Abstract
Clinical experience supports a critical role for nutrition in patients with spinal muscular atrophy (SMA). Three-day dietary intake records were analyzed for 156 visits in 47 SMA type I patients, 25 males and 22 females, ages 1 month-13 years (median 9.8 months) and compared to dietary reference intakes for gender and age along with anthropometric measures and dual-energy x-ray absorptiometry (DEXA) data. Using standardized growth curves, twelve patients met criteria for failure to thrive (FTT) with weight for age < 3rd percentile; eight met criteria based on weight for height. Percentage of body fat mass was not correlated with weight for height and weight for age across percentile categories. DEXA analysis further demonstrated that SMA type I children have higher fat mass and lower fat free mass than healthy peers (p<0.001). DEXA and dietary analysis indicates a strong correlation with magnesium intake and bone mineral density (r=0.65, p<0.001). Average caloric intake for 1–3 year olds was 68.8 ±15.8 kcal/kg - 67% of peers’ recommended intake. Children with SMA type I may have lower caloric requirements than healthy age-matched peers, increasing risk for over and undernourished states and deficiencies of critical nutrients. Standardized growth charts may overestimate FTT status in SMA type I.
Keywords: Spinal muscular atrophy type I, body composition, bone density, nutrient and caloric intake, dual-energy x-ray absorptiometry, growth status, nutrient deficiencies
Introduction
Spinal muscular atrophy (SMA) is one of the most common lethal recessive genetic diseases, with an incidence of 1 in 10,000 births (1, 2). The condition is medically serious, associated with significant motor disability, respiratory insufficiency, and death in infancy or childhood in more than 50% of affected children. Homozygous deletion and/or mutation of the survival motor neuron 1 (SMN1) gene on 5q13 is disease-causing, and the severity inversely correlates with SMN2 copy number (3, 4). SMA type I, also known as Werdnig-Hoffmann disease, is the most common and severe form of the disorder (5). Muscle weakness is typically evident by six months of age, and affected infants never achieve the ability to sit unsupported (6). Historically, the majority of children diagnosed with SMA type I did not survive past their second birthday due to the development of severe, progressive respiratory insufficiency.
The severity of the phenotype in infants with SMA type I significantly impacts nutritional status and overall health. Early bulbar dysfunction results in failure to gain weight, weight loss, and/or acute or chronic aspiration (7). Infants with low muscle mass are more likely to develop hypoglycemia in the setting of a catabolic state, since one of the main energy sources during fasting comes from skeletal muscle (8). In addition, children with SMA have demonstrated metabolic abnormalities consistent with a generalized defect in fatty acid metabolism, which make them more vulnerable to acidosis in this setting (9). Finally, gastroesophageal reflux and impaired gastrointestinal motility can make provision of optimal nutritional intake challenging, even when deficits are recognized (7,10). These challenges include limited intake volume and fat intolerance.
Due to increasingly proactive interventions in clinical care in recent years, survival rates in children with SMA type I have improved, with an increasing percentage surviving beyond 2 years of age (11). Recent studies in SMA animal models support an independent and critical adjuvant role for nutrition in the absence of proactive respiratory care interventions (12, 13). These data, and clinical experience, suggest a critical role for nutrition on survival and quality of life in SMA. The aims of this observational study were to provide an overview of caloric, macronutrient and micronutrient intakes, body composition, and bone mineral density in subjects with SMA type I, to assess the suitability of the use of standardized growth charts, and to identify and highlight areas where further nutritional research is needed.
Patients and Methods
Study Population
All subjects participating in a broader outcomes study of SMA at the University of Utah, “Clinical and Genetic Studies in SMA” were asked to provide dietary data in conjunction with clinical visits. For purposes of this analysis, we reviewed a subset of dietary and DEXA data in those with confirmed homozygous deletion and/or mutation in SMN1 for whom the clinical phenotype was consistent with type I. Specifically, all children in this cohort were sufficiently weak and hypotonic such that they were unable to sit unsupported at any point in their clinical course.
