Abstract
The disease mechanisms and histology of plaque development associated with atherosclerosis remain incredibly complex and not entirely understood. Recent investigations have emphasized the importance of inflammation in atherosclerosis. Several studies have also indicated heterotopic, or extraskeletal, bone formation in atherosclerotic vessels. The mechanisms behind heterotopic ossification (HO) appear to have similarities to those underlying atherosclerosis, with inflammation being a key inductive component to HO. Therefore, in the present study, we evaluated the histology associated with pathologies of atherosclerosis and HO in 271 coronary vessel tissue samples. We examined the prevalence and features of the inflammatory response, as well as new vessel and bone formation. Inflammation and neovascularization was observed both in the adventitia and within the atherosclerotic lesions of the vessels themselves. Intriguingly, neural changes, including collections of inflammatory cells and expression of neuro-inflammatory factors, were detected in the adventitial nerves of the vessels. Mature lamellar bone was found in 18 coronary vessels (7%), often with hematopoietic elements and active bone remodeling. Brown adipocytes, which pattern heterotopic bone formation, were present within the atherosclerotic lesions (28%, 75/271). As expected, there was a strong correlation between the presence of cholesterol and plaque formation (P<0.0001), but there also seemed to be a trend towards a connection between the presence of brown adipocytes and plaque. From this histological evaluation, along with cholesterol and dystrophic calcification, we noted a novel appearance of brown adipocytes, as well as neural changes, which may provide new insights to further our understanding of atherosclerosis.
Keywords: atherosclerosis, heterotopic ossification, nerve, brown adipocyte, bone, bone morphogenetic protein
1. Introduction
Atherosclerosis, which is the common cause of heart disease and stroke, two major morbidities worldwide, involves the formation of plaques within the arterial blood vessel [1–3]. These plaques, or lesions, are typified by continuous lipid accumulation and inflammatory infiltration within the arterial wall. Inflammatory mechanisms have emerged as a central player in atherosclerotic plaque development [2,4,5]. However, drug therapies targeting the inflammatory response have had mixed results, not always showing a measureable effect on clinical outcome [5–7]. This phenomenon most likely results from the complex biology governing plaque formation and highlights the necessity of a fresh assessment of the histology of this process.
Adding to this complexity are reports of de novo or heterotopic bone formation within a subset of these plaques [8–10]. Recently, we [11] and others [12] have shown that inflammation in sensory neurons is a key regulator of heterotopic ossification (HO) in skeletal muscle. This neuro-inflammation is associated with nerve remodeling, or degradation of the epineurial-endoneurial matrix, and the release of cells for the rapid production of brown adipose within the tissues [11,13,14]. One of the primary osteoinductive factors that appears to evoke neuro-inflammation is bone morphogenetic protein 2 (BMP2) [11,12]. Increased BMP2 signaling has recently been observed during atherosclerotic plaque formation and been shown to induce expression of proteins, such as CD68 and E-selectin, which are involved in the infiltration of inflammatory cells, such as monocytes [15]. Interestingly, cells expressing these same markers appear to infiltrate the site of de novo bone formation in response to neuro-inflammation [12,16,17].
Sensory nerves innervate the arterial wall and are predominantly found in the adventitial layer [18]. These nerves have been shown to be associated with unique microvasculature, termed the vasa vasorum, in the adventitia (for review see [19]). This microvasculature has been implicated in plaque formation and associated with two neuro-inflammatory factors, substance P (SP) and calcitonin gene related peptide (CGRP) [19–21].
Therefore, in the present retrospective study, we evaluated histological features of postmortem vessel tissue, with an emphasis on examining specific pathologies related to HO, including neuro-inflammation, adipose, and bone. We investigated the incidence of each of these features in the human plaques and show the presence of altered peripheral nerves, consistent with the sensory nerve remodeling observed in HO, as well as the presence of brown adipose in these tissues. We used this histological examination to view atherogenesis from a different perspective and to potentially identify new features within atherosclerotic plaques.
2. Methods
2.1 Histologic Examination of Vessels
Histologic slides from autopsy cases diagnosed with atherosclerosis plaque formation were retrieved from the files of the Michael E. DeBakey VA Medical Center in Houston following approved IRB protocols. Section retrieval was limited to the years of 1998–2004. Vessels identified with atherosclerotic plaques were identified by one of the authors (FHG). This vascular subset of patient slides were then examined by two of the authors (FHG and ES) for the following variables: inflammation, neovascularization, neuronal changes, brown fat formation, foam cells, cholesterol crystal formation, calcification, and ossification. All vessel types (coronary, aorta, iliac) represented within these patient slides were examined for these specific features. However, the representative histology shown focuses on these characteristics in the coronary vessels.
