Abstract
Context
Pain, fatigue and sleep disturbance commonly co-occur in patients receiving treatment for advanced cancer.
Objectives
A pilot randomized controlled trial was conducted to assess initial efficacy of a patient-controlled cognitive-behavioral (CB) intervention for the pain, fatigue, sleep disturbance symptom cluster.
Methods
Eighty-six patients with advanced lung, prostate, colorectal, or gynecologic cancers receiving treatment at a comprehensive cancer center were stratified by recruitment clinic (chemotherapy, radiation therapy) and randomized to intervention or control groups. Forty-three patients were assigned to receive training in and use of up to 12 relaxation, imagery, or distraction exercises delivered via MP3 player for two weeks during cancer treatment. Forty-three patients were assigned to a waitlist control condition for the same two-week period. Outcomes included symptom cluster severity and overall symptom interference with daily life measured at baseline (Time 1) and two weeks later (Time 2).
Results
Eight participants dropped out; 78 completed the study and were analyzed (36 intervention, 42 control). Participants used the CB strategies an average of 13.65 times (SD= 6.98). Controlling for baseline symptom cluster severity and other relevant covariates, symptom cluster severity at Time 2 was lower in the intervention group (MAdj=2.99, SE=0.29) than in the waitlist group (MAdj=3.87, SE=0.36), F(1,65)=3.57, P=0.032. Symptom interference with daily life did not differ between groups. No significant adverse events were noted with the CB intervention.
Conclusion
Findings suggest that the CB intervention may be an efficacious approach to treating the pain, fatigue, sleep disturbance symptom cluster. Future research is planned to confirm efficacy and to test mediators and moderators of intervention effects.
Keywords: Cancer, fatigue, pain, sleep, symptoms, cognitive behavior therapy, guided imagery, relaxation therapy
Introduction
Pain, fatigue, and sleep disturbance are among the most prevalent symptoms in persons with advanced cancer. This cluster of symptoms occurs in over 50% of patients receiving treatment for advanced cancer [1–7]. The pain, fatigue, sleep disturbance symptom cluster has negative consequences on patient outcomes such as functional status and quality of life [8]. Yet, few investigators have tested interventions that specifically target this or any other symptom cluster.
Among those few investigators, some have elected to develop and test interventions designed to address a wide array of cancer-related symptoms, as opposed to a symptom cluster per se. For example, Given and colleagues [9–11] tested 8- and 20-week cognitive-behavioral (CB) interventions in which patients were trained to use different self-management strategies for a number of cancer-related symptoms including pain, fatigue, nausea, vomiting, insomnia, dyspnea, weakness, anorexia, fever, dry mouth, constipation, mouth sores, and depressive symptoms. No overall effects of the interventions were seen, but select subgroups of patients reported improvement in some symptoms. Jarden and colleagues [12] tested a relaxation, exercise and psychoeducational intervention on 21 symptoms experienced by hospitalized patients undergoing hematopoietic stem cell transplant. The investigators first conducted a factor analysis on baseline symptom scores to group the 21 symptoms into clusters (gastrointestinal, cognitive, affective, functional/pain, mucositis), and then compared scores on those symptom clusters between treatment and control groups across eight subsequent data collection points (weeks 1, 2, 3, 4, 5, 6, three months, and six months). They found significantly lower symptom severity scores over time in the intervention group compared to the control group for four of the five clusters.
Another approach used by a growing number of investigators is to test interventions designed for a single symptom and to then evaluate if the intervention has secondary effects on other symptoms known to be related or clustered with the target symptom. For example, Dirksen and Epstein [13], and Espie and colleagues [14] tested CB interventions using stimulus control, sleep restriction, sleep hygiene, and/or sleep education for insomnia and demonstrated primary effects on sleep disturbance and secondary effects on fatigue. Barsevick [15] recently developed and tested a psychoeducational intervention using energy conservation, and activity and sleep management strategies for fatigue and sleep disturbance and tested secondary effects on pain and depression. No overall effects of the intervention were seen on primary or secondary outcomes.
Investigators are just beginning to test interventions targeted to a specific symptom cluster. Chan et al. [16] tested a psychoeducational intervention to control the anxiety, breathlessness, fatigue symptom cluster in patients with lung cancer receiving radiation therapy. Using a randomized controlled design, 140 patients were assigned to receive usual care or the experimental intervention including preparatory information, discussion of symptoms and their meanings, advice on self-care strategies, and training in daily use of progressive muscle relaxation. Controlling for baseline symptom cluster score (an average of anxiety, breathlessness and fatigue scores), the six-week symptom cluster score was significantly lower in patients who received the psychoeducational intervention than in those who received usual care, as were scores on each of the three component symptoms. Similar studies that test interventions targeting treatment of other symptom clusters are necessary.
The pain, fatigue, sleep disturbance symptom cluster may be considered a priority cluster for intervention development, given that these three symptoms are known to frequently co-occur, and that this particular cluster of symptoms affects a large number of patients receiving treatment for advanced cancer [1–7]. In a systematic review of CB strategies, Kwekkeboom et al. [17] found that relaxation, distraction, and guided imagery had each been shown to be effective for at least two of the three symptoms of interest (pain, fatigue, and sleep disturbance) when tested in single-symptom trials. The CB strategies may work for all three symptoms by changing patients’ beliefs, by changing appraisals related to the symptom experience, or by enhancing coping skills. All three symptoms are exacerbated by negative psychological reactions [18–21], and failure to relieve one symptom may result in the others worsening. Conversely, reduction in one symptom may reduce or prevent escalation in the others. Combining these CB strategies into a single intervention offers a relatively simple and pragmatic approach to treating the pain, fatigue, sleep disturbance symptom cluster in a patient population that would otherwise need to balance several different single-symptom management strategies.