Methods
The primary caregiver was instructed to record all dietary intake and supplements over a three-day period including two weekdays and one weekend date. Growth parameters were obtained as part of the clinical evaluation with each visit, and included weight, length, and head circumference. Reliability of height measurements was supported by comparison to other collected anthropometric measures such as arm span. If the subject was unable to attend clinic for one or more visits, growth parameters were obtained at their local doctor’s office or at home using standard measurement methods. Growth was plotted and categorized across percentiles using the Centers for Disease Control and Prevention (CDC) growth charts (14). Three day dietary records were analyzed using ESHA Food Processor (version 9.1.0, and 10.5.2, 2003 and 2009 ESHA Research, Salem, Oregon) nutritional analysis software. Dietary intake for each patient’s individual food record was calculated by the ESHA Food Processor and adjusted for any additional supplements that were reported. Energy, fat (total, linoleic, and alpha linolenic fatty acids), protein, and certain vitamin and mineral intakes were compared to the dietary reference intakes (DRIs) and recommended dietary allowances (RDAs) for gender and age according to the DRI reports from the Food and Nutrition Board, the National Academies of Sciences (15–19).
Parents and primary care providers made decisions about type of formula, and we did not advocate one type of formula or a specific diet over another. There were no specific dietary interventions employed between visits. A dietitian was not available to families to provide ongoing nutritional support and feedback, but did review or analyze the dietary records (during the time period in which this data was collected). However, the physician or study dietitian did consult with families during or after each visit to provide feedback if there were caloric or obvious macronutrient deficiencies based on age-related growth chart deviations. The dietary records from the first visit were collected prior to any dietary feedback from this study.
Dual-energy X-ray absorptiometry (DEXA) scanning for body composition was performed at the time of the first visit unless contraindicated by medical status. Norland DEXA (XR-36 software version 3.3.1, Fort Atkinson, Wisconsin) for small subjects was used to assess whole body composition.
Norland DEXA scans for whole body composition were also collected from 65 healthy peers (age birth-36 months) between 1998 and 2009 at the University of Utah under a separate Institutional Review Board approved study and used for comparison purposes. These controls were Caucasian and healthy, without any chronic illness. Control subjects were of normal size, falling between the 25th and 75th percentile for weight and height on standardized growth curves.
Serum calcium laboratory values were also collected during the visits as part of a metabolic panel and analyzed at Primary Children’s Medical Center Laboratory.
Statistics
Data were analyzed using Statistical Analysis Software (version 9.1.2, 2010, SAS Institute Inc., Cary, NC, USA). Pearson’s coefficient of correlation was used to analyze associations. Student’s t-tests were used to evaluate differences between groups. Mixed effects analyses were used to evaluate percent fat mass (PFM), bone mineral density (BMD) and calcium and magnesium intakes, and fat mass (FM) increases over time. Fixed effects analyses were used to compare BMD, FM, fat free mass (FFM), and lumbar BMD for SMA patients and age-matched healthy peers. The level of significance for all analyses was set at p<0.05.
Ethics
Written informed parental consent (subjects < 18 years) and assent (subjects > 7 years) were obtained for all subjects. The Institutional Review Board at the University of Utah approved this study.
Results
Subject Characteristics
Detailed three day dietary records for 156 visits were collected from 47 children with genetically confirmed type I SMA evaluated in the pediatric motor disorders clinic at Primary Children’s Medical Center and the University of Utah or via a home visit (11 of 156 visits). Two patients with analyzed food records were not evaluated for at least one visit in our clinic, due to medical fragility. Among 47 subjects, there are 25 males and 22 females, birth to 13 years of age; 34 had food records for multiple visits. Forty-two subjects were Caucasian (23 males and 19 females) and three were Asian (2 males and 1 female); 41 were non-Hispanic (22 males and 19 females) while two were Hispanic (1 male and 1 female). The racial background of two subjects and ethnicity of four subjects was not reported. The number of SMN2 copies was 2 (n=41), 3 (n=3), or not available (n=3).
