose during the pre-weaning period. This suggests that access to highly palatable foods during critical developmental periods can have long-lasting effects on offspring ingestive behavior. These data complement earlier findings, which show that maternal obesity or excess fat-intake can promote increased body weight in the offspring, as well as increase preference for palatable foods [7–13, 19, 20]. They also provide additional behavioral support for the alterations in brain-reward regions that have previously been reported in offspring exposed to highly-palatable diets in utero [10, 15–18, 21, 22].
4.1. Palatable-diet exposure in utero or during pre-weaning affects the body weight of the offspring
Both under- and over-nutrition during the perinatal period are risk factors for the development of obesity and metabolic syndrome in adulthood [36], and likewise, low body weight at birth has been associated with increased risk for metabolic syndrome and obesity later in life [37, 38]. In the present study, we observed selected decreases in pre-weaning body weights. In Exp. 2, despite the fact that dams were maintained on calorically-sufficient, nutritionally-complete diets during pregnancy and nursing, female offspring born to dams fed HFCS or sucrose, or nursed by HFCS dams, had low body weights compared to chow-fed rats at P15. This effect of perinatal diet leading to decreased pre-weaning body weights has been reported by others in the offspring of animals nursed by “junk-food”-consuming dams [11, 21]. Some of the dams consuming sucrose or HFCS may have, in fact, been undernourished, given their depressed daily caloric intake of the nutritionally-complete rodent chow compounded by the large proportion of calories derived from the sugar supplement. It has been suggested that decreased pre-weaning bodyweight might be driven by decreased protein intake, which has been linked to lower birth weight as well as perturbations in mesocortical-DA controlled behaviors [38, 39]. By decreasing daily chow intake to compensate for increased calories obtained from sugar, the dams in Exp. 2 were essentially decreasing their protein intake, potentially contributing to the low body weights observed prior to weaning. This idea that the supplemental sugar access may result in a nutrient deficiency is further supported by the findings from Exp. 1, showing that the offspring of dams consuming a nutritionally-complete, fat-rich diet maintained normal body weights prior to weaning.
It is interesting that this effect of diet-induced, low pre-weaning weight was seen only in the female offspring. Most of the male offspring from Exp. 2 were, in fact, heavy prior to weaning and into adulthood compared to offspring with prenatal and pre-weaning exposure to chow. Studies suggest that there are sex-dependent differences in the metabolic adaptations to diet in utero. Recently, it has been shown that female offspring are more vulnerable to the adverse, long-term consequences of low body weight at birth [40]. Also, early work showed that prenatal undernutrition at select times during gestation can produce delayed-onset obesity in male offspring but not females [4]. Thus, there appear to be sex-dependent as well as gestational-time-dependent differences in the effects of diet during the perinatal period, underscoring the role of sex in the transmission and inheritance of traits that are generated in the perinatal environment [41].
Despite the fact that pre-weaning body weights were normal in the rats from Exp. 1 on a HFD, the offspring were significantly heavier as adults compared to the offspring of the control-fed dams. This is in alignment with the literature, which reports a HFD during the perinatal period leading to increased body weight later in life, preference for high-fat foods, and alterations in reward-related gene expression [7, 10, 19]. Later on, as adults, some groups from Exp. 2, exposed to sucrose in utero or nursed by HFCS-fed dams, were also overweight compared to controls, underscoring that sugar-rich food can also promote obesity later in life. Collectively, these studies and the present findings suggest that there may be multiple mechanisms through which diet during the perinatal period can promote increased body weight later in life. Maternal nutrition-deficiency could decrease pre-weaning body weight, while HFD exposure could induce brain changes that lead to hedonically-driven, food seeking later in life.