We designed a patient-controlled CB (PC-CB) intervention that provides a variety of relaxation, distraction, and imagery recordings on an MP3 player (Sony Walkman™), allowing patients to self-administer their preferred selection of strategies at whatever time and place they are needed. The intervention was designed to be brief (e.g., single training session), with self-administration of strategies as needed, given the limited capacities of many patients with advanced disease, and the fluctuating nature of many treatment-related symptoms. A single-group feasibility study was conducted with 30 patients experiencing co-occurring pain, fatigue, and sleep disturbance during treatment for advanced cancer [22]. Subjects completed baseline measures of symptom severity and received instructions to use the intervention daily during the subsequent two weeks of cancer therapy. Symptom severity measures were completed again after the two-week study period. Participants also kept a log, recording symptom severity before and after each time they used a CB strategy. Ninety percent of participants completed the study; only one person dropped out because of difficulty self-administering the strategies with the MP3 player. Participants used the CB strategies an average of 12 times over two weeks (range 2–22). Although symptom ratings at two weeks were not significantly different from baseline, significant improvements in all three symptoms were documented at the time participants used the CB strategies (i.e., change in ratings from before to after using a CB strategy).
Given that the PC-CB intervention produced some positive effects in the feasibility study, a pilot of a randomized trial was warranted. The primary aims of the pilot study were: 1) to explore the patterns in use of recorded CB strategies, and 2) to test initial efficacy of the intervention on symptom cluster severity and symptom interference with daily life during cancer treatment. We hypothesized that patients who received the intervention would report lower symptom severity and less symptom interference with daily life than persons who received a waitlist control condition. This pilot study focused on testing short-term symptom outcomes (initial efficacy) during a two-week period of cancer treatment, over which time symptoms were likely to exacerbate. Depression, which is sometimes identified as clustering with pain, fatigue, and sleep disturbance, was not targeted in this trial, as depression is a complex and persistent psychological condition not likely to respond to brief intervention.
Methods
Design
The study used a randomized controlled design with two groups (PC-CB intervention or waitlist control) and two measurement times over the two-week study period (baseline = Time 1, end of week 2 = Time 2). Participants were stratified by recruitment clinic (chemotherapy, radiation therapy) and randomized with equal allocation to intervention or control groups. The randomization sequence was created by the study’s statistician using RAN2 [23]. The sequence was generated within strata (chemotherapy, radiation therapy) in blocks of four, and individual assignments sealed in opaque envelopes. After baseline measures were collected, a research nurse selected and opened the next consecutive envelope from the appropriate strata to assign participants to groups. Neither the patient nor research nurse was blinded to group assignment.
Study procedures were reviewed and approved by the Health Sciences Institutional Review Board at the University of Wisconsin, and all patients provided informed consent prior to initiating study procedures.
Participants
A convenience sample of 86 patients was recruited between July 2009 and November 2010 from the outpatient chemotherapy or radiation therapy clinics at a National Cancer Institute-designated Comprehensive Cancer Center in the midwest U.S. Participants were receiving treatment for advanced (metastatic or recurrent) colorectal, lung, prostate or gynecologic cancers, and had experienced pain, fatigue, and sleep disturbance in the past week. To qualify, severity of at least two of the three symptoms had to be rated as three or more on a 0–10 numeric rating scale (NRS). Research staff recruited patients on a day when patients were receiving chemotherapy or were in the final weeks of radiation therapy, in anticipation of symptoms becoming worse in the subsequent two weeks. In our feasibility study, 86% of participants who experienced at least two of the three symptoms upon recruitment went on to report all three symptoms during the two-week study period [22]. Patients with post-operative or neuropathic pain were excluded, as were persons who had been hospitalized for mental health reasons within the last three months. Although the sample may seem heterogeneous with regard to cancer diagnosis, symptom management recommendations do not differ by diagnosis, nor is diagnosis expected to influence effects of symptom management strategies. All participants had advanced disease and experienced at least minimal levels of symptoms as an eligibility criterion.
Intervention
Patient-Controlled Cognitive-Behavioral Intervention
Participants assigned to the PC-CB intervention group received a single one-on-one training session from a research nurse. Training sessions were audio-recorded and intervention fidelity was assessed with a checklist. Intervention components included: a) information about the causes of pain, fatigue, and sleep disturbance during treatment for advanced cancer, b) rationale for how the CB strategies were expected to effect symptoms, c) overview of the 12 CB strategies offered for the study, and d) individualized recommendations for practice based on the patient’s usual symptom patterns and preferences for CB strategies. Patients were encouraged to use the CB strategies as often as desired, but at least once per day. An educational booklet was given to the participant and used to guide the training.
The 12 CB strategies were presented in four categories: symptom-focused imagery, nature-focused imagery, relaxation exercises, and nature sounds. The three symptom-focused imagery strategies were 1) pain-focused imagery, in which patients imagined draining pain from the body and using a special glove to change any remaining pain to a more pleasant sensation; 2) fatigue-focused imagery, in which patients imagined circulating a ball of healing energy throughout their bodies; and 3) sleep-focused imagery, in which patients imagined floating through the night sky into a peaceful sleep. The three nature-focused imagery strategies were 1) beach imagery, 2) mountain imagery, and 3) meadow imagery.
The three relaxation exercises were 1) progressive muscle relaxation, 2) jaw relaxation, and 3) focused breathing relaxation. The three nature sound recordings were 1) rainstorm sounds, 2) sounds of surf and waves, and 3) forest sounds [24].
Scripts for all of the imagery and relaxation exercises were developed for this line of research and were recorded in the same female voice. Some of the exercises were brief (e.g., jaw relaxation was 4 minutes), but most were approximately 20 minutes long. The recordings did not include any musical background. Recordings were loaded on an MP3 player provided to participants for the length of the study. All participants were provided with their choice of earbud or over-the-ear style headphones. The research nurse demonstrated how to use the MP3 player. Written instructions were provided in the patient education booklet and duplicated on a small laminated card carried in a case with the MP3 player. Study participants were given an opportunity to practice and then were asked to provide a return demonstration by locating, playing, and adjusting the volume of a selected recording.
