Abstract
We analyzed data from case groups of 538 children with autism spectrum disorders (ASD) and 163 with developmental delays (DD), and from 421 typically developing controls to assess associations with maternal influenza or fever during pregnancy. Exposure information was obtained by telephone interviews, and outcomes were clinically confirmed. Though neither ASD nor DD was associated with influenza, both were associated with maternal fever during pregnancy: OR’s (odds ratios) were 2.12 (95 % CI 1.17, 3.84) and 2.50 (95 % CI 1.20, 5.20) respectively. However, the fever-associated ASD risk was attenuated among mothers who reported taking antipyretic medications (OR = 1.30, 95 % CI 0.59, 2.84), but remained elevated for those who did not (OR = 2.55, 95 % CI 1.30, 4.99).
Keywords: Maternal influenza, Fever, Autism, Anti-fever medication
Introduction
Previous studies have suggested an association between maternal infections during pregnancy and neurodevelopmental disorders like autism (Atladóttir et al. 2010) and schizophrenia (Brown et al. 2004). Specific infectious organisms reported to be risk factors for autism include congenital rubella (Chess 1971), and maternal prenatal measles, mumps (Deykin and MacMahon 1979), cytomegalovirus (Yamashita et al. 2003), bacteria (Atladóttir et al. 2010), and influenza (Deykin and MacMahon 1979). In the United States, the prevalence of the first three viruses is very low due to effective vaccination programs and hence they are unlikely to play a role in current cases of autism. Influenza viruses are prevalent worldwide and they cause about 36,000 deaths every year in the U.S. according to the Centers for Disease Control and Prevention (CDC). During the recent influenza (flu) pandemic, pregnant women were among the most vulnerable groups to experience severe illnesses (Louie et al. 2009). The effects of the viruses on the developing fetus remain to be determined. In animal experiments, offspring of dams infected with human influenza viruses during pregnancy exhibited autistic-like behaviors (Patterson 2002) pointing to the possibility of an association between influenza infection and risk of autism in humans.
Very few epidemiological studies have investigated the possible association between maternal influenza infection during gestation and autism. In a case–control study Deykin and MacMahon (1979) determined that mothers of cases were more likely to have had or been exposed to influenza during gestation compared with controls (RR = 4, p < 0.001). However, Dassa et al. (1995) did not find any association between the community rate of death attributed to influenza and risk of autism. The two previous studies were different in design and both had some limitations that include recall bias, low power, and misclassification of exposure (through ecological measures) and/or outcome.
Clinical symptoms of influenza infection usually include the occurrence of fever, which in previous animal and epidemiological studies has been suggested to be associated with neural tube defects when it occurred in the first trimester (Edwards 2006; Moretti et al. 2005). Moreover, maternal fever exceeding 38°C in labor has been associated with increased risk of unexplained cerebral palsy (Grether and Nelson 1997). A recent large epidemiological study, including 587 people with cerebral palsy and 1,154 controls, found a fivefold increased risk when mothers experienced fever during pregnancy (O’Callaghan et al. 2011). Studies using animal models also indicate that fever in mid gestation may produce behavioral abnormalities in the offspring (Edwards 2006). The literature on maternal fever and autism is sparse and reported results are inconsistent. Very few studies (Deykin and MacMahon 1979; Wilkerson et al. 2002) have reported a positive association between fever and autism. One report (Maimburg and Vaeth 2006) in which maternal fever was determined by medical records, thereby not capturing mild cases or those not brought to medical attention, found no link. None of the previous cited studies investigated whether the use of medications to control symptoms of fever had an effect on their results.
Given the inconclusive nature of the literature, we conducted a case–control study with a large population-based sample to investigate the association between maternal influenza infection or fever during pregnancy and risk for autism and developmental delays. Unlike previous studies, many of which relied on administrative data, the present analysis included a population of children who had their diagnoses confirmed by standardized instruments, and potential confounding was controlled by multivariate methods. We additionally assessed whether associations were modified by maternal use of medications for influenza or fever symptoms. Sampling weights were applied to adjust for self-selection, thereby rendering results more generalizable to the target population. The study was approved by the Institutional Review Boards for the Protection of Human Subjects of the State of California and of the University of California, Davis.
Methods
Study Population
Cases and controls in the present analysis are part of a large ongoing population-based case–control investigation known as the Childhood Autism Risk from Genetics and Environment (CHARGE) Study. The broad goals of the CHARGE Study are to determine the contributions of environmental exposures, characterize phenotypes, and examine genetic factors and their interactions with environment in the etiology of autism (Hertz-Picciotto et al. 2006).
To be eligible, both case and control children have to meet the following criteria: age between 24 and 60 months, live with at least one biological parent, have a parent who speaks English or Spanish, were born in California, and reside in the study catchment areas of California covering 20 counties. Participants in the CHARGE study are sampled from three strata: children with autism, children with developmental delay but not autism, and children from the general population.