At the time of the first submitted dietary record, the median age was 9 months (1 month– 13 years). For females the median age was 11 months (1 month – 13 years), and for males the median age was 7 months (1 month – 4.8 years). The 13 year old female had a tracheostomy with ventilation. Although her age was an outlier, including her data did not significantly alter the results of this study. For the first visit, 3 subjects (2 females, 1 male) had tracheostomies with ventilation (subject ages at first visit 18 months, 58 months, 13 years). Of 47 subjects enrolled, 34 had dietary records for multiple visits. We elected to analyze and report aggregate results for the last dietary record available in each subject, in order to help determine what changes had been made in terms of dietary alterations over the course of time. Based on the information for the last dietary record submitted, the overall median age was 23 months (8 – 69 months), including 17 females (median age 28 months, range 12–64 months) and 17 males (median age 20.5 months, range 8–69 months). For the last dietary record, 3 subjects(2 females, 1 male) had tracheostomies with ventilation (subject ages 19 months, 22 months, and 55 months). Only one of these subjects (the 22 month old) had tracheostomy with ventilation from the first visit. The two additional subjects had tracheostomies with ventilation prior to the last dietary record.
At the time of the first dietary record, 24 subjects had a gastrostomy-tube (G-tube) and Nissen fundoplication procedure (9 male, 15 female), while 6 only had a G-tube (4 male, 2 female). The mean age at the time of G-tube surgery was 9 months (1 – 31 months) for males and 11 months (2 – 49 months) for females. By the time of the last visit, all but one subject had received a G-Tube with or without Nissen, and even this subject received supplemental feeds via nasogastric (NG) tube placement.
Dietary Intake
Table 1 provides a summary of types of dietary intake and supplementation between the first and last visits. The number of subjects taking elemental formula increased from the first visit from 18 of 47 (38%) to the last visit to 23 of 34 (68%). The average length of time between the first and last visit was 18 months. The most commonly received elemental formulas were Tolerex® or Pediatric Vivonex® (Nestle Nutrition, New Jersey) and the most common peptide based or hydrolyzed protein formulas included Peptamen Jr. ® (Nestle Nutrition, New Jersey) and Nutramigen® (Mead Johnson, Illinois). The most common source of fat supplementation was safflower oil.
Table 1.
Dietary intake summary at first and last visit
| Type of Diet | First Visit N = 47 n (%) | Last Visit N = 34 n (%) |
|---|---|---|
| Elemental Formulas | 18 (38%) | 23 (68%) |
| Non-elemental soy or milk based formulas | 26 (55%) | 8 (24%) |
| Peptide-based or Hydrolyzed Formulas | 2 (4%) | 2 (6%) |
| Breast Milk Only | 3 (6%) | 0 (0%) |
| Supplemental Breast Milk | 14 (30%) | 3 (9%) |
| Supplemental Multivitamin | 10 (21%) | 13 (38%) |
| Supplemental Oil | 7 (15%) | 13 (38%) |
Growth Parameters
At the first visit, 32 subjects (68%) had a weight for age in the normal range (3rd – 85th percentile). Thirteen subjects, or 28%, met the usually defined clinical criteria for failure to thrive (FTT) with a weight for age of less than the 3rd percentile; 6 were male and 6 were female. Eight of 13 subjects (62%), also met criteria for failure to thrive based on weight for height less than the 3rd percentile. Only 2 subjects (both males) had a weight for age over the 97th percentile indicating significant obesity based on standard definitions (19). At the time of the last dietary analysis, 21 of the 34 subjects, only 62%, had a weight for age in the normal range of 3rd – 85th percentile. Twelve of 34 subjects, or 35%, met clinical criteria for failure to thrive with a weight for age of less than the 3rd percentile, a higher percentage of patients as compared to the first visit (5 males and 7 females). Eleven of these subjects (92%) also met the criteria for failure to thrive based also on a weight for height less than the 3rd percentile, an increase compared to the first visit. One male subject had a weight for age between the 90th – 97th percentile indicating obesity (19).
For linear growth measures at the time of their first dietary evaluation, 37 subjects, (79%) were within 3rd – 97th percentile of expected height for age; 21 subjects were between the 5th and 50th percentiles, while 16 were between the 50th and 97th percentiles. There were six subjects (1 male and 5 females) below the 3rd percentile height for age and three subjects (2 males and 1 female) had a height above 97th percentile for age. Head circumference measurements were recorded in 35 subjects at the time of the first dietary record evaluation. Twenty-nine subjects had a normal head circumference, four were macrocephalic (> 97th percentile) and two were microcephalic (< 5th percentile) (19). At the last dietary assessment, anthropometric measures were similar in terms of category percentiles. However, six subjects (four males and two females) were above the 97th percentile for height for age.