Waitlist Control Condition
Participants assigned to the waitlist control group were asked to follow usual care for their symptoms during the two-week study period. None of the recruitment clinics routinely provide patient education about CB symptom management strategies. Waitlist participants were offered the PC-CB intervention at the end of the two-week study period.
Procedures
Clinic staff identified patients who met eligibility criteria regarding diagnosis and treatment, and obtained patient permission for a research nurse to meet with them to discuss the study. The research nurse met with interested patients, determined if they met symptom criteria, explained study purposes and procedures, and obtained written consent.
After providing informed consent, participants completed a demographic questionnaire, and measures of concurrent symptoms, mood (anxiety, depression), and Time 1 measures of symptom severity, and symptom interference with daily life. All measures were completed in the clinic, unless patients asked to take them home and return them to clinic the next day. When completed questionnaires were returned, the research nurse opened a randomization envelope to reveal group assignment and provided intervention or waitlist instructions. In addition, participants in the intervention group were shown how to complete a log recording each use of the CB strategies, and all participants (intervention and waitlist control) were taught to complete a daily symptom diary. Follow-up phone calls were made to participants, in both the intervention and waitlist groups, one to two days after the initial meeting, on day 7, and at the end of the two-week period to resolve any questions or concerns, to assess for adverse events, and to remind participants of upcoming study activities.
Time 2 measures of symptom severity and symptom interference with life were mailed to participants, with a reminder to complete them at home, at the end of the two-week period. Participants sealed the completed questionnaires along with their symptom diaries and CB logs in an envelope and brought them to their next clinic visit for a final study meeting. At the final meeting, the research nurse collected the packet of completed measures and the MP3 player. All participants were reimbursed $70 for their time and effort. Waitlist participants were offered the PC-CB intervention at this time, but no additional data were collected from these participants.
Measures
Demographic Questionnaire
Patients provided information about their age, gender, education, race and ethnicity. Medical records were reviewed to collect information about diagnosis, current therapy, and supportive medications used during the study period.
Symptom Cluster Severity
Subsets of items from validated symptom assessment questionnaires were selected to minimize participant burden and item overlap. Pain severity was measured with four pain severity items from the Brief Pain Inventory [25]. Participants rated pain at its “worst,” “least,” and “average” in the last 24 hours, and pain “now” on a 0–10 NRS. A pain summary score was created by averaging the four ratings, with higher scores indicating more severe pain (Cronbach’s alpha was 0.90 at Time 1 and 0.92 at Time 2). Fatigue severity was measured with four fatigue severity items from the Brief Fatigue Inventory [26]. Participants rated fatigue at its “worst,” “least,” and “usual” in the last 24 hours, and fatigue “now” on a 0–10 NRS. A fatigue summary score was created by averaging the four ratings, with higher scores indicating more severe fatigue (Cronbach’s alpha was 0.85 at Time 1 and 0.88 at Time 2). Sleep disturbance was measured with a 0–10 numeric rating of “worst” sleep disturbance in the past 24 hours, and 1–4 (very good - very bad) rating of sleep quality from the Pittsburgh Sleep Quality Index [27]. A sleep disturbance summary score was calculated by averaging the z-scores for the two items and transforming the average to a 0–10 scale, with higher scores indicating greater sleep disturbance. Finally, a symptom cluster severity score was calculated by averaging the pain, fatigue, and sleep disturbance summary scores.
Symptom Interference With Daily Life
Symptom interference with daily life was measured using the symptom interference subscale from the M. D. Anderson Symptom Inventory (MDASI) [28]. The subscale includes six ratings (0, did not interfere to 10, interfered completely) of how all cancer-related symptoms interfered in the past 24 hours with general activity, mood, work, relations with others, walking, and enjoyment of life. The overall interference score was calculated by averaging responses across the six items, with higher scores indicating greater interference (Cronbach’s alpha = 0.92 at both time points).
Concurrent Symptoms
The experience of 10 other symptoms was assessed with items from the MDASI [28]. Symptoms including nausea, vomiting, lack of appetite, dry mouth, numbness, shortness of breath, distress, trouble remembering, drowsiness, and sadness were rated at their worst in the past 24 hours using a 0–10 NRS. Each symptom was coded as “present” if its severity rating was one or greater.
Mood (Anxiety / Depression)
Anxious and depressed moods were measured with subscales from the Profile of Mood States–Short Form (POMS-SF). The anxiety subscale consists of six adjectives for anxious mood, and the depression subscale comprises nine adjectives for depressed mood. Participants rated each adjective on a 0 (not at all) to 4 (extremely) scale. Items were summed for each subscale, with higher scores indicating more anxious (range 0 to 24) or depressed mood (range 0 to 36). The POMS-SF is well validated in cancer populations [29–30]. Cronbach’s alpha in this use was 0.89 for anxiety and 0.93 for depression.
Symptom Severity and Distress Before and After CB Use
Participants in the PC-CB intervention group completed a log with each use of the CB strategies. They recorded the date, time, and CB strategy used and made pre- and post-treatment ratings of the severity and distress of current pain, fatigue, and sleep disturbance, using 0–10 NRSs.
Data Analysis
Based on effect sizes from meta-analyses of CB interventions for cancer symptom management [31–32], and covariate contribution to variability cited in previous studies [33–37], we estimate a true effect size of dAdj = 0.65. An a priori power analysis indicated a need for 39 subjects in each group (N=78) to have 80% power for detecting an estimated effect size of d=0.65, with alpha = 0.05. One-tailed P-values were used in testing directional hypotheses with regard to symptom cluster severity and pain, fatigue, and sleep disturbance individually, as previous research has demonstrated beneficial effects of the selected CB strategies on these specific symptoms [17]. Intervention effect on symptom interference with daily life was evaluated with a two-tailed test, as the symptom interference score includes the impact of all cancer-related symptoms, not just pain, fatigue, and sleep disturbance.