A large proportion of autism and developmental delay cases are identified through the California Department of Developmental Services system, which includes 21 Regional Centers across the state. Other sources of cases include the Medical Investigations of Neurodevelopmental Disorders (MIND) Institute clinic patients, participants in other MIND Institute studies, the CHARGE Study website, and referrals from the Regional Centers, friends, and health or service providers. The control group was sampled from the general population, using the state birth file, by random selection with frequency-matching to the projected age and Regional Center catchment area distribution of the autism cases and sampled with a 4:1 male:female ratio.
Children aged 2 to 5 years old whose mothers consented to participate between January 2003 and September 2010 were included in the present analysis. Siblings of cases or controls were not included in this analysis.
Diagnostic Classification
Standardized clinical assessments were conducted by trained clinicians to confirm each child’s diagnostic group. The Mullen Scales of Early Learning (Mullen 1995) were used to evaluate cognitive function and the Vineland Adaptive Behavior Scales (Sparrow et al. 1984) to assess adaptive function in all children. Autism case status was assessed using the Autism Diagnostic Interview-Revised (Le Couteur et al. 1989) and the Autism Diagnostic Observation Schedules-Generic (Lord et al. 2000). Developmental delay cases and general population controls were evaluated following the same protocol as the autism cases except that their parents completed the Social Communication Questionnaire, and only if they scored at or above the a priori cut point of 15 were the Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedules administered.
Autism case status was defined as meeting criteria on the communication, social and repetitive behavior domains of the Autism Diagnostic Interview-Revised with onset prior to 36 months and scoring at or above the autism cutoff on the communication plus social interaction total of the Autism Diagnostic Observation Schedules module 1, 2, or 3. Children who did not meet criteria for autism but (1) scored at least 7 on the Autism Diagnostic Observation Schedules-Generic Module 1 or at least 8 on Module 2; (2) met the cutoff value for section A or B and were within 2 points of the cutoff value on A or B (whichever did not meet cutoff value) in the Autism Diagnostic Interview-Revised; and (3) met the cutoff value on section D in Autism Diagnostic Interview-Revised were classified as autism spectrum disorder. In the present study, our first case group includes children with either autistic disorder or autism spectrum disorders and will be referred to as the autism spectrum disorder group (ASD). ASD onset type was determined by parental report and categorized as “early” (no statement of loss of social and/or language skills, or “regressive” (clear loss of previously acquired language and/or social skills).
The developmental delay group includes children who did not meet suspicion for ASD using the Social Communication Questionnaire and obtained composite scores of ≤69 on the Mullen Scales of Early Learning and the Vineland Adaptive Behavior Scales. Children recruited from the general population who scored ≥70 on both Mullen and Vineland Adaptive Behavior Scales and showed no evidence of an ASD on the Social Communication Questionnaire were classified as typically developing.
Exposure Data
A telephone interview was conducted by trained bilingual (English and Spanish) interviewers with the mother regarding preconceptional, prenatal, and early childhood exposures and experiences of the index child. Standardized questions were used to collect information about maternal influenza, other infectious diseases, and fever associated with each condition, as well as fever from unknown cause. Specifically, mothers were asked if, during their pregnancy, they had the “Flu”. If they responded yes, they were asked during which month(s) of the pregnancy they had the “Flu”. In addition, they were asked if they had fever with their “Flu” and if they took any medication for either their “Flu” or fever and if so, which medication they had taken for each condition. During the interview we also asked if, during the pregnancy, they had a fever for which they did not know the cause. We also asked about influenza vaccination. Relevant parts of the questionnaire are attached (Appendix 1 of Supplementary material). The interviewees had the option to answer yes, no, don’t know or refuse to any of the questions. To increase the accuracy of the definition of exposure and minimize misclassification, e.g., of the common cold, we defined influenza during pregnancy as maternal self reported influenza infection accompanied by fever. For sensitivity analysis we also utilized the self reported influenza infection alone. We conducted a separate analysis to examine the hypothesis that fever from any cause during gestation might be associated with ASD.
Statistical Analysis
We performed univariate descriptive analysis to check for data completeness, outliers, and distributional assumptions of the variables. Bivariate analyses were conducted between the main exposures (self reported influenza or fever) and selected covariates previously reported to be associated with increased risk of autism or that we suspected to be potential confounders. We also examined the association between the potential confounders and diagnostic categories using the odds ratio. The covariates included maternal age (< 25, 25–29, 30–34, and ≥35 years), education (High school education or less, and more than high school), place of residence (grouped by catchment areas of the California Department of Developmental Services Regional Centers), smoking 3 months before conception or during pregnancy, periconceptional vitamin supplementation, parity (1 vs. ≥2 children), type of health insurance coverage (government vs. private), child sex, and race/ethnicity (Hispanic, non-Hispanic white, and other). Variables that were associated with influenza or fever and with the developmental categories were considered potential confounders and entered in a multivariate model predicting either ASD or developmental delay.