Inflammation was defined as the presence of white blood cells, either acute or chronic, outside of established vessels, singly or in loose aggregates, anywhere in the tissues examined. Acute inflammation was classified as primarily neutrophils with rare eosinophils noted. Chronic inflammation was defined as lymphocytes, plasma cells, or monocytes. The accumulation of inflammatory cells was then further defined to be either in the intimal/medial layers of the vessel or in the outer, adventitial layer.
Neovascularization (new blood vessel formation) was defined as an accumulation of thin vascular spaces. Like the inflammatory components, these vascular changes were characterized as either intralesional (intimal/medial layers) or within the outermost layer of the vessel wall (adventitial layer).
Neuronal changes were identified as accumulations of inflammatory cells and evidence of hemorrhage within the nerves, features outside the normal histologic appearance of nerves. Nerves were noted almost exclusively in the adventitia.
A histologic identification of brown fat cells was attempted when identifying foam cells. Foam cells were defined as macrophages with varying degrees of finely granular cytoplasmic accumulations, usually noted in proximity to ischemic areas of the plaques. Brown fat cells were identified as cells with discrete globules of lipid-like material filling the cytoplasm and displacing the small, dark nucleus laterally. This gave the appearance of a “soap-bubble” cytoplasm that was distinct from the foam cells. In instances where the morphologic features were indistinct, the cells were classified as foam cells. Immunohistochemistry for uncoupling protein 1 (UCP1), a well known marker of brown adipocytes [22], was used to confirm the presence of brown adipose.
Cholesterol accumulation was characterized by “needle-like” areas of clearing within the plaques and was usually noted in clusters. These accumulations were identified both with and without accompanying inflammation or foreign body-like giant cells.
Calcification was categorized as amorphous aggregates of basophilic crystalline material that appeared as basophilic staining of tissues on decalcified sections.
Bone was defined as an eosinophilic matrix deposition. These depositions were often accompanied by osteoblasts and osteoclasts. Marrow accumulation was defined as hematopoietic elements in various stages of maturation within the osseous elements. All foci of ossification were confirmed by polarized light microscopy.
2.2 Immunohistochemical Evaluation of Vessels
Vessel sections identified by hematoxylin and eosin staining to contain brown adipocytes or neuronal changes were further serially sectioned at a 5 μm thickness and used for immunohistochemical and immunofluorescent staining, following previously published methods [11,13]. Primary antibodies were used as follows: UCP1 (1:50 dilution; Chemicon, Billerica, MA), SP (8ug/mL; R&D Systems, Minneapolis, MN), S100 (2ug/mL; R&D Systems). To evaluate the subtype of lymphocytic infiltration, slides were stained for CD20 (B cell marker; DAKO USA) at a dilution of 1:150 and CD3 (T cell marker; DAKO USA) at a dilution of 1:110. For immunofluorescence, secondary antibodies conjugated to Alexa Fluor 488 or 594 (Invitrogen, Carlsbad, CA) were used at a dilution of 1:500.
2.3 Statistical Analysis
Statistical analyses were performed to investigate possible correlations between the histological features examined within the vessels. We focused on the correlations between the presence of plaque and the presence of the other histological features described in Table 1. We performed tetrachoric correlation analyses, as the variables are binary representations of continuous phenomena. The tetrachoric test makes assumptions about the underlying data and can therefore only provide an estimate of the existence and strength of associations. Data from all vessels, coronary, aorta, and iliac, was included in the analyses. Statistical analyses were made with Stata/MP (Version 11.0).
TABLE 1.
Histological Features of Coronary Vessels
| Histological Feature | Absence, n (%) | Presence, n (%) |
|---|---|---|
| Plaque | 4 (1) | 267 (99) |
| Cholesterol deposits | 72 (27) | 199 (73) |
| Neovascularization in the adventitia | 97 (36) | 174 (64) |
| Neovascularization in the media/ intima | 118 (44) | 153 (56) |
| Inflammatory cell infiltration in the adventitia | 95 (35) | 176 (65) |
| Inflammatory cell infiltration in the media/ intima | 141 (52) | 130 (48) |
| Neural inflammation/ hemorrhage | 219 (81) | 52 (19) |
| Foam cells | 139 (51) | 132 (49) |
| Brown adipocytes | 196 (72) | 75 (28) |
| Dystrophic calcification | 89 (33) | 182 (67) |
| Bone | 253 (93) | 18 (7) |
3. Results
3.1 Histological Characterization of the Adventitia
A total of 271 coronary vessels, from autopsy material, were examined for specific histological features (Table 1). Atherosclerotic plaques were identified in 267 (99%) of the coronary vessels and further analyzed for the histological variables outlined below. We also distinguished the presence of these variables within the specific layers (adventitial, medial, or intimal) of the vessel wall (Fig. 1).