Data were analyzed using SPSS version 19.0 (SPSS, Inc., Chicago IL). Descriptive statistics were used to summarize all baseline variables and data regarding CB strategy use. Strategy use was evaluated with respect to the overall number of uses per participant over the two-week study period, the number of days on which a strategy was used, frequency by time of day, and frequency of specific recordings. The effects of the PC-CB intervention on primary outcomes of symptom cluster severity and symptom interference with daily life were assessed in a per protocol analysis by comparing the intervention and waitlist control group post-intervention means within an analysis of covariance. The stratification variable, recruitment clinic (chemotherapy, radiation therapy), and variables widely recognized as related to the symptom outcomes, including age, gender, anxiety, depression, number of supportive medications, and Time 1 score on the outcome of interest, were included as covariates in analyses. Effect sizes are reported as partial eta-squared. Confidence intervals were obtained using methods described in Serlin and Lapsley [38] and Steiger and Fouladi [39]. Outcomes of the per protocol analysis were compared to those obtained in an intent-to-treat analysis which used the “last value carried forward” approach to replace missing data.
To further explore intervention effects, we examined symptom ratings made before and after each use of the CB strategies. Symptom ratings from logs kept by participants in the PC-CB intervention group were averaged across all CB strategy uses to create mean severity and distress scores before CB strategy use and mean severity and distress scores after CB strategy use for each of the three symptoms. Mean scores before and after CB strategy use were compared using paired t-tests.
Results
Sample Characteristics
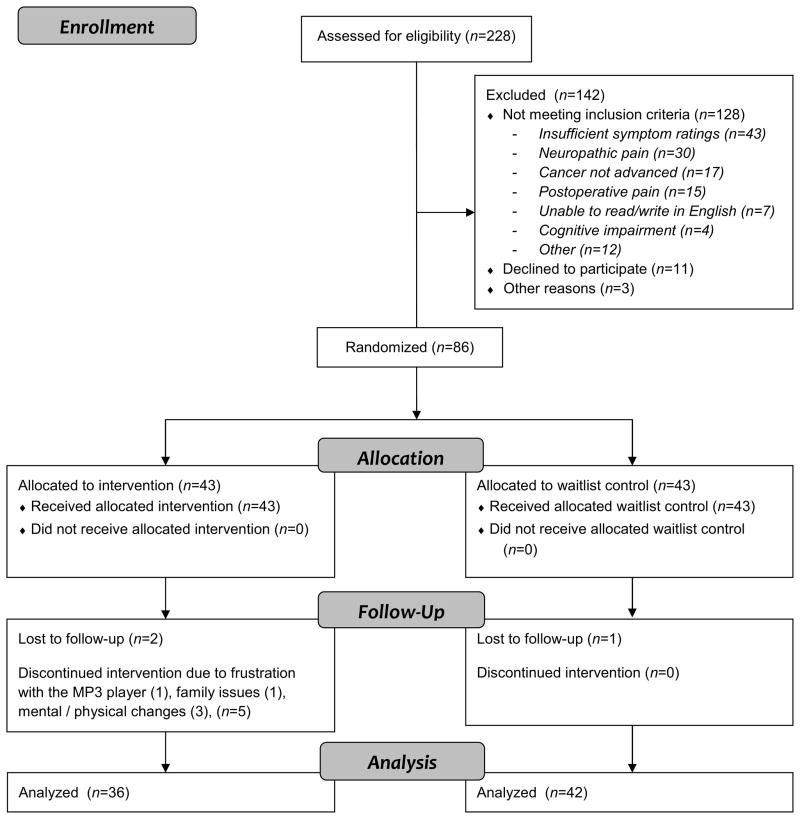
Subjects were recruited from July 2009 through December 2010. The flow of participants through the trial is provided in Fig. 1. Eighty-six patients were randomly assigned, 43 to the intervention group and 43 to the control group. All patients completed baseline measures, were randomized, and received instructions appropriate to their group. Fidelity checklists indicated that intervention components were delivered with more than 90% accuracy. Baseline demographic and clinical characteristic for the sample are reported in Table 1. All subjects assigned to the waitlist group completed the two-week study, but one did not return Time 2 measures. Similarly, two subjects in the intervention group completed the two-week study, but did not return Time 2 measures. Five subjects discontinued participation from the intervention group for reasons including mental or physical changes and family issues that precluded completing study activities, and one subject was frustrated by the MP3 player. Thus, per protocol outcome analyses were conducted with data from 42 control and 36 intervention subjects. Persons with colorectal cancer made up a greater percentage of the group without Time 2 data for analysis (n=4, 50%) than the group with Time 2 data (n=6, 8%), Fisher’s exact test, P = 0.005. There were no differences on study variables at Time 1 between those with Time 2 data for analysis and those without (Table 2).
Figure 1.
CONSORT Flow Diagram
Table 1.