We fitted a series of multivariate regression models to the data, initially including exposures of interest and all potential confounders identified in the bivariate analysis. Covariates were retained in the model only if they changed the magnitude of the beta coefficient of maternal flu or fever during pregnancy by at least 10 %. All matching variables (regional center catchments areas, child sex, and age) were included in all multivariate analyses regardless of impact on beta coefficient. We used both the logistic regression and the survey logistic regression procedure of SAS® (Statistical Analysis Software, Cary, North Carolina version 9.2) (SAS 2002). We report odds ratios (OR) as measures of association between developmental outcome and maternal self reported influenza (with fever) or any self reported fever during pregnancy, along with their 95 % confidence intervals (CI). We also calculated the OR by trimester of exposure to influenza or to fever. We explore if antipyretic medications modified the relationship of influenza or fever with autism spectrum disorders. The list of reported medications for influenza and fever symptoms is in Table 1.
Table 1.
List of medications that women reported they took for influenza and fever symptoms, and list of medications that we determined were antipyretic. The California CHARGE study 2003–2010
| List of reported medication taken for flu | List of reported medication taken for fever | Our list of medications considered anti fever |
|---|---|---|
| Advil | Acyclovir | Advil |
| Aleve | Amoxicillin | Darvocet |
| Amoxicillin | Antibiotic | Excedrin |
| Antibiotic | Augmentin | Ibuprofen |
| Azithromycin | Azithromycin | Motrin |
| Benadryl | Ciprofloxacin | Nyquil |
| Promethazine | Dayquil | Theraflu |
| Chloraseptic Spray | Darvocet | Tylenol |
| Dithenoxyoate | Ibuprofen | Advil |
| Excedrin | Keflex | |
| Motrin | Nyquil | |
| Mylanta/peptobismal | Penicillin | |
| Nyquil | Prodciden | |
| Phenergan with codeine | Sudafed | |
| Phenergan | Tylenol | |
| Robitussin | ||
| Saline Nasal Spray | ||
| Sudafed | ||
| Theraflu | ||
| Tylenol |
We conducted weighted multivariate analyses, with the goal to make our sample better represent the target population with regard to the distribution of socio-demographic factors, i.e., to adjust for differential response rates among cases and controls. The weights were determined based on the probabilities of participating in the study as a function of the three sampling strata, maternal education, age, country of birth, type of payment for child’s delivery (private insurance or not), and child race/ethnicity.
Our study included 538 children with autism spectrum disorders, 163 with developmental delay but not autism, and 421 typically developing children. Assuming exposure rates during pregnancy between 15 and 20 % for the mothers of the controls, we anticipated that our study would have between 82 and 89 % power to detect odds ratios as small as 1.65, when comparing ASD cases to typical developing controls. We anticipated a power of 80–86 % to detect odds ratios of 1.95 or higher when comparing the developmental delay group to typically developing controls.
Results
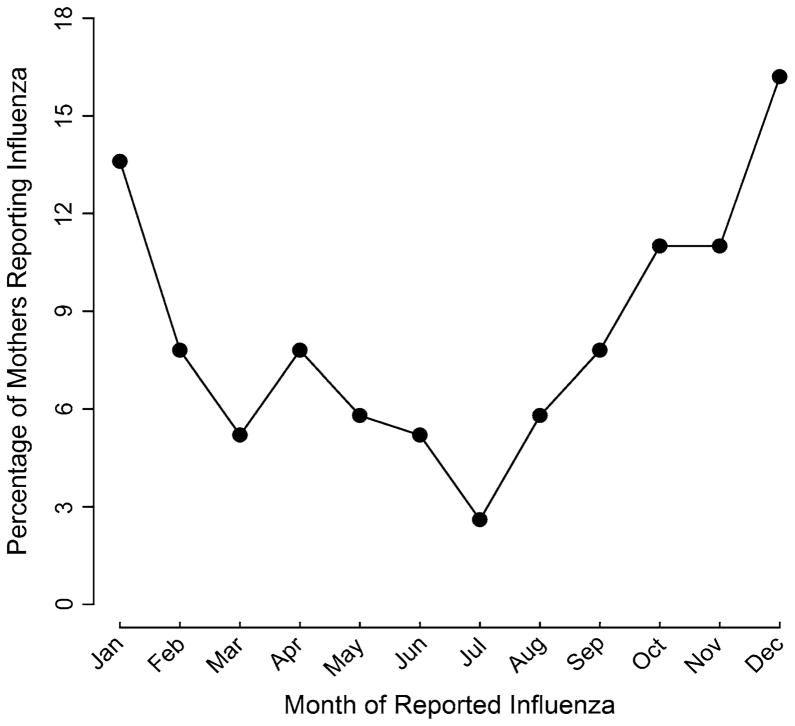
Of the eligible contacted families, 26 % of children from the general population and 30 % of children in the autism spectrum disorders group were enrolled. The proportions of maternal self-reported influenza among cases and controls were respectively 9 and 8 %. For fever during pregnancy, the proportions were 18 % for mothers of cases compared to 15 % for mothers of controls. Influenza infection in our sample followed the traditional influenza season in California. A greater number of the infections occurred in the winter and fall seasons (Fig. 1).
Fig. 1.