Energy and Macronutrient Intake
Caloric intake per day, calories per kg weight, calories per cm height, protein per kg weight, and percent daily fat intake were measured at each dietary analysis for all subjects; average values for each are given based on the following criteria: all subjects, “ideal” weight for age (3rd – 75th percentile), failure to thrive, and obese patients and are summarized for the first and last visit in Table 2. Caloric intake per day, calories per kg weight, calories per cm height, protein per kg weight, percent daily fat intake, and DRI recommendations for protein and RDA for calories are also given based on age for the first and last dietary analyses in Table 3. Note the progressive decrease in caloric intake relative to age-matched RDA recommendations in kcal/kg from infancy to 3 years. Caloric intake was not correlated across percentile categories for either weight for age or weight for length. Type of formula, either elemental or nonelemental, also was not correlated across percentile categories.
Table 2.
Caloric, fat, and protein intake for SMA type I children at the first and last visit
| Patient Description | N | Caloric Intake (kcal/day) | Caloric Intake by Height (kcal/cm) | Caloric Intake by Weight (kcal/kg) | Protein Intake (g/kg) | Fat Intake (%) |
|---|---|---|---|---|---|---|
| All Patients | ||||||
| First Visit | 47 | 672 ± 203 | 8.89 ± 1.88 | 83.2 ± 28.2 | 1.99 ± 0.88 | 34.7 ± 16.4 |
| Last Visit | 34 | 742 ± 171 | 8.22 ± 1.66 | 66.1 ± 17.7 | 1.55 ± 0.59 | 27.4 ± 12.8 |
| Failure to Thrive | ||||||
| <3rd percentile | ||||||
| First Visit | 13 | 665 ± 221 | 8.91 ± 2.41 | 91.0 ± 32.3 | 2.60 ± 1.20 | 32.8 ± 15.6 |
| Last Visit | 12 | 738 ± 150 | 8.58 ± 1.34 | 78.5 ± 14.4 | 1.80 ± 0.64 | 23.1 ± 13.7 |
| Obese | ||||||
| >75th percentile | ||||||
| First Visit | 2 | 544 ±126 | 7.57 ± 0.20 | 51.4 ± 0.5 | 1.15 ± 0.55 | 44.5 ± 14.8 |
| Last Visit | 2 | 985 ± 66 | 9.44 ± 0.79 | 57.8 ±15.2 | 1.68 ± 1.04 | 35.0 ± 2.80 |
| “ideal” weight | ||||||
| 3rd – 75th percentile | ||||||
| First Visit | 32 | 682 ± 201 | 9.05 ±1.69 | 82.0 ± 26.2 | 1.80 ± 0.58 | 34.8 ± 17.1 |
| Last Visit | 20 | 719 ± 175 | 7.88 ± 1.84 | 59.4 ± 16.1 | 1.38 ± 0.48 | 29.3 ± 12.4 |
Table 3.