Demographic and Clinical Characteristics by Treatment Group (N=86)
| Intervention | Waitlist | Total | |
|---|---|---|---|
| n = 43 | n = 43 | N=86 | |
| n (%) | n (%) | n (%) | |
| Age, mean (SD) | 60.44 (10.76) | 60.14 (11.54) | 60.29 (11.09) |
| Gender | |||
| Male | 14 (33%) | 21 (49%) | 35 (41%) |
| Female | 29 (67%) | 22 (51%) | 51 (59%) |
| Ethnicity | |||
| Non-Hispanic / Latino | 43 (100%) | 43 (100%) | 86 (100%) |
| Race | |||
| White | 41 (96%) | 39 (91%) | 80 (93%) |
| Black | 1 (2%) | 3 (7%) | 4 (5%) |
| Asian | 0 (0%) | 1 (2%) | 1 (1%) |
| Native American / Alaskan Native | 1 (2%) | 0 (0%) | 1 (1%) |
| Education | |||
| < HS diploma | 1 (2%) | 2 (5%) | 3 (4%) |
| HS diploma / GED | 8 (19%) | 11 (26%) | 19 (22%) |
| Undergrad college | 26 (60%) | 23 (53%) | 49 (57%) |
| Graduate school | 8 (19%) | 7 (16%) | 15 (17%) |
| Cancer Diagnosis | |||
| Lung | 10 (23%) | 15 (35%) | 25 (29%) |
| Prostate | 6 (14%) | 9 (21%) | 15 (17%) |
| Colorectal | 6 (14%) | 4 (9%) | 10 (12%) |
| Gynecologic | 21 (49%) | 15 (35%) | 36 (42%) |
| Current Treatment | |||
| Chemotherapy | 30 (70%) | 32 (75%) | 62 (72%) |
| Radiation Therapy | 9 (21%) | 10 (23%) | 19 (22%) |
| Chemo + Radiation | 4 (9%) | 1 (2%) | 5 (6%) |
| Concurrent Symptoms | |||
| Nausea | 20 (47%) | 26 (60%) | 46 (53%) |
| Distress | 34 (79%) | 37 (86%) | 71 (83%) |
| Shortness of Breath | 24 (56%) | 28 (65%) | 52 (60%) |
| Trouble Remembering | 33 (77%) | 30 (70%) | 63 (73%) |
| Lack of appetite | 28 (65%) | 29 (67%) | 57 (66%) |
| Drowsiness | 42 (98%) | 40 (93%) | 82 (95%) |
| Dry Mouth | 31 (72%) | 33 (77%) | 64 (74%) |
| Sadness | 27 (63%) | 33 (77%) | 60 (70%) |
| Vomiting | 4 (9%) | 5 (12%) | 9 (10%) |
| Numbness | 23 (53%) | 25 (58%) | 48 (56%) |
| Supportive Meds Ordered | |||
| Opioids | 25 (58%) | 26 (61%) | 51 (59%) |
| Steroids | 32 (74%) | 29 (67%) | 61 (71%) |
| Psychostimulants | 3 (7%) | 2 (5%) | 5 (6%) |
| Hypnotics / Sedatives | 17 (40%) | 15 (35%) | 32 (37%) |
| Antidepressants | 9 (21%) | 5 (12%) | 14 (16%) |
| Antiemetics | 37 (86%) | 37 (86%) | 74 (86%) |
| Colony stim. factors | 1 (2%) | 3 (7%) | 4 (5%) |
| Other | 24 (56%) | 19 (44%) | 43 (50%) |
Table 2.
Mean (SD) of Time 1 Variables by Completion Status (N=86)
| Those Without Time 2 Data | Those With Time 2 Data | |||
|---|---|---|---|---|
| n = 8 | n = 78 | |||
| M (SD) | M (SD) | t | P | |
| Symptom cluster severity | 3.63 (1.89) | 3.77 (1.62) | −0.195 | 0.85 |
| Pain severity | 2.63 (2.05) | 2.19 (1.76) | 0.579 | 0.58 |
| Fatigue severity | 3.78 (1.62) | 3.91 (2.05) | −0.214 | 0.84 |
| Sleep dist. Severity | 4.50 (2.54) | 5.21 (2.54) | −0.753 | 0.47 |
| Symptom interference | 4.81 (2.12) | 4.04 (2.58) | 0.959 | 0.36 |
| Number concurrent symptoms | 5.88 (1.96) | 6.47 (2.16) | −0.753 | 0.45 |
| Number supportive meds | 3.88 (1.73) | 3.24 (1.35) | 1.003 | 0.35 |
| Anxiety | 8.13 (5.19) | 9.27 (5.42) | −0.591 | 0.57 |
| Depression | 9.88 (7.32) | 7.86 (7.20) | 0.743 | 0.48 |
Baseline Data
Among the 78 participants analyzed per protocol, the PC-CB intervention and waitlist control groups did not differ on study variables at Time 1, with the exception of depressed mood. Participants in the waitlist group reported more depressed mood (mean [SD] 10.07 [8.24]) than participants in the intervention group (mean [SD] 5.28 [4.66]), t(76) = 3.09, P = 0.003.
Patterns of CB Strategy Use
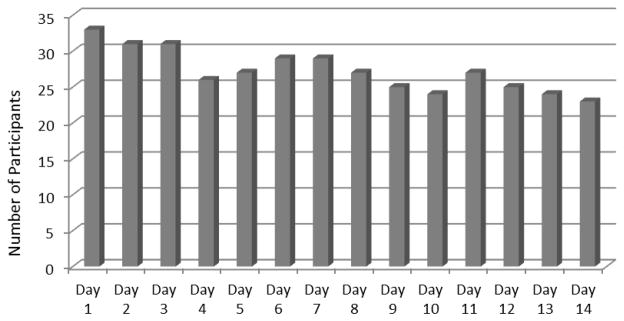
Participants used the CB strategies an average (SD) of 13.65 (6.98) times during the two- week study period (range 1 to 32 uses). The majority of participants (n=20, 59%) recorded use of the CB strategies on all 14 days (Fig. 2). Participants used the CB strategies more frequently in daytime (mean [SD] 6.13 [5.72]) and evening hours (mean [SD] 5.59 [3.83]) than during the nighttime (mean [SD] 1.72 [1.92]), F(2, 62) = 9.80, P = 0.001. The four categories of CB strategies were used with nearly equal frequency. The most commonly used CB strategies were rainstorm nature sounds (n=52 uses), fatigue-focused imagery (n=51 uses), focused breathing relaxation (n=47 uses) and beach imagery (n=45 uses). No significant adverse events were reported among intervention participants.
Figure 2.