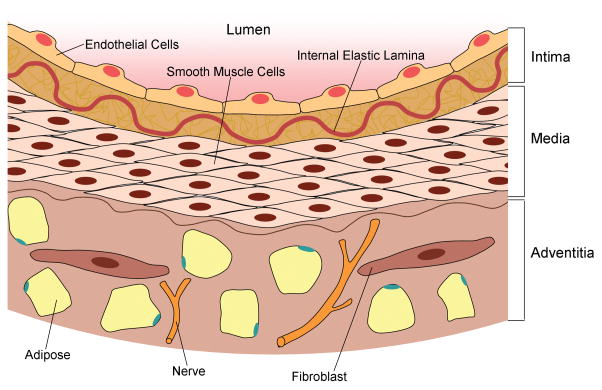
Fig. 1.
Anatomy of a normal arterial blood vessel, which is comprised of three distinct layers. The intima is the innermost layer of the vessel, which is composed of a single layer of endothelial cells that line the lumen of the vessel and extracellular connective tissue, including collagen and proteoglycans. The internal elastic lamina (IEL) separates the intima and media. The media is the middle layer of the vessel, which is made of smooth muscle cells. The adventitia, the outermost layer of the vessel wall, consists of connective tissue, adipose, and interspersed fibroblasts. The adventitial layer also contains peripheral nerves that supply the vessel.
Neovascularization was observed in the adventitial layer, surrounding the atherosclerotic vessel wall (Fig. 2A–B), in 174 (64%) of the samples. In some instances, we noted cells with the histological appearance of brown adipocytes adjacent to this new vessel formation (Fig. 2B). Inflammatory cells were also present in the adventitial layer of the vessel wall in 65% of the coronary vessels. Fig. 2C shows a representative image of this inflammation within the adventitia that appears to then invade the muscular wall of the vessel.
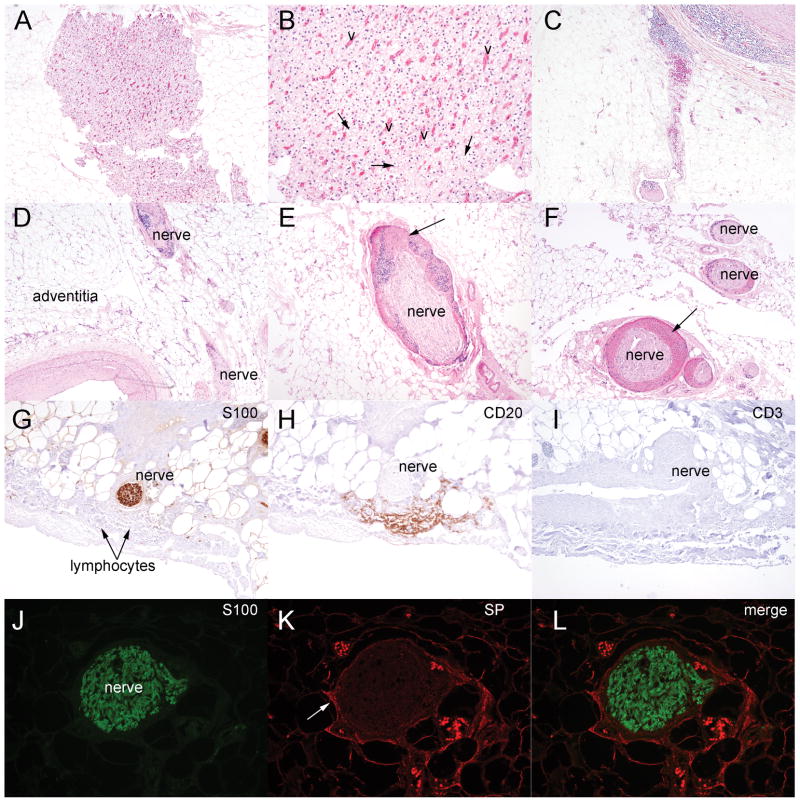
Fig. 2.
Histological changes in the adventitial layer of coronary vessels with atherosclerosis. (A) Photomicrograph of vascular changes, adjacent to fat cells, within the adventitia (hematoxylin and eosin, original magnification × 40). (B) Higher-power photomicrograph of neovascularization (v) and brown adipocytes (arrow) (original magnification × 100). (C) Low-power photomicrograph of inflammatory cells within the adventitia of a vessel with plaque (original magnification × 40). (D) Low-power view of nerves (nerve) surrounded by clusters of inflammatory cells within the adventitia (original magnification × 40). (E & F) Nerves with hemorrhage and inflammation (arrow) (original magnification × 100 (E) and × 40 (F)). (G) S-100 immunostaining (brown) marking nerves in the adventitial layer. (H&I) Immunostaining of serial tissue sections shown the presence of B lymphocytes (CD20 positive brown cells) and the absence of T lymphocytes (lack of CD3 positive brown cells) (original magnification × 100). (J–L) S100-positive nerves in the adventitia (green) express the neuro-inflammatory molecule SP (red color, arrow) (original magnification × 200).