Percentage of self-reported influenza by calendar month for mothers of both cases and controls combined. The California CHARGE Study 2003–2010
Socio-demographically, there were no differences between the mothers of children with autism, and the mothers of children with typical development. However, mothers of children with developmental delays were less likely to have an education level greater than high school [OR = 0.54, 95 % CI (0.35, 0.82)], and more likely to have their deliveries paid by a government sponsored insurance (OR = 2.32, 95 % CI (1.49, 3.57) compared to mothers of typically developing children (Table 2). Child race/ethnicity and type of maternal insurance coverage were associated with influenza, fever, and developmental categories and therefore were included in all the multivariate models in addition to the matching variables. For the analysis where fever was the main exposure, maternal influenza during pregnancy was found to be a confounder, i.e., it changed the beta coefficient of fever by more than 10 %.
Table 2.
Characteristics (frequency, %) of children with autism spectrum disorders (ASD), developmental delays (DD), or typical developing (TD) and their mothers. CHARGE Study, California, 2003–2010
| Developmental category
|
|||
|---|---|---|---|
| ASD (n = 538) | DD (n = 163) | TD (n = 421) | |
| Exposure variables | |||
| Influenza during pregnancy | |||
| Yes | 47 (8.7) | 16 (9.8) | 33 (7.8) |
| No | 491 (91.3) | 147 (90.2) | 388 (92.2) |
| Fever during pregnancy | |||
| Yes | 97 (18) | 32 (19.6) | 62 (14.7) |
| No | 441 (82.0) | 131 (80.4) | 359 (85.3) |
| Other covariates | |||
| Anti-fever or anti-inflammatory medication taken during pregnancy | |||
| Yes | 32 (6.0) | 13 (8.0) | 31 (7.4) |
| No | 506 (94.0) | 150 (92.0) | 390 (92.6) |
| Maternal periconceptional vitamin supplementation (3 months before conception up to the first month of pregnancy)a | |||
| Yes | 263 (48.8) | 75 (46.0) | 236 (56.0) |
| No | 213 (39.6) | 65 (39.9) | 115 (27.3) |
| Maternal age at child’s birth in year | |||
| < 25 | 75 (13.9) | 36 (22.01) | 68 (16.1) |
| 25–29 | 143 (26.5) | 41 (25.1) | 95 (22.5) |
| 30–34 | 171 (31.8) | 43 (26.4) | 158 (37.5) |
| ≥35 | 149 (27.8) | 43 (26.4) | 100 (23.7) |
| Maternal educationb | |||
| >High school | 460 (85.5) | 118 (72.4) | 349 (82.9) |
| ≤High school | 77 (14.3) | 45 (27.6) | 72 (17.1) |
| Type of insurance coverage at child deliveryc | |||
| Government | 82 (15.2) | 45 (27.6) | 60 (14.3) |
| Private | 455 (84.6) | 118 (72.4) | 360 (85.5) |
| Parity | |||
| 2 children or more | 297 (55.2) | 104 (63.8) | 244 (58.0) |
| 1 | 241 (44.8) | 59 (36.2) | 177 (42.0) |
| Maternal residence by regional center at child birth | |||
| North Bay | 71 (13.2) | 16 (9.8) | 62 (14.8) |
| San Andreas, East Bay, and Golden Gate | 95 (17.6) | 16 (9.8) | 75 (17.8) |
| Southern California | 93 (17.3) | 10 (6.1) | 27 (6.4) |
| Valley Mountain and Central Valley | 88 (16.3) | 37 (22.7) | 68 (16.1) |
| Alta | 191 (35.5) | 84 (51.5) | 189 (44.9) |
| Race/ethnicity | |||
| Hispanic | 146 (27.1) | 50 (30.6) | 97 (23.0) |
| Others | 125 (23.2) | 43 (26.3) | 101 (24.0) |
| White | 267 (49.7) | 70 (42.9) | 223 (53.0) |
| Maternal place of birth | |||
| USA | 404 (75.1) | 127 (77.9) | 341 (81.0) |
| Mexico | 42 (7.8) | 26 (16.0) | 31 (7.4) |
| Others | 92 (17.1) | 10 (6.1) | 49 (11.6) |
| Maternal smoking 3 months before or during pregnancyd | |||
| Yes | 66 (12.2) | 11 (6.8) | 31 (7.4) |
| No | 430 (79.9) | 133 (81.6) | 322 (76.5) |
| Child sex | |||
| Male | 459 (85.3) | 106 (65.0) | 327 (77.7) |
| Female | 79 (14.7) | 57 (35.0) | 94 (22.3) |
62 ASD, 23 DD, and 70 TD children had missing maternal per-iconceptional vitamin supplementation
One ASD case had missing maternal education
One ASD and one typical child had missing maternal insurance status
42 ASD, 19 DD, and 68 typical children had missing smoking status
In a multivariate analysis controlling for race/ethnicity and type of insurance coverage in addition to the matching variables, the weighted odds ratio (wOR) comparing self-reported influenza between mothers of ASD children and those of children with typical development was 1.26, 95 % CI (0.73, 2.19). We did not see a difference in the proportion of self-reported influenza during pregnancy between mothers of children with developmental delays and those of children with typical development [wOR = 1.15, 95 % CI (0.54, 2.47)] (Table 3). The results did not change when they were adjusted for medication use for influenza symptoms (column 1 of Table 1). These findings did not change based on the reported trimester of infection (Results not shown). When we stratified the analysis by anti-pyretic medication, we found that for women who reported taking these medications for their influenza (Had flu and took medication: 22 cases, 18 controls) the wOR for the association between maternal reported influenza and ASD was 1.00, 95 % CI (0.49, 2.07). For those who did not report taking antipyretics (Had flu but did not take medication for it: 25 cases, 15 controls) the wOR was 1.60, 95 % CI (0.72, 3.55).