Number of Participants Using one or more CB Strategies by Study Day
Intervention Effects
After adjusting for covariates (i.e., recruitment clinic, age, gender, supportive medications, Time 1 anxiety, Time 1 depression, and Time 1 score on the outcome of interest), persons in the PC-CB intervention group had lower symptom cluster scores at Time 2 (MAdj = 2.99, SE = 0.29) than did persons in the waitlist control group (MAdj = 3.87, SE = 0.36, F = 3.57, P= 0.032), [effect size partial η2 = 0.052, CI η2 > 0.004]. Examining individual cluster component symptoms, significant differences between groups were observed in pain and fatigue, but not in sleep disturbance. Persons in the PC-CB intervention group reported less pain severity at Time 2 (MAdj = 1.99, SE = 0.30) compared to the control group (MAdj = 3.23, SE = 0.37, F = 6.70, P = 0.006), [effect size partial η2 = 0.093, CI η2 > 0.021]. Similarly, the PC-CB intervention group reported less fatigue at Time 2 (MAdj = 3.43, SE = 0.33) than the control group (MAdj = 4.31, SE = 0.40, F = 2.81, P = .049), [effect size partial η2 = 0.041, CI η2 > 0.002]. Scores on symptom interference with daily life did not differ between the intervention and control groups. Unadjusted mean (SD) scores on outcome variables at Time 1 and Time 2 are reported by group in Table 3.
Table 3.
Mean (SD) of Outcome Variables at Time 1 and Time 2 by Group (Unadjusted)
| Time 1
|
Time 2
|
|||
|---|---|---|---|---|
| Intervention(n = 43) | Control(n = 43) | Intervention(n=36) | Control(n=42) | |
| Symptom cluster severitya | 3.59 (1.46) | 3.92 (1.79) | 2.89 (1.48) | 3.53 (1.82) |
| Pain severityb | 1.97 (1.64) | 2.49 (1.88) | 1.65 (1.61) | 2.23 (1.96) |
| Fatigue severitya | 3.77 (1.76) | 4.03 (2.23) | 3.32 (1.74) | 3.96 (1.82) |
| Sleep dist. severity | 5.04 (2.49) | 5.25 (2.59) | 3.71 (2.15) | 4.39 (2.70) |
| Symptom Interference | 3.82 (2.55) | 4.40 (2.53) | 3.34 (2.58) | 3.98 (2.35) |
Note: Sleep disturbance severity scores were computed as z-scores transformed to a 0 to 10 scale.
Significant differences observed in covariate adjusted means,
P< 0.05,
P < 0.01.
We repeated the same analyses using an intent-to-treat approach, with the last value carried forward for all participants, including those who dropped or did not return Time 2 questionnaires. All findings were the same as the per protocol analysis.
When participants in the PC-CB intervention group used the CB strategies, pain, fatigue, and sleep disturbance severity ratings decreased significantly from before to after using a CB strategy. Comparisons between symptom distress ratings revealed similar reductions at the times CB strategies were used (Table 4).
Table 4.
Mean (SD) of Pre- and Post-CB Strategy Symptom Severity and Distress Ratings (Intervention Group)
| Pre-CB Strategy M (SD) |
Post-CB Strategy M (SD) |
t(df) | P | |
|---|---|---|---|---|
| Pain Severity | 3.27 (1.67) | 2.26 (1.47) | t(31) = 9.18 | 0.000 |
| Fatigue Severity | 4.31 (1.52) | 3.03 (1.64) | t(32) = 8.89 | 0.000 |
| Sleep dist. Severity | 4.23 (2.09) | 2.73 (1.99) | t(29) = 6.85 | 0.000 |
| Pain Distress | 3.32 (1.57) | 1.94 (1.29) | t(28) = 7.11 | 0.000 |
| Fatigue Distress | 3.71 (1.57) | 2.33 (1.59) | t(32) = 9.47 | 0.000 |
| Sleep dist. Distress | 3.83 (2.19) | 2.21 (1.94) | t(29) = 6.99 | 0.000 |
Discussion
The PC-CB intervention demonstrated initial efficacy in this pilot study, when compared to a waitlist control condition. Symptom cluster severity at the end of the two-week study period was lower in the intervention group compared to control, with statistically significant differences in the cluster severity score and in severity scores for two of the three component symptoms, pain and fatigue. In addition, significant reductions in all three symptoms were reported at the time of CB strategy use for persons in the intervention group. Symptom interference scores, however, did not differ between intervention and control groups.
Findings from this study are consistent with those of other investigators who have reported improvement in two or more related symptoms with the use of CB strategies. For example, Demiralp and colleagues [40] reported significant improvements in both fatigue and sleep among women with early stage breast cancer assigned to a progressive muscle relaxation intervention. Chan et al. [16] reported improvement in the anxiety, breathlessness, fatigue symptom cluster in patients with lung cancer using patient education and progressive muscle relaxation. Adding exercise to relaxation, Rabin and colleagues [41] reported reduced fatigue and improved sleep in early stage breast cancer patients, and Jarden et al. [12] reported improvement in gastrointestinal, cognitive, functional / pain, and mucositis symptom clusters experienced by hematopoietic stem cell transplant patients. CB strategies such as relaxation, distraction, and imagery may be particularly useful in treating multiple related symptoms that share a psychological component; that is, symptoms that are exacerbated by negative psychological reactions.
In the current study, our CB intervention seemed to have less effect on sleep disturbance and overall symptom interference with daily life, than on pain and fatigue. Although sleep disturbance scores were reduced, the difference between intervention and control groups was not statistically significant. Symptom education provided to patients in the intervention group described the range of experiences comprising sleep disturbance (e.g., sleeping too much during the day). This education may have expanded what intervention participants considered in their report of sleep disturbance at Time 2. In addition, some physiologic causes of interrupted sleep, such as needing to urinate, cannot be controlled with CB strategies. We hoped that the CB strategies may have helped patients return to sleep more quickly after using the bathroom, but they would not treat nocturia, per se. Given that sleep disturbance was a problem for participants in this trial, it is surprising that there was not more nighttime use of the CB strategies. Our earlier feasibility study [22] revealed greater use of the CB strategies at night (22%) than was observed in the current sample (approximately 8%). We advised patients with sleep concerns to keep the MP3 player beside their bed. But some patients may have felt too burdened to use the player at night – to locate the player, turn on the room lights to make pre-use ratings of symptom severity and distress, and to view MP3 operating keys. It is also possible that sleep disturbance is not sensitive to our CB intervention and requires more specific strategies focusing on sleep hygiene.