Given the contribution of the peripheral nerves to the initial inflammatory response in heterotopic ossification [11,12,14], we evaluated the nerves, located in the adventitia, and found alterations from the normal histological appearance in 52 (19%) of the atherosclerotic coronary vessels. These changes included nerves surrounded by clusters of inflammatory cells and nerves exhibiting signs of hemorrhage (Fig. 2D–F). Further immunohistochemical analysis of the inflammatory cells surrounding the nerves revealed these cells to predominately consist of B lymphocytes, as evidenced by expression of CD20 (Fig. 2G–I). In addition, these nerves expressed the pro-inflammatory neuropeptide Substance P (Fig. 2J–K).
3.2 Histological Characterization of the Media and Intima
Neovascularization and inflammatory cell infiltration was also observed in the medial and intimal layers of the vessel in 153 (56%) and 130 (48%) of the specimens respectively. Representative images of these characteristics are shown in Fig. 3A–D. The inflammatory response within these layers of the vessel has been detailed and reviewed previously 5, but we confirmed these classic histological features within our sample population. Cholesterol deposits were also found within atherosclerotic lesions in a majority (73%) of the vessels, and calcification was detected in 67% of the vessels (Fig. 3E, F). Finally, we observed instances of necrotic debris within the plaques, although we did not quantify the presence of this feature.
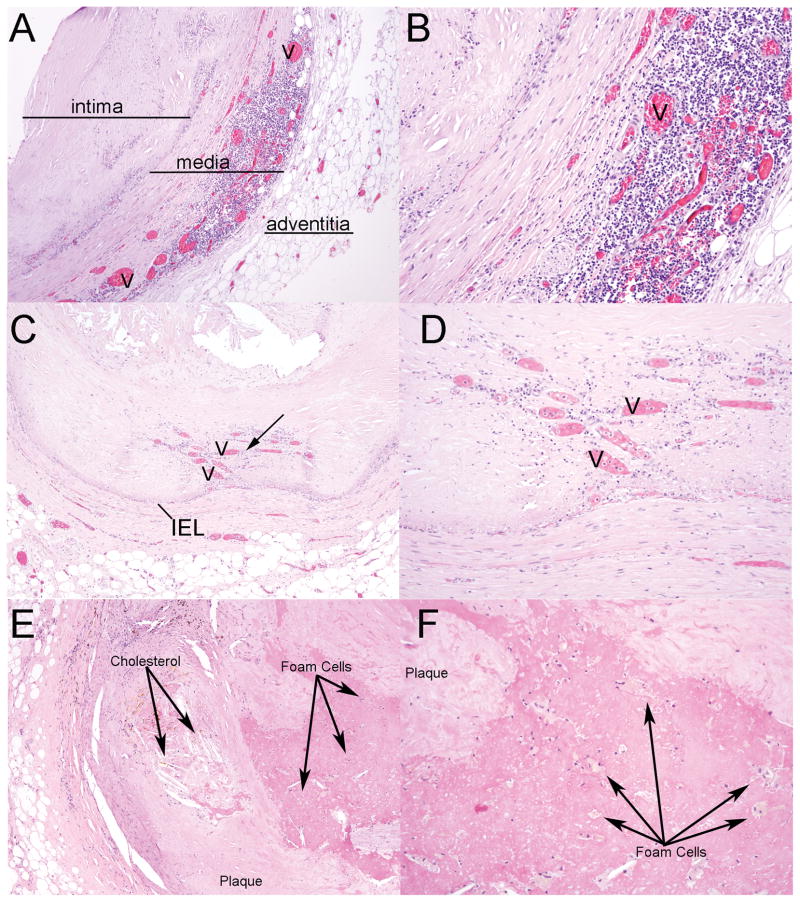
Fig. 3.
Histological changes in the medial and intimal layers of coronary vessels with atherosclerosis. (A) Image of neovascularization (v) and inflammatory cells within the muscular layer of the coronary vessel (hematoxylin and eosin, original magnification × 40). (B) Higher-power image (original magnification × 100). (C) Image of neovascularization (arrow) that is in the intimal region and is adjacent to dystrophic calcification (original magnification × 40). (D) Higher-power image (original magnification × 100). (E) Photomicrograph of cholesterol accumulation within an atherosclerotic lesion with calcification (original magnification × 40). (F) Higher power image highlighting the presence of foam cells within the plaque (original magnification × 100). Cholesterol and foam cells indicated as labeled. IEL=internal elastic lamina.