Table 3.
Weighted multivariate adjusted odds ratios (wOR) and 95 % confidence intervals (CI) for maternal self-reported influenza and fever during pregnancy in relation to child’s developmental outcome. The California CHARGE Study, 2003–2010
| Developmental outcome
|
||||
|---|---|---|---|---|
| ASD
|
DD
|
|||
| wORa | (95 % CI) | wORa | (95 % CI) | |
| Exposure during pregnancy | ||||
| Influenza | 1.26 | (0.73, 2.19) | 1.15 | (0.54, 2.47) |
| Fever | 2.12 | (1.17, 3.84) | 2.50 | (1.20, 5.20) |
| Other covariates | ||||
| Type of insurance | ||||
| Government | 0.94 | (0.62, 1.42) | 1.92 | (1.16, 3.12) |
| Private | 1.00 | 1.00 | ||
| Race/ethnicity | ||||
| Hispanics | 1.03 | (0.70, 1.50) | 1.65 | (0.96, 2.83) |
| Others | 0.91 | (0.63, 1.31) | 1.52 | (0.89, 2.58) |
| White | 1.00 | 1.00 | ||
Results of the multivariate analysis were also adjusted for the matching variables (child age, sex, and maternal place of residence at child’s birth)
In a subset of children whose mother reported information on influenza vaccination (N = 426), we found that this variable was not a confounder of the association between influenza infection and ASD or developmental delay. However, we found that a significantly higher proportion of mothers of ASD cases reported not being vaccinated for flu compared to mothers of controls [67 % cases vs. 50 % controls, wOR = 2.30, 95 % (CI 1.32, 3.99)]. The proportion of self-reported influenza during pregnancy was the same among mothers of children of ASD with early onset or regression and mothers of controls (8 % in each group) When we defined exposure to influenza as only self-reported without the inclusion of fever, the wOR of the association of influenza and ASD was 0.90, 95 % CI (0.60, 1.40).
In the analysis of associations between fever and ASD, the odds that mothers of children with ASD reported fever during pregnancy was doubled that of mothers of typically developing controls [wOR = 2.12, 95 % CI (1.17, 3.84)]. Again, using typically developing children as referents, a higher proportion of mothers of children with developmental delays reported fever during pregnancy [wOR = 2.50, 95 % CI (1.20, 5.20)] (Table 3). Trimester analysis showed that children exposed to first and second trimester fevers were more vulnerable to ASD than those exposed to a third trimester fever (Table 4).
Table 4.
Multivariate adjusted odds ratios (OR) and 95 % confidence intervals (CI) for the association between maternal fever during each trimester of pregnancy and developmental categories. The California CHARGE Study, 2003–2010
| Developmental outcome
|
||||
|---|---|---|---|---|
| ASD
|
DD
|
|||
| wOR | (95 % CI) | wOR | (95 % CI) | |
| Exposure to fever by trimester of pregnancya | ||||
| First | 2.23 | (0.97, 5.11) | 1.79 | (0.60, 5.37) |
| Second | 2.60 | (1.14, 5.95) | 1.83 | (0.63, 5.29) |
| Third | 1.45 | (0.59, 3.53) | 2.62 | (0.92, 7.45) |
Each trimester was modeled separately, with adjustment for maternal anti-pyretic medication use, place of residence, type of insurance coverage, race/ethnicity, child age, and child’s sex
Women who did not report fever also did not report taking anti-pyretic medication. Using this group as referents, we found that the wOR for ASD was 2.55, 95 % CI (1.30, 4.99) for children whose mothers reported fever but did not take anti-pyretic medication. For women who reported fever and took anti-pyretic medications, the wOR for ASD = 1.30, 95 % CI (0.59, 2.84) (Table 5). Maternal anti-pyretic medication did not modify the results of the association between fever and developmental delays.
Table 5.