We hypothesized that reductions in the pain, fatigue, sleep disturbance symptom cluster would also reduce symptom interference with daily life. It did not. The MDASI symptom interference items ask participants to reflect on how all of their cancer-related symptoms interfered with daily activities, not just interference from pain, fatigue, and sleep disturbance. It is possible that these patients experienced the new onset or exacerbation of a range of treatment-related symptoms during the study period, which countered any improvement in interference related to our three target symptoms.
The PC-CB intervention was designed such that CB strategies would be used at times when patients experienced symptom exacerbations, and thus individualized to their specific symptom management needs. The CB strategies are short, with effects of some strategies, like distraction, only expected to be effective during use or for a short period after use. Thus, it is possible that the PC-CB intervention has more intermittent than sustained effects. This possibility is borne out by the significant reductions reported in all three symptoms at the time that the CB strategies were being used. Similar to short acting analgesics or sedatives, these CB strategies are beneficial when administered as needed, but need to be used more frequently and routinely to see a sustained effect over time. In this study, participants who used the strategies more frequently were not necessarily those who obtained greater symptom relief, as using the strategies frequently in the context of the study could mean that the CB strategies are effective or that participants were unsuccessfully searching for relief.
Participants used the full range of CB strategies available with fairly similar frequency. No one type of CB strategy (imagery-based vs. non-imagery based, nature vs. symptom-focused) was used substantially more frequently than the others. This finding underscores the variation in the types of strategies that patients prefer and the need to offer a wide range of strategies to meet the needs of a diverse population. Many of the scripts for relaxation and imagery interventions assume that listeners have similar perceptions of what are peaceful or relaxing experiences (e.g., rural nature images as opposed to urban images, experienced in solitude as opposed to with others). Future research should address the different preferences for images and contexts based on demographic characteristics such as race/ethnicity, gender, and rural versus urban residence.
There are some limitations of this pilot randomized controlled trial. A greater number of patients left the intervention group than the control group. This differential dropout may be related to the burden of using the CB strategies at least once a day, using the MP3 player, and / or completing the log of pre- and post-use symptom ratings. However, it also may be that those patients who dropped out did so because they were not experiencing any beneficial effect of the CB strategies. If symptoms actually worsened among participants who dropped out, then our intent-to-treat analysis using the last value carried forward resulted in biased findings. Depression scores at baseline were higher in the waitlist control group than the intervention group, despite random assignment. Our analysis controlled Time 1 depression scores as a covariate; however, this already depressed group may have been further disappointed to learn they had not been assigned to receive the intervention right away, which could have contributed to greater distress and symptom persistence. Future research also should include an attention control condition, to account for the effect of time spent using a novel device (i.e., MP3 player) and engaging in a daily activity, and for attention from the research staff in training to use the CB strategies.
Despite these limitations, the study provides evidence supporting the beneficial effects of using the PC-CB intervention to simultaneously control pain, fatigue, and sleep disturbance in patients with advanced cancer. Combined with findings from the earlier feasibility testing, this study supports pursuit of further research questions related to effects of the PC-CB intervention, including identification of moderating variables that explain individual differences in intervention effects, and physical and psychological mediating variables that may explain the mechanisms by which the intervention exerts its effects (changes in outcome expectancy, perceived control and stress biomarkers). Future research also should include a longer intervention period to determine if clinical impact of the intervention increases with time / practice and to test for sustained effects. Sleep strategies also may need enhancement in subsequent testing.
Clinicians may consider recommending CB strategies to their patients with concurrent pain, fatigue, and sleep disturbance, particularly as they become overwhelmed with managing multiple symptoms in advanced disease. Addressing clusters of symptom with a single intervention, such as the PC-CB intervention tested here, reduces patient burden and has the potential to improve symptom severity, distress, ability to engage in daily activities, and improve patients’ quality of life.
Acknowledgments
This work was supported by funding from the National Institute of Nursing Research (Grant Number R21 NR010746, PI: Kristine Kwekkeboom, UW-Madison School of Nursing).