4.2. Palatable-diet exposure in utero affects triglyceride levels, HFD and alcohol intake in offspring
In Exp. 1, HFD in utero was associated with increased triglyceride levels in the offspring. Hyperlipidemia is a symptom of metabolic syndrome and often seen in obesity [42]. Further, increased triglyceride levels have been reported in adult animals consuming a highly-palatable diet [43], as well as in the offspring of obese dams or those consuming an energy-rich diet [10]. The rats exposed to HFD in utero consumed more HFD in acute tests than those exposed to a control diet. The increased intake of HFD supports the findings of previous studies, in which a palatable diet in utero can stimulate preference for palatable foods later in life [10, 11, 19, 20] and even increase milk consumption in nursing pups [45]. Another explanation for the changes in triglyceride levels seen in Exp. 1 could be related to alcohol consumption. The rats exposed to HFD in utero also consumed more alcohol than those exposed to a control diet. There is a relationship between alcohol intake, triglyceride levels, and HFD intake. Alcohol intake can itself increase circulating triglyceride levels [33], and triglyceride-attenuating drugs can suppress alcohol intake [32]. Further, a HFD can promote alcohol intake, and this is associated with increased triglyceride levels and changes in hypothalamic orexigenic peptides [33]. Of particular relevance to the findings in the present study is the evidence that HFD in utero results in increased triglycerides, as well as the proliferation and differentiation of neurons and their migration toward hypothalamic areas where ultimately a greater proportion of the new neurons express orexigenic peptides [10]. While the present study does not allow us to determine whether the elevated triglycerides are due to the in utero HFD exposure or to alcohol intake later in life, the existing literature leads us to hypothesize a HFD-induced increase in triglycerides, which may contribute to increased future alcohol intake. Since the offspring exposed to HFCS or sucrose failed to exhibit any change in triglycerides, however, other mechanisms, perhaps related to changes in dopamine (DA) and the opioids [19], may also be involved in promoting alcohol intake.
4.3 Palatable-diet exposure in utero or during pre-weaning is associated with sensitivity to amphetamine
In Exp. 2, male rats with prenatal or pre-weaning exposure to HFCS or sucrose were hyperactive in response to a low dose of the dopaminergic agonist, amphetamine. This finding suggests a functional application to the studies revealing neurochemical alterations in the offspring of dams consuming a highly palatable diet. These include: (1) increased mRNA expression of the μ-opioid receptor and decreased levels of dopamine reuptake transporter (DAT) in the mesolimbic brain regions [21]; (2) increased tyrosine hydroxylase expression in the ventral tegmental area (VTA) and nucleus accumbens (NAc) and increased DA and metabolite contents in the NAc [22]; and (3) increased expression of both μ-opioid receptor and preproenkephalin in the NAc, prefrontal cortex, and hypothalamus in the brains of offspring from dams that consumed palatable, high-fat diets [19]. Further, the findings of Exp. 2 complement previous studies, which have noted that maternal obesity leads to hyperactivity in the offspring [46] and protein restriction (as discussed above) in utero and during the pre-weaning stage leads to hyperactivity and increased cocaine-induced locomotion [39]. Conversely, Naef et al. [22] found that dams with a HFD only during the final week of gestation and the pre-weaning period produced offspring that showed reduced amphetamine-precipitated locomotor activity but increased DA and DOPAC levels in the NAc. In another study, Naef et al. [44] reported that animals born to HFD dams are more sensitive than controls to quinpirole (a selective D2/3 receptor agonist), following the initial, immediate inhibitory locomotor effect. While it is clear that the exact mechanisms underlying prenatal effects of diet on the mesolimbic DA system require further investigation, it is important to note that the previous studies have investigated fat-rich diets, while the current study examined dams consuming sugar-rich diets. Since it has been shown that fat- vs. sugar-rich foods can differentially affect reward-related behavior (e.g., aspects of addiction) [28], the nutrient used in the studies described above may potentially be driving the observed differences.
4.4. Differences between HFCS and sucrose
In Exp. 2, we tested the effects of two different sugars, sucrose and HFCS, on behaviors that result from gestational or pre-weaning exposure. Sugar-sweetened beverage consumption is on the rise and has been linked to the obesity epidemic [47], and excessive intake of sugar may produce a state that behaviorally and neurochemically resembles an “addiction,” which may make consumption of sweet foods difficult to curtail for some individuals [49, 50]. Further, the intake of sweet foods in obese women is the strongest predictor of offspring body weight [48]. In adult animals, limited daily access to a supplement of HFCS for 8 weeks results in increased body weight compared to sucrose-supplemented rats, and this increase in body weight is not accounted for by hyperphagia [29]. Similarly, a clinical study revealed that HFCS-sweetened beverages result in different acute metabolic effects compared with sucrose, including increased levels of serum uric acid, systolic blood pressure, and the relative bioavailability of glucose [30]. However, in the present study, there were negligible behavioral differences observed in the gestational or pre-weaning effects of these sugars. Of note, sucrose-consuming dams ingested more total kcals than both chow-fed or HFCS-fed dams, and male offspring nursed by HFCS-consuming dams had elevated triglyceride levels. Thus, both HFCS and sucrose exposure during the perinatal period can affect body weight, alcohol intake and amphetamine-induced locomotor activity.