Weighted multivariate analysis of the association between self-reported fever during pregnancy and developmental outcome. Results stratified by maternal report of anti-pyretic medication. The California CHARGE Study, 2003–2010
| Developmental outcome
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ASD
|
DD
|
TD
|
||||||||
| N | % | ORa | (95 % CI) | N | % | ORa | (95 % CI) | N | % | |
| Had fever but took anti-pyretic medication | 32 | 5.95 | 1.30 | (0.59, 2.84) | 13 | 7.98 | 2.05 | (0.78, 5.36) | 31 | 7.36 |
| Had fever but did not take anti-pyretic medication | 65 | 12.08 | 2.55 | (1.30, 4.99) | 19 | 11.66 | 2.73 | (1.19, 6.28) | 31 | 7.36 |
The ORs were adjusted for maternal report of influenza, type of insurance coverage during pregnancy, race/ethnicity in addition to the matching variables (child age, sex, and maternal place of residence at child birth). The reference group was mothers with no fever; none took medications for fever
Discussion
In this case–control study, odds ratios were elevated but not significantly for maternal influenza and either autism spectrum disorders (ASD) or developmental delays, but fever during pregnancy was associated with both sets of conditions. Maternal anti-fever medication modified the association between fever and ASD. Specifically, the OR for ASD was higher for children whose mothers reported having fever during pregnancy but not taking anti-fever medication, compared to children whose mothers did not have fever. However, children whose mothers reported fever but took anti-fever medication had comparable odds for ASD as those whose mothers did not have fever during pregnancy. Our results parallel those reported in animal model studies of maternal immune activation where the neutralization of the induced pro-inflammatory cytokines in pregnant dams minimizes the development of behavioral abnormality otherwise seen in the offspring (Smith et al. 2007). Contrary to a previous report hypothesizing that use of anti-fever medication can be detrimental to the developing brain and thus lead to autism (Torres 2003) we report that their usage during the prenatal period by mothers to control fever may be beneficial to the developing fetus with regard to autism spectrum disorders.
Our finding of an association between maternal fever and ASD is similar to that previously reported by Wilkerson et al. (2002). However, their patient population ranged from age 2 to 40 years, whereas our study had a fairly homogeneous age range of children. By including participants with a wide age range, it is likely that the diagnostic criteria in the previous study were not uniform. In contrast to two previous case–control studies (Zhang et al. 2010; Deykin and MacMahon 1979), our results do not support an overall association between maternal self-reported influenza during pregnancy and autism. Zhang et al. (2010) reported an unadjusted OR of 3.29 (95 % CI 1.02, 10), but the results were not adjusted for other covariates because of a small sample size (95 cases of autism and 95 controls). Mothers of only 12 cases and four controls were exposed to influenza. Deykin and MacMahon (1979) reported a relative risk of 4.1, p < 0.001 for the association between maternal influenza infection and autism. Their study had 163 cases and 355 controls with 17 and 11, respectively, having mothers who reported being exposed to influenza, but also did not control for potential confounders such as maternal socio economic factors. Our study included 538 cases of ASD, more than both previous studies and adjusted for maternal place of residence during pregnancy, type of insurance coverage, child age, race/ethnicity, and sex. In the study by Deykin and MacMahon (1979) the association between prenatal influenza and autism was stronger when the investigators included women who reported either themselves or someone else in the household having influenza, suggesting possible mis-classification of exposure that could have biased the results. In addition, both previous studies may be subject to substantial recall errors because the duration between the pregnancy and the interview of the parents of the cases and controls ranged from 3 to 27 years, in comparison with CHARGE Study intervals of 3–5 years from pregnancy to interview, which may have produced higher accuracy of exposure information. An additional difference was the source of diagnoses of cases, which was either administrative or hospital data in previous studies, compared with a standardized assessment by trained clinicians in the CHARGE Study, a far more rigorous diagnostic process. Nevertheless, some non-differential misclassification of exposure may have biased our results on influenza illness in pregnancy towards the null.
We found that fewer mothers of children with ASD reported receiving influenza vaccine compared to mothers of typical controls. This result should be considered with caution because they did not report the exact timing of the influenza vaccination. More research needs to be conducted on this subject to fully investigate the effect of influenza vaccine on the risk of autism.
Similar to previous reports, our results can potentially be affected by recall bias if mothers of cases remember and recall more accurately than controls. We did not have serological confirmation of influenza infection, and the medical records were incomplete with regard to documentation of the occurrence of either influenza or fever. Hence we relied on self-reported information, raising the possibility of either a differential or non differential misclassification, or a combination of the two, for maternal influenza or fever during pregnancy. We attempted to mitigate the possibility of errors arising if mothers reported a common cold as the flu, by defining influenza to be self-reported influenza with fever. We also found that self-reported influenza events followed the expected seasonal pattern in this region of California, suggesting broad validity. Nevertheless, we could not preclude a modest but potentially meaningful association with maternal influenza of an OR of 1.65 or lower. An additional limitation is that if maternal influenza during pregnancy increases risk for autism, and if there is a critical vulnerable time window within the pregnancy period, our study might not have been able to detect such possible association because our numbers did not allow fine subdivision into shorter time periods.
Despite the limitations, the CHARGE study has major strengths. Detailed standardized questionnaires were used to gather exposure information. In addition, all CHARGE study participants had their diagnosis confirmed by standard diagnostic assessments administered by trained, research-reliable clinicians. Thus, misclassification of outcome is very unlikely. We used a fairly large and ethnically diverse population-based sample instead of a clinic sample and adjusted for selection bias through a weighted analysis.