Calories, protein, and fat intake for SMA type I children by age at first and last visits
| Age | N | Caloric Intake (kcal/day) | Caloric Intake by Height (kcal/cm) | Caloric Intake by Weight (kcal/kg) | RDA Caloric Intake by Weight (kcal/kg) | Protein (g/kg) | DRI Protein Intake (g/kg) | Fat Intake (%) |
|---|---|---|---|---|---|---|---|---|
| 0 – 6 Months | 15 | 507 ± 123 | 8.24 ± 2.05 | 94.9 ± 36.4 | 108 | 1.9 ± 0.7 | 2.2 | 45.2 ± 13.9 |
| First Visit | (88% RDA) | |||||||
|
| ||||||||
| 7 – 12 Months | 12 | 663 ± 103 | 9.53 ± 1.53 | 85.9 ± 20.7 | 98 | 2.02 ± 0.72 | 1.6 | 41.9 ± 11.7 |
| First Visit | (88% RDA) | |||||||
|
| ||||||||
| 7 – 12 Months | 5 | 638 ± 165 | 8.8 ± 2.1 | 77.1 ± 17.1 | 98 | 1.7 ± 0.5 | 1.6 | 41.4 ± 15.3 |
| Last Visit | (79% RDA) | |||||||
|
| ||||||||
| 1 – 3 Years | 15 | 731 ± 166 | 9.3 ± 1.9 | 77.3 ± 20.3 | 102 | 2.44 ± 1.11 | 1.2 | 26.9 ± 11.8 |
| First Visit | (76% RDA) | |||||||
|
| ||||||||
| 1 – 3 Years | 17 | 716 ± 149 | 8.2 ± 1.6 | 68.8 ± 15.8 | 102 | 1.5 ± 0.5 | 1.2 | 26.2 ± 12.1 |
| Last Visit | (67% RDA) | |||||||
|
| ||||||||
| 4 – 8 Years | 4 | 1002 ± 269 | 9.28 ± 2.23 | 69.7 ± 12.4 | 70–90 | 1.40 ± 0.28 | 1.0–1.2 | 13.8 ± 4.3 |
| First Visit | ||||||||
|
| ||||||||
| 4 – 8 Years | 12 | 821 ± 181 | 8.0 ± 1.7 | 57.7 ± 18.0 | 70–90 | 1.5 ± 0.8 | 1.0–1.2 | 23.3 ± 9.1 |
| Last Visit | ||||||||
DEXA Studies
Thirty of 47 subjects had a DEXA scan performed at one or more visits. Percent fat mass (PFM) and weight for age, weight for height, and weight for length were not significantly correlated. However, mixed effects analysis over time found that PFM and weight per age were different for the higher percentiles. Those with a weight for age greater than the 90th percentile had significantly more PFM than those in the 50th to 75th percentile (p=0.05) and nearly significantly more PFM compared to those with weight for age less than the 3rd percentile (p=0.06). Time (increasing age) is associated with increasing PFM (p<0.01). No linear relationship between PFM and percentile categories was observed, although there are differences between the categories (p=0.02). Subjects using elemental formula had a significantly higher PFM (p=0.02). The PFM difference between elemental and nonelemental formula types was not significant over time (p=0.06), indicating time (age) was the primary factor. Subjects using elemental formulas had a significantly lower fat intake as a percentage of total energy intake compared to those on regular, peptide-based or hydrolyzed formulas. At the first visit, those on elemental formula had a fat intake of 21.3+/−12.2% of total energy intake compared to 42.5+/−13.4% of total energy intake for those not using elemental formula (p<0.0001). However, there is no statistical relationship between the percent fat intake and weight for age and weight for length percentile categories.
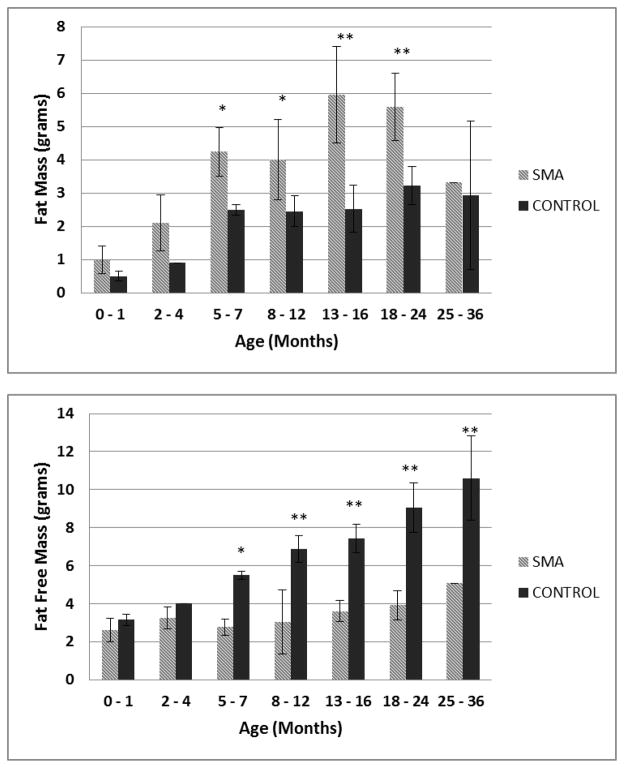
DEXA results for body composition compared by age group and age-matched healthy controls are summarized in Figure 1. DEXA data in children with SMA was compared to age-matched controls within and across age groups. Children with SMA type I under 36 months have significantly increased FM and decreased FFM when compared to healthy controls in a fixed effects analysis (p<0.0001). Fat mass increased significantly over time (p=0.0002) while FFM did not, in a mixed effects model. Whole body bone mineral density (BMD), as well as lumbar spine BMD was lower than age-matched healthy controls (fixed effects analysis p=0.04 and p=0.04, respectively). There was an overall age effect on BMD and lumbar spine BMD (p<0.001). The age interaction was not statistically significant for lumbar spine BMD.