3.2.1 Histological identification of brown adipocytes
As brown adipocytes play a crucial role in the mechanisms of HO [13], the histological analysis was extended to evaluate the appearance of these cells within atherosclerotic plaques (Fig. 4). Brown adipocytes have a unique histological phenotype, characterized by a considerable amount of cytoplasm with multiple lipid-like droplets. These cells were identified in 75 (28%) of the coronary vessels (Fig. 4A, B), and were distinct from foam cells found in 48% (Fig. 3E, F and Fig. 4E, F). Immunohistochemical analysis confirmed this presence of brown adipocytes by expression of the brown adipocyte specific marker UCP1 (Fig. 4C, D).
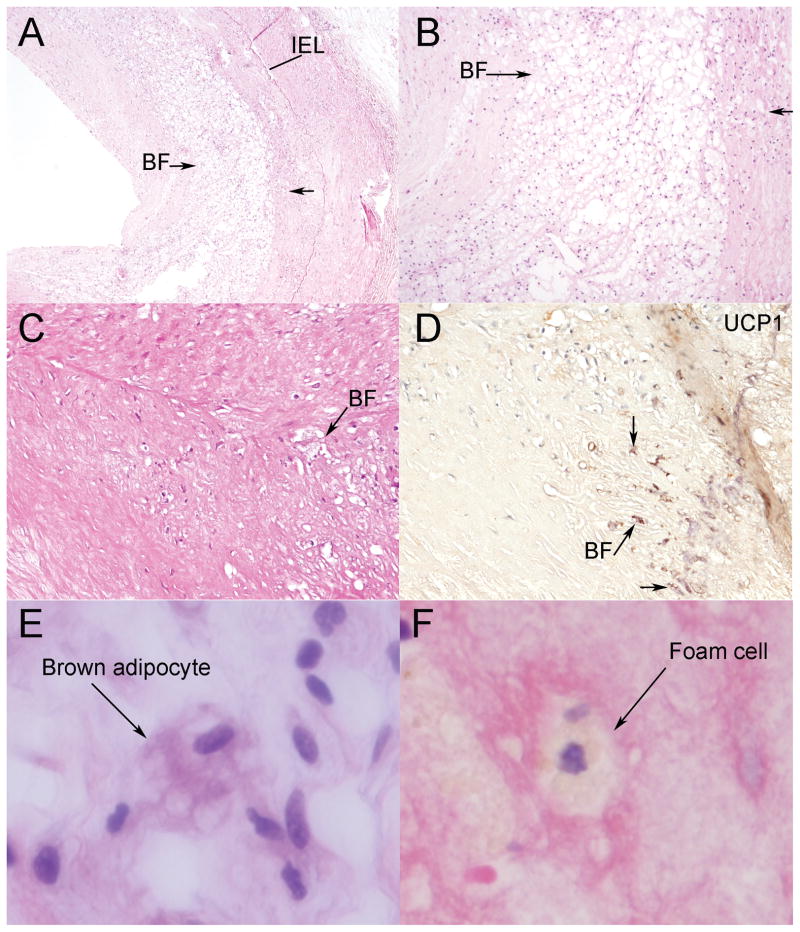
Fig. 4.
Appearance of brown adipocytes. (A) Low (original magnification × 40) and (B) higher-power (original magnification × 100) view of brown fat cells (arrow) within atherosclerotic plaques in the intima of coronary vessels. (C&D) Representative image of brown fat cells (arrow) on hematoxylin and eosin staining, shown with immunohistochemistry to express the brown fat marker UCP1 (brown) (original magnification × 200). (E&F) High power image (original magnification × 400) showing the typical “soap-bubble” cytoplasm of the brown adipocytes (E), distinct from the morphology of a foam cell (F). IEL=internal elastic lamina; BF= brown fat.