5. Conclusions
The majority of women who are at child-bearing age are overweight, and this is mostly due to overeating. Thus, it is important to understand the effects that ubiquitous, highly palatable foods can have on the health and well-being of offspring that are exposed to such diets in utero and/or after parturition. Further, increasing rates of prenatal and childhood obesity, as well as the rise in the number of youths abusing drugs and alcohol, warrants further investigation into the potential etiology of this growing problem. The current study supports the existing literature on the changes in body weight and diet preference in the offspring of dams consuming a HFD. Further, it reveals enhanced sensitivity, susceptibility and intake of some drugs of abuse and assesses different types of sweeteners that are prevalent in today’s eating environment. These results may have implications for suggested prenatal diets in clinical populations.
Highlights.
Offspring of dams eating a palatable diet show altered body weight.
Female offspring of HFD- and sugar-consuming dams consume excess alcohol.
Male offspring of sugar-consuming dams have increased amphetamine-induced locomotion.
Acknowledgments
This research was supported by AA-019623 (MEB), DA-03123 (NMA), AA-12882 (SFL/BGH), and Kidelhoj-Santini (BGH/NMA). We thank Olga Karatayev for her assistance with some of the triglyceride assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canani RB, Di Costanzo M, Leone L, Bedogni G, Brambilla P, Cianfarani S, Nobili V, Pietrobelli A, Agostoni C. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011:1–8. doi: 10.1017/S0954422411000102. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, Rodford J, Slater-Jefferies JL, Garratt E, Crozier SR, Emerald BS, Gale CR, Inskip HM, Cooper C, Hanson MA. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60(5):1528–34. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152(6):2228–36. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones AP, Assimon SA, Friedman MI. The effect of diet on food intake and adiposity in rats made obese by gestational undernutrition. Physiol Behav. 1986;37(3):381–6. doi: 10.1016/0031-9384(86)90194-0. [DOI] [PubMed] [Google Scholar]
- 5.Jones AP, Simson EL, Friedman MI. Gestational undernutrition and the development of obesity in rats. J Nutr. 1984;114(8):1484–92. doi: 10.1093/jn/114.8.1484. [DOI] [PubMed] [Google Scholar]
- 6.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–53. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan EL, Smith MS, Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93(1):1–8. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalevss U, Tylor A, Schuster K, Frate C, Tobin S, Woodside B. Long-term physiological and behavioral effects of exposure to a highly palatable diet during the perinatal and post-weaning periods. Physiol Behav. 2010;101(4):494–502. doi: 10.1016/j.physbeh.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Rooney K, Ozanne SE. Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. Int J Obes (Lond) 2011;35(7):883–90. doi: 10.1038/ijo.2011.96. [DOI] [PubMed] [Google Scholar]
- 10.Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28(46):12107–19. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98(4):843–51. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- 12.Nivoit P, Morens C, Van Assche FA, Jansen E, Poston L, Remacle C, Reusens B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia. 2009;52(6):1133–42. doi: 10.1007/s00125-009-1316-9. [DOI] [PubMed] [Google Scholar]
- 13.Rajia S, Chen H, Morris MJ. Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by postweaning diet. J Neuroendocrinol. 2010;22(8):905–14. doi: 10.1111/j.1365-2826.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- 14.Knittle JL, Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968;47(9):2091–8. doi: 10.1172/JCI105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Morris MJ. Differential responses of orexigenic neuropeptides to fasting in offspring of obese mothers. Obesity (Silver Spring) 2009;17(7):1356–62. doi: 10.1038/oby.2009.56. [DOI] [PubMed] [Google Scholar]
- 16.Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes (Lond) 2009;33(1):115–22. doi: 10.1038/ijo.2008.213. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Srinivasan M, Thamadilok S, Patel MS. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol. 2009;200(3):293–300. doi: 10.1677/JOE-08-0429. [DOI] [PubMed] [Google Scholar]
- 18.Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 2006;20(8):1257–9. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- 19.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151(10):4756–64. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162(4):924–32. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong ZY, Muhlhausler BS. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011;25(7):2167–79. doi: 10.1096/fj.10-178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology (Berl) 2008;197(1):83–94. doi: 10.1007/s00213-007-1008-4. [DOI] [PubMed] [Google Scholar]