If maternal fever is associated with autism spectrum disorders or developmental delays, what might be the biological mechanism? When the human body is invaded by exogenous organisms such as bacteria or viruses, leukocytes and other cell types are stimulated to respond by releasing pro-inflammatory cytokines, most notably, inter-leukins (IL) IL-1, IL-6, tumor necrosis factor, and interferon-γ. One of the clinical manifestations of such response is the elevation in the body temperature (fever). The mechanism by which these interleukins induce fever is complex and may involve multiple pathways. After their release into the bloodstream in response to pathogenic agents, cytokines reach the central nervous system where they induce the synthesis of prostaglandins leading to fever (Netea et al. 2000). For a review of the effect of cytokines on fever see (Conti et al. 2004).
Experimental studies have shown that levels of cytokines can be altered in the placenta and amniotic fluid after pregnant animals are exposed to infectious agents (Ashdown et al. 2006; Gilmore et al. 2004; Meyer et al. 2006) or when the maternal immune system is activated (Patterson 2011). These types of studies have also found that IL-2, IL-6 and IL-8 can cross the placenta and enter the fetal environment (Ponzio et al. 2007; Zaretsky et al. 2004). Cytokines are capable of altering the release of neurotransmitters like acetylcholine, dopamine, serotonin, or norepinephrine in the brain (Libbey et al. 2005; Ponzio et al. 2007) leading to decreased cerebral cortical neuronal survival during brain development. Moreover, injection of IL-6 into pregnant mice was found to lead to the development of behavioral abnormalities that are suggestive of autistic or schizophrenic behaviors (Smith et al. 2007). A previous epidemiological investigation that used banked specimens from a prospective study (Brown et al. 2004) found that second trimester IL-8 was elevated in serum of mothers of patients with schizophrenia. However, unlike the animal model studies, no association was found between IL-6 and schizophrenia. Recent epidemiological studies reported significant differences in maternal cytokine and chemokine levels during pregnancy between autism cases and controls. Goines et al. (2011) found that maternal mid-gestation interferon gamma, IL-4 and IL-5 were elevated in mothers of autism cases compared to controls. Abdallah et al. (2011) reported elevated levels of IL-4, IL-10 and tumor necrosis factors α and β in amniotic fluids of ASD patients as well as increased levels of amniotic chemokines (Abdallah et al. 2012).
Previous researchers on maternal infections and risk of neurodevelopmental disorders have postulated that maternal reaction to infections rather than the direct effects of the infectious agents may be the mechanism by which infections in mothers were associated with disorders like schizophrenia and autism (Shi et al. 2003). In the present study, the majority of the reported fever is associated with infections, despite the null association of influenza infection with autism spectrum disorders. We did not have measurements of maternal cytokines during pregnancy, but fever represents a clinical manifestation of pro-inflammatory cytokines due to either infections or other unknown causes. Elevated levels of cytokines like Il-6 have also been observed in pregnant women with fever during labor in the absence of infections (Goetzl et al. 2002). This type of fever has been found to be associated with early unexplained seizure in some infants (Lieberman et al. 2000). In addition to the cytokine hypothesis, maternal antibodies raised against fetal brain tissue have been proposed as a possible mechanism of maternal immune activation that can lead to neurodevelopment abnormalities (Grether et al. 2010; Martin et al. 2008).
In conclusion, we did not find an association between maternal influenza infection during pregnancy and either ASD or developmental delay. However, mothers whose children had autism spectrum disorders at ages 2–5 years were more likely to report fever from any cause during pregnancy compared to those of similarly aged children with typical development. This was also true of mothers whose child had developmental delay. Our results additionally suggest that anti-fever medication used to control fever during pregnancy can reduce or eliminate the association we observed between maternal fever and autism.
Supplementary Material
Acknowledgments
The authors would like to thank Paula Krakowiak and Lora Delwiche both from the Department of Public Health Sciences at the University of California Davis for their great data management skills, Dr. Daniel Tancredi, PhD from the Department of Pediatrics at the University of California Davis for his statistical analysis advice.
Funding The present work was supported by grants R01-ES015359 and P01-ES11269 from the National Institute of Environmental Health Sciences; # R-829388 & R-833292 from the U.S. Environmental Protection Agency’s STAR program; and the UC Davis MIND Institute.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10803-012-1540-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Ousseny Zerbo, Email: ozerbo@ucdavis.edu, Department of Public Health Sciences, MS1C, University of California, Davis, CA 95616, USA.
Ana-Maria Iosif, Department of Public Health Sciences, MS1C, University of California, Davis, CA 95616, USA.
Cheryl Walker, Department of Public Health Sciences, MS1C, University of California, Davis, CA 95616, USA.
Sally Ozonoff, The UC Davis Medical Investigation of Neurodevelopmental Disorders (MIND) Institute, Sacramento, CA, USA.
Robin L. Hansen, The UC Davis Medical Investigation of Neurodevelopmental Disorders (MIND) Institute, Sacramento, CA, USA. Department of Pediatrics, University of California, Davis, CA, USA
Irva Hertz-Picciotto, Department of Public Health Sciences, MS1C, University of California, Davis, CA 95616, USA. The UC Davis Medical Investigation of Neurodevelopmental Disorders (MIND) Institute, Sacramento, CA, USA.