3.2.2 Characterization of bone within the plaque
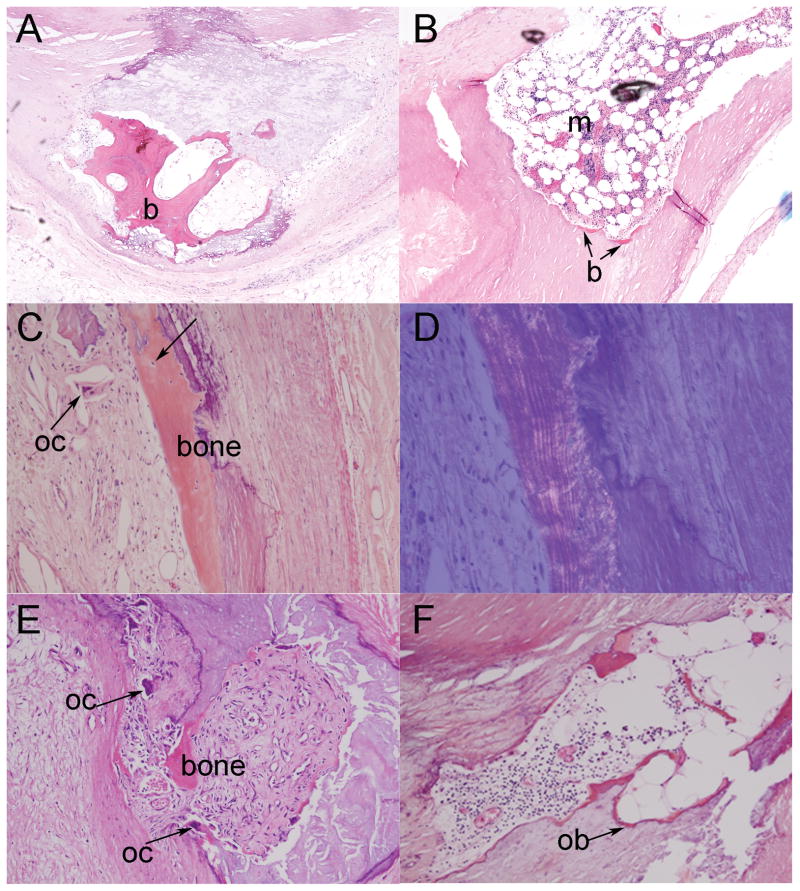
Bone was detected in 7% of the coronary vessels examined (Fig. 5). This ossification followed a linear, layered pattern of collagen when viewed under polarized light, indicative of mature, lamellar bone (Fig. 5C, D). Hematopoietic elements were noted within the bone structure of some samples, suggesting marrow accumulation (Fig. 5B). In addition, we observed distinct bone cell types associated with the osseous structure, including osteocytes embedded within the bone matrix (Fig. 5C), multinucleated osteoclasts within resorption spaces (Fig. 5E), and osteoblasts on the bone forming surface (Fig. 5F).
Fig. 5.
Bone with hematopoietic elements and active remodeling within plaques of human coronary vessels. (A) Photomicrograph of mature bone (b) within calcified matrix of the progressing atherosclerotic lesion (original magnification × 40). (B) Photomicrograph of tentative marrow accumulation (m), with distinctive stem and fat cells, within the plaque wall (original magnification × 40). (C) Low-power image of mature bone (bone) with osteogenic cells, including osteocytes (arrow) and osteoclasts (oc) (original magnification × 100). (D) Same field of view shown under polarized light microscopy (original magnification × 200) reveals the linear, lamellated bright areas characteristic of mature bone. (E) Photomicrograph of bone with osteoclasts (oc) (original magnification × 100). (F) Photomicrograph of osetoblasts (ob) on the bone surface within the plaque (original magnification × 100).
3.3 Correlation between histological features and plaque formation
We evaluated any correlations between the presence of plaque and the presence of the histological features discussed above by tetrachoric correlation analyses (correlation coefficient=rtet). We found a strong correlation between plaque and cholesterol (rtet=0.795, p<0.0001), plaque and calcification (rtet=0.7563, p<0.0001), and plaque and neovascularization within the media and intima (rtet=0.7458, p<0.001). In addition, the presence of plaque was moderately correlated to the presence of neovascularization within the adventitia (rtet=0.6034, p<0.0001), inflammation both in the adventitia (rtet=0.5528, p<0.001) and the media and intima (rtet=0.6899, p<0.001), and foam cells (rtet=0.615, p<0.001). Interestingly, there was a trend towards a moderate association between brown adipocytes and plaque within the vessel (rtet=0.6845, p=0.07). We did not observe a significant association between plaque appearance and either neural changes in the adventitial layer or bone.
4. Discussion
Atherosclerosis is a slowly progressing disease of the arterial walls, commonly leading to coronary artery disease (CAD), peripheral artery disease, and stroke [23]. The classic histological features of atherosclerotic plaques have been previously described [24,25] and refined [26], and are the basis for the current histological grading system for atherosclerotic lesions established by the American Heart Association (AHA) [26,27]. These plaques continue to evolve throughout one’s life, exhibiting complex histological changes that can vary from one individual to another and within each individual [28]. Given the heterogeneous and complicated nature of plaque formation and development, in this study we have focused on identifying the incidence of key features that may be common to both heterotopic ossification and atherosclerotic lesion progression. From this approach, we have identified novel features, including abnormal peripheral nerves, brown adipocytes, and neovascularization within the adventitia and media/intima of vessels containing atherosclerotic plaques and true heterotopic bone.