Footnotes
Disclosures
The authors have no financial or other conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. ClinicalTrials.gov Identifier: NCT00946803
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck S, Dudley WN, Barsevick AM. Using a mediation model to test a symptom cluster: pain, sleep disturbance, and fatigue in cancer patients. Oncol Nurs Forum. 2005;32:E48–E55. doi: 10.1188/04.ONF.E48-E55. [DOI] [PubMed] [Google Scholar]
- 2.Esper P. Symptom clusters in individuals living with advanced cancer. Semin Oncol Nurs. 2010;26:168–174. doi: 10.1016/j.soncn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Lidstone V, Butters E, Seed PT, et al. Symptoms and concerns amongst cancer outpatients: identifying the need for specialist palliative care. Palliat Med. 2003;17:588–595. doi: 10.1191/0269216303pm814oa. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen AT, Petersen MA, Pedersen L, Groenvold M. Symptoms and problems in a nationally representative sample of advanced cancer patients. Palliat Med. 2009;23:491–501. doi: 10.1177/0269216309105400. [DOI] [PubMed] [Google Scholar]
- 5.Kirkova J, Walsh D, Davis M, Rybicki L. Symptom prevalence and symptom severity across different primary sites in 1000 advanced cancer patients. J Palliat Care. 2006;22:245. [Google Scholar]
- 6.Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Spichiger E, Müller-Fröhlich C, Denhaerynck K, et al. Symptom prevalence and changes of symptoms over ten days in hospitalized patients with advanced cancer: a descriptive study. Euro J Oncol Nurs. 2011;15:95–102. doi: 10.1016/j.ejon.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Dodd MJ, Miaskowski C, Paul S. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 9.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22:507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood P, Given BA, Given CW, et al. A cognitive-behavioral intervention for symptom management in patients with advanced cancer. Oncol Nurs Forum. 2005;32:1190–1198. doi: 10.1188/05.ONF.1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikorskii A, Given C, Given B, et al. Symptom management for cancer patients: a trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34:253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarden M, Nelausen K, Hovgaard D, Boesen E, Adamsen L. The effect of a multimodal intervention on treatment-related symptoms in patients undergoing hematopoietic stem cell transplantation: a randomized controlled trial. J Pain Symptom Manage. 2009;38:174–190. doi: 10.1016/j.jpainsymman.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood, and quality of life in breast cancer survivors. J Adv Nurs. 2007;61:664–675. doi: 10.1111/j.1365-2648.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 14.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trials of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 15.Barsevick A, Beck SL, Dudley W, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage. 2010;40:200–216. doi: 10.1016/j.jpainsymman.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CWH, Richardson A, Richardson J. Managing symptoms in patients with advanced lung cancer during radiotherapy: results of a psychoeducational randomized controlled trial. J Pain Symptom Manage. 2011;41:347–357. doi: 10.1016/j.jpainsymman.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Kwekkeboom KL, Cherwin CH, Lee JW, Wanta B. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage. 2010;39:126–138. doi: 10.1016/j.jpainsymman.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50:440–447. doi: 10.1176/appi.psy.50.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins SC, Montgomery GH, Raptis G, Bovbjerg DH. Effect of pretreatment distress on daily fatigue after chemotherapy for breast cancer. J Oncol Pract. 2008;4:59–63. doi: 10.1200/JOP.0822002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen SA. Negative affect and sensitization to pain. Scand J Psychol. 2002;43:131–137. doi: 10.1111/1467-9450.00278. [DOI] [PubMed] [Google Scholar]
- 21.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 22.Kwekkeboom KL, Abbott-Anderson K, Wanta B. Feasbility of a patient-controlled cognitive-behavioral intervention for pain, fatigue, and sleep disturbance in cancer. Oncol Nurs Forum. 2010;37:E151–E159. doi: 10.1188/10.ONF.E151-E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical recipes in FORTRAN: The art of scientific computing. 2. New York: Cambridge University Press; 1992. [Google Scholar]
- 24.Grout J. Relaxing sounds of nature [Audio recording] Clearwater FL: Passport Music; 2000. [Google Scholar]
- 25.Cleeland C. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Issues in pain management. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 26.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer: The M. D Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: psychometric and structural evaluation. Psychooncology. 2002;11:273–281. doi: 10.1002/pon.564. [DOI] [PubMed] [Google Scholar]
- 30.Shacham S. A shortened version of the Profile of Mood States. J Person Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 31.Devine EC. Meta-analysis of the effect of psychoeducational interventions on pain in adults with cancer. Oncol Nurs Forum. 2003;30:75–89. doi: 10.1188/03.ONF.75-89. [DOI] [PubMed] [Google Scholar]
- 32.Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: A meta-analysis of randomized experiments. Health Psychol. 1995;14:101–108. doi: 10.1037//0278-6133.14.2.101. [DOI] [PubMed] [Google Scholar]
- 33.Francoeur RB. The relationship of cancer symptom clusters to depressive affect in the initial phase of palliative radiation. J Pain Symptom Manage. 2005;29:130–155. doi: 10.1016/j.jpainsymman.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto JM, Blanch J, Atala J, et al. Clinical factors associated with fatigue in haematologic cancer patients receiving stem-cell transplantation. Eur J Cancer. 2006;42:1749–1755. doi: 10.1016/j.ejca.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Stone P, Richards M, A’Hern R, Hardy J. Fatigue in patients with cancers of the breast or prostate undergoing radical radiotherapy. J Pain Symptom Manage. 2001;22:1007–1015. doi: 10.1016/s0885-3924(01)00361-x. [DOI] [PubMed] [Google Scholar]
- 36.Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23:8296–8304. doi: 10.1200/JCO.2005.10.167. [DOI] [PubMed] [Google Scholar]
- 37.Mystakidou K, Tsilika E, Parpa E, et al. Psychological distress of patients with advanced cancer. Cancer Nurs. 2006;29:400–405. doi: 10.1097/00002820-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Serlin RC, Lapsley DK. Rational appraisal of methodological research and the good-enough principle. In: Keren G, Lewis C, editors. Methodological and quantitative issues in the analysis of psychological data. Hillsdale NJ: Lawrence Erlbaum Associates; 1993. pp. 199–228. [Google Scholar]
- 39.Steiger JH, Fouladi RT. Noncentrality interval estimation and evaluation of statistical models. In: Harlow LL, Mulaik SA, Steiger JH, editors. What if there were no significance tests? Mahwah NJ: Lawrence Erlbaum Associates; 1997. pp. 221–257. [Google Scholar]
- 40.Demiralp M, Oflaz F, Komurcu S. Effects of relaxation training on sleep quality and fatigue in patients with breast cancer undergoing adjuvant chemotherapy. J Clin Nurs. 2010;19:1073–1083. doi: 10.1111/j.1365-2702.2009.03037.x. [DOI] [PubMed] [Google Scholar]
- 41.Rabin C, Pinto B, Dunsiger S, Nash J, Trask P. Exercise and relaxation intervention for breast cancer survivors: feasibility, acceptability, and effects. Psychooncology. 2009;18:258–266. doi: 10.1002/pon.1341. [DOI] [PubMed] [Google Scholar]