References
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid inflammatory cytokines: Potential markers of immunologic dysfunction in autism spectrum disorders. World Journal of Biology Psychiatry. 2011 doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid chemokines and autism spectrum disorders: An exploratory study utilizing a Danish Historic Birth Cohort. Brain, Behavior, and Immunity. 2012;26(1):170–176. doi: 10.1016/j.bbi.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: Implications for schizophrenia. Molecular Psychiatry. 2006;11(1):47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of General Psychiatry. 2004a;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2004b;161(5):889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- CDC. CDC; http://www.cdc.gov/flu/about/season/flu-season.htm. [Google Scholar]
- Chess S. Autism in children with congenital rubella. Journal of autism and childhood schizophrenia. 1971;1(1):33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Frontiers in Bioscience. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Dassa D, Takei N, Sham PC, Murray RM. No association between prenatal exposure to influenza and autism. Acta Psychiatrica Scandinavica. 1995;92(2):145–149. doi: 10.1111/j.1600-0447.1995.tb09558.x. [DOI] [PubMed] [Google Scholar]
- Deykin EY, MacMahon B. Viral exposure and autism. American Journal of Epidemiology. 1979;109(6):628–638. doi: 10.1093/oxfordjournals.aje.a112726. [DOI] [PubMed] [Google Scholar]
- Edwards MJ. Review: Hyperthermia and fever during pregnancy. Birth Defects Research Part A, Clinical and Molecular Teratology. 2006;76(7):507–516. doi: 10.1002/bdra.20277. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29(7):1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- Goetzl L, Evans T, Rivers J, Suresh MS, Lieberman E. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. American Journal of Obstetrics and Gynecology. 2002;187(4):834–838. doi: 10.1067/mob.2002.127135. [DOI] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased mid-gestational IFN-gamma, IL-4, and IL-5 in women giving birth to a child with autism: A case-control study. Molecular Autism. 2011;2(1):13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether JK, Croen LA, Anderson MC, Nelson KB, Yolken RH. Neonatally measured immunoglobulins and risk of autism. Autism Research. 2010;3(6):323–332. doi: 10.1002/aur.160. [DOI] [PubMed] [Google Scholar]
- Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278(3):207–211. [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, et al. Autism Diagnostic Interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19(3):363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. Journal for Neurovirology. 2005;11(1):1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- Lieberman E, Eichenwald E, Mathur G, Richardson D, Heffner L, Cohen A. Intrapartum fever and unexplained seizures in term infants. Pediatrics. 2000;106(5):983–988. doi: 10.1542/peds.106.5.983. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. New England Journal of Medicine. 2009;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatrica Scandinavica. 2006;114(4):257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain, Behavior, and Immunity. 2008;22(6):806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Experimental Brain Research. 2006;173(2):243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- Moretti ME, Bar-Oz B, Fried S, Koren G. Maternal hyperthermia and the risk for neural tube defects in offspring: Systematic review and meta-analysis. Epidemiology. 2005;16(2):216–219. doi: 10.1097/01.ede.0000152903.55579.15. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines MN: American Guidance Services Inc; 1995. [Google Scholar]
- Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clinical Infectious Diseases. 2000;31(Suppl 5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- O’Callaghan ME, MacLennan AH, Gibson CS, McMichael GL, Haan EA, Broadbent JL, et al. Epidemiologic associations with cerebral palsy. Obstet Gynecol. 2011;118(3):576–582. doi: 10.1097/AOG.0b013e31822ad2dc. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends in Molecular Medicine. 2002;17(7):384–389. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends Molecular Medicine. 2011 doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Annals of the New York Academy of Sciences. 2007;1107:118–128. doi: 10.1196/annals.1381.013. [DOI] [PubMed] [Google Scholar]
- SAS. SAS (Statistical Analysis Software) 9.1.3. Cary, NC: Statistical Analysis Software Institute; 2002. [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. Journal of Neuroscience. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales Interview Edition Expanded Form Manual. Circle Pines: American Guidance Services Inc; 1984. [Google Scholar]
- Torres AR. Is fever suppression involved in the etiology of autism and neurodevelopmental disorders? BMC Pediatrics. 2003;3:9. doi: 10.1186/1471-2431-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson DS, Volpe AG, Dean RS, Titus JB. Perinatal complications as predictors of infantile autism. International Journal of Neuroscience. 2002;112(9):1085–1098. doi: 10.1080/00207450290026076. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Fujimoto C, Nakajima E, Isagai T, Matsuishi T. Possible association between congenital cytomegalovirus infection and autistic disorder. Journal of Autism and Developmental Disorders. 2003;33(4):455–459. doi: 10.1023/a:1025023131029. [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstetrics and Gynecology. 2004;103(3):546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lv CC, Tian J, Miao RJ, Xi W, Hertz-Picciotto I, et al. Prenatal and perinatal risk factors for autism in China. Journal of Autism and Developmental Disorders. 2010;40(11):1311–1321. doi: 10.1007/s10803-010-0992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.