We observed a unique reaction, characterized by hemorrhaging and inflammation, of the nerves within the adventitia. To our knowledge, this is the first report of the abnormal histological appearance of the nerves surrounding the vessel wall. It is interesting to speculate whether neuro-inflammatory mediators play a key role in atherosclerosis similar to heterotopic ossification. These adventitial nerves were expressing the neuro-inflammatory molecule substance P and had associated lymphocytic infiltration of B-lymphocytes. B-lymphocytes and mast cells have been demonstrated to be uniquely present in early heterotopic ossification in human skeletal muscle from patients who have the genetic disease fibrous dysplasia ossificans progressiva [29,30]. Blocking SP binding to its receptor suppresses this process, again showing a key functional role for these factors [12]. Within cardiac vessels, sensory peptinergic nerves, producing SP, are found in close association with the micro-vasculature, or vasa vasorum, within the adventitia [20,21]. There has been interest in the vasa vasorum not only because of its interaction with the intima [31], but also because of its potential role in atherosclerosis [32]. In this study, we identified both inflammation and neovascularization within the adventitial layer of atherosclerotic vessels. Further, this new blood vessel formation and inflammation extended to both the medial and intimal layers, thereby perpetuating the inflammatory cascade in atherosclerosis. Together, this data suggests the nerve may contribute to the inflammatory response and the vascular changes observed during plaque formation.
In addition, we observed the appearance of brown adipocytes within the vessel intima, which have been implicated in regulating the local oxygen environment to pattern heterotopic bone formation [13,33]. In the setting of HO, neural inflammation induces progenitor cell recruitment, and the brown adipocyte generates the local environment for progenitor cell differentiation [11,13,33]. On one hand, brown adipocytes undergo oxidative metabolism, lowering local oxygen levels necessary for chondrocyte differentiation [13]. On the other hand, brown adipocytes promote neovascularization, both by activation of the hypoxia inducible factor (HIF) pathway and secretion of vascular endothelial growth factor-A and -D (VEGF) [13,33]. These factors encourage new vessel formation, increasing local oxygen levels necessary for osteoblast differentiation. Since these brown adipocytes play a role in establishing new vessels during HO, we speculate that they may perform a similar function in the vascular remodeling needed in atherosclerosis. In addition, brown adipose tissue has been shown to be a critical mediator of triglyceride homeostasis [34]. Furthermore, based on the ability of brown adipocytes to modulate local oxygen levels, these cells may be well suited for pathological conditions with an altered oxygen environment, such as atherosclerosis. Whether these brown adipocytes appear within the plaque to aid in vascular remodeling, triglyceride homeostasis, or simply as a consequence of the surrounding environmental conditions, to our knowledge, this is the first report on the incidence of such a cell type within a human disease process, such as atherosclerosis. Interestingly, our recent data shows that the brown adipocytes rapidly observed in HO arise from unique progenitors within the epineurial region of the nerve (Salisbury et al, unpublished). Further, their release is dependent not only on neuro-inflammation, but also remodeling of the epineurial region of the nerve itself.
As lesion development proceeded, we recognized cholesterol accumulation and calcification within the plaque, in addition to mature bone. This bone exhibited a lamellar bone structure under polarized light and signs of active remodeling displayed through the presence of osteoblasts, osteoclasts, and osteocytes. Some of the bone even contained hematopoietic elements.
When examining any associations between plaque and the identified histological features related to HO, we noted a possible trend between atherosclerotic plaques and brown adipocytes. However, our statistical analyses did not reveal significant correlations with either changes in the nerve or bone. The aberrant nature of bone within the plaque may account for the lack of an observed association. Furthermore, the absence of a significant association between abnormal nerves in the adventitia and plaque may be due to the fact that this inflammation within the nerves represents an early initiating stage of plaque formation, similar to HO, and was consequently underrepresented in the sample population examined. Similarly, if bone formation is a very late event in atherosclerosis, then death may occur before its formation in some patients. There are likely few patients where one would see all stages of the disease, both early and late, as atherosclerosis develops over a decade or more [35].
The present histological analysis within the coronary vessels examined stages of HO in atherosclerotic vessels. This analysis provides the foundation for future studies to define the role of peripheral nerves and brown adipose in the advancement of atherosclerosis, which may identify novel mechanisms to regulate or suppress this process. Future studies should aim to discover the role of these elements in the setting of atherosclerosis, which could provide further clues to the unwanted pathological consequences of the disease. As current studies have highlighted the contribution of inflammation to plaque formation, knowledge of the complete picture of this inflammatory component, including the potential participation of the nerves in this process, may provide new opportunities for prevention and risk assessment of the progression of atherosclerosis. Furthermore, by examining atherosclerosis in the context of heterotopic ossification, markers of nerves, brown adipocytes, vessel formation, and osteoblasts could be used to highlight the changes in plaque progression over time. This could lead to future diagnostic and imaging studies to follow and/or identify very early in vivo changes that precede atherosclerotic plaque formation.