- 23.Dyrskog SE, Gregersen S, Hermansen K. High-fat feeding during gestation and nursing period have differential effects on the insulin secretory capacity in offspring from normal Wistar rats. Rev Diabet Stud. 2005;2(3):136–45. doi: 10.1900/RDS.2005.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra A, Alvers KM, Crump EM, Rowland NE. Effect of high-fat diet during gestation, lactation, or postweaning on physiological and behavioral indexes in borderline hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R20–8. doi: 10.1152/ajpregu.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled study. Eur J Clin Nutr. 2011;65(1):10–9. doi: 10.1038/ejcn.2010.225. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 2007;15(4):986–93. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 27.Belzer LM, Smulian JC, Lu SE, Tepper BJ. Food cravings and intake of sweet foods in healthy pregnancy and mild gestational diabetes mellitus. A prospective study. Appetite. 2010;55(3):609–15. doi: 10.1016/j.appet.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139(3):623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav. 2010;97(1):101–6. doi: 10.1016/j.pbb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, Segal MS, Johnson RJ, Johnson JA. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61(5):641–51. doi: 10.1016/j.metabol.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avena NM, Carrillo CA, Needham L, Leibowitz SF, Hoebel BG. Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol. 2004;34(2–3):203–9. doi: 10.1016/j.alcohol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, Leibowitz SF. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol. 2009;43(6):433–41. doi: 10.1016/j.alcohol.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31(2):249–59. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 34.Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Circulating triglycerides after a high-fat meal: predictor of increased caloric intake, orexigenic peptide expression, and dietary obesity. Brain Res. 2009;1298:111–22. doi: 10.1016/j.brainres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odaka Y, Nakano M, Tanaka T, Kaburagi T, Yoshino H, Sato-Mito N, Sato K. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity (Silver Spring) 2010;18(9):1688–94. doi: 10.1038/oby.2009.513. [DOI] [PubMed] [Google Scholar]
- 36.Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res. 2006;65(Suppl 3):83–9. doi: 10.1159/000091511. [DOI] [PubMed] [Google Scholar]
- 37.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4(2B):611–24. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 38.Bieswal F, Ahn MT, Reusens B, Holvoet P, Raes M, Rees WD, Remacle C. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring) 2006;14(8):1330–43. doi: 10.1038/oby.2006.151. [DOI] [PubMed] [Google Scholar]
- 39.Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168(2):359–70. doi: 10.1016/j.neuroscience.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitaker KW, Totoki K, Reyes TM. Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2011.02.007. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn GA, Morgan CP, Bale TL. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59(3):290–5. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Hill MJ, Metcalfe D, McTernan PG. Obesity and diabetes: lipids, ‘nowhere to run to’. Clin Sci (Lond) 2009;116(2):113–23. doi: 10.1042/CS20080050. [DOI] [PubMed] [Google Scholar]
- 43.Barson JR, Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Effect of dietary fatty acid composition on food intake, triglycerides, and hypothalamic peptides. Regul Pept. 2012;173(1–3):13–20. doi: 10.1016/j.regpep.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 2011;176:225–36. doi: 10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 45.Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KL. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav. 2011;104(3):474–9. doi: 10.1016/j.physbeh.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandes C, Grayton H, Poston L, Samuelsson AM, Taylor PD, Collier DA, Rodriguez A. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.164. [DOI] [PubMed] [Google Scholar]
- 47.Lasater G, Piernas C, Popkin BM. Beverage patterns and trends among school-aged children in the US, 1989–2008. Nutr J. 2011;10:103. doi: 10.1186/1475-2891-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phelan S, Hart C, Phipps M, Abrams B, Schaffner A, Adams A, Wing R. Maternal Behaviors during Pregnancy Impact Offspring Obesity Risk. Exp Diabetes Res. 2011;2011:985139. doi: 10.1155/2011/985139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avena NM, Bocarsly ME, Hoebel BG, Gold MS. Overlaps in the Nosology of Substance Abuse and Overeating: the Translational Implications of “Food Addiction”. Curr Drug Abuse Rev. 2011;4(3):133–9. doi: 10.2174/1874473711104030133. [DOI] [PubMed] [Google Scholar]
- 50.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):2–039. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]