Acknowledgments
Grant Support: American Heart Association (AHA 10815339F), Kirschstein-NRSA (T32 HL092332-08)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 3.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moubayed SP, Heinonen TM, Tardif JC. Anti-inflammatory drugs and atherosclerosis. Curr Opin Lipidol. 2007;18:638–44. doi: 10.1097/MOL.0b013e3282f0ee11. [DOI] [PubMed] [Google Scholar]
- 7.Zenovich AG, Taylor DA. Atherosclerosis as a disease of failed endogenous repair. Front Biosci. 2008;13:3621–36. doi: 10.2741/2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt JL, Fairman R, Mitchell ME, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–19. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 9.Mohler ER, 3rd, Gannon F, Reynolds C, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–28. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 10.Herisson F, Heymann MF, Chetiveaux M, et al. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis. 2011;216:348–54. doi: 10.1016/j.atherosclerosis.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Salisbury E, Rodenberg E, Sonnet C, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112:2748–58. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kan L, Lounev VY, Pignolo RJ, et al. Substance P signaling mediates BMP dependent heterotopic ossification. J Cell Biochem. 2011;112:2759–72. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olmsted-Davis E, Gannon FH, Ozen M, et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–32. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salisbury E, Sonnet C, Heggeness M, et al. Heterotopic ossification has some nerve. Crit Rev Eukaryot Gene Expr. 2010;20:313–24. doi: 10.1615/critreveukargeneexpr.v20.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, Bennett BJ, Wang X, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafer J, Davis AR, Gannon FH, Fouletier-Dilling CM, et al. Oxygen tension directs chondrogenic differentiation of myelo-monocytic progenitors during endochondral bone formation. Tissue Eng. 2007;13:2011–19. doi: 10.1089/ten.2006.0063. [DOI] [PubMed] [Google Scholar]
- 17.Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011;26:1685–93. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- 18.Olesen IJ, Gulbenkian S, Valenca A, et al. The peptidergic innervation of the human superficial temporal artery: immunohistochemistry, ultrastructure, and vasomotility. Peptides. 1995;16:275–87. doi: 10.1016/0196-9781(94)00165-0. [DOI] [PubMed] [Google Scholar]
- 19.Baikoussis NG, Apostolakis EE, Papakonstantinou NA, et al. The implication of vasa vasorum in surgical diseases of the aorta. Eur J Cardiothorac Surg. 2011;40:412–17. doi: 10.1016/j.ejcts.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 20.Milner P, Crowe R, Loesch A, et al. Neurocompensatory responses to balloon-catheter-induced injury of the rat carotid artery. J Vasc Res. 1997;34:31–40. doi: 10.1159/000159199. [DOI] [PubMed] [Google Scholar]
- 21.Herbst WM, Eberle KP, Ozen Y, et al. The innervation of the great saphenous vein: an immunohistochemical study with special regard to regulatory peptides. Vasa. 1992;21:253–57. [PubMed] [Google Scholar]
- 22.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–88. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 24.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 25.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 26.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–78. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 27.Dalager S, Paaske WP, Kristensen IB, et al. Artery-related differences in atherosclerosis expression: implications for atherogenesis and dynamics in intima-media thickness. Stroke. 2007;38:2698–705. doi: 10.1161/STROKEAHA.107.486480. [DOI] [PubMed] [Google Scholar]
- 28.Insull W., Jr The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122:S3–S14. doi: 10.1016/j.amjmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Gannon FH, Glaser D, Caron R, et al. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol. 2001;32:842–48. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 30.Gannon FH, Valentine BA, Shore EM, et al. Acute lymphocytic infiltration in an extremely early lesion of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998:19–25. [PubMed] [Google Scholar]
- 31.Stefanadis CI, Karayannacos PE, Boudoulas HK, et al. Medial necrosis and acute alterations in aortic distensibility following removal of the vasa vasorum of canine ascending aorta. Cardiovasc Res. 1993;27:951–56. doi: 10.1093/cvr/27.6.951. [DOI] [PubMed] [Google Scholar]
- 32.Barger AC, Beeuwkes R, 3rd, Lainey LL, et al. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–77. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 33.Dilling CF, Wada AM, Lazard ZW, et al. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner Res. 2010;25:1147–56. doi: 10.1359/jbmr.091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 35.Hagg S, Salehpour M, Noori P, et al. Carotid plaque age is a feature of plaque stability inversely related to levels of plasma insulin. PLoS One. 2011;6:e18248. doi: 10.1371/journal.pone.0018248. [DOI] [PMC free article] [PubMed] [Google Scholar]