Figure 1.
Fat Mass and Fat Free Mass age group comparisons between SMA type I subjects and age-matched normal controls (0 – 1 mo, n = 3 and n=18; 2 – 4 mo, n=6 and n=1; 5 – 7 mo, n=4 and n=5; 8 – 12 mo, n=6 and n=15; 13 – 16 mo, n=4 and n = 4; 18 – 24 mo, n=4 and n=12; and 25 – 36 mo, n=1 and n=10, respectively). * P<0.01 **P<0.0001
Overall, calcium intake did not correlate with whole body BMD. However, using a mixed effects analysis, increased calcium intake is predictive of increased BMD (p<0.01). Mixed effects modeling comparing calcium intakes above DRI and normal intake, randomized for subject, reveals significantly higher BMD at higher intakes of calcium compared to those with DRI recommended calcium intakes (p=0.02). Bone mineral density after four visits were significantly increased in this cohort of subjects when compared to the first visit (p<0.0001). Eighty-four serum calcium levels were obtained over the first four visits (n=29,23,18,14). Subjects with serum calcium levels higher than those with levels in the normal range(>9.8 mg/dL) also had increased BMD levels (p=0.02) in a mixed effects model. Increased serum calcium levels were evident throughout the visits; in the first visit, 18 of 29 were above normal range. More surprisingly, increased magnesium intake also predicts increased BMD (p<0.01), and magnesium intake significantly correlates with overall BMD (r=0.65, p<0.001).
Micronutrient Intake
An apparent inadequate intake for a variety of nutrients is evident with dietary record analysis in this population. Inferred inadequate intakes were noted if the patient’s calculated dietary intake for a given macronutrient, vitamin or mineral was lower than the DRI guidelines based on gender and age. These results are summarized in Table 4. Common nutrients considered at greatest risk for deficiency due to intake below the current DRI for age in 25% or more of this cohort includes: alpha-linolenic fatty acid, linoleic fatty acid, vitamin A, vitamin D, vitamin E, vitamin K, folate, calcium, iron, and magnesium.
Table 4.
Nutrients with intakes less than dietary reference intakes at first and last visit
| Nutrient at Risk | Inadequate Intake first visit (N = 47)(%) | Inadequate Intake last visit (N = 34)(%) |
|---|---|---|
| Linoleic Fatty Acid | 36 (77%) | 29 (85%) |
| Linolenic Fatty Acid | 38 (81%) | 28 (82%) |
| Vitamin A | 15 (32%) | 2 (6%) |
| Vitamin D | 18 (38%) | 5 (15%) |
| Vitamin E | 24 (51%) | 13 (38%) |
| Vitamin K | 10 (21%) | 16 (47%) |
| Thiamin | 10 (21%) | 2 (6%) |
| Riboflavin | 9 (19%) | 0 (0%) |
| Niacin | 3 (6%) | 1 (3%) |
| Vitamin B6 | 7(15%) | 3 (9%) |
| Vitamin B12 | 4 (8.5%) | 0 (0%) |
| Vitamin C | 8 (17%) | 2 (6%) |
| Folate | 13 (28%) | 20 (59%) |
| Calcium | 12 (25.5%) | 10 (29%) |
| Iron | 17(36%) | 14 (41%) |
| Magnesium | 15 (32%) | 10 (29%) |
| Zinc | 6 (13%) | 1 (3%) |
Discussion
Analysis of dietary intake in a relatively large cohort of SMA type I subjects in this study indicates that children with SMA type I have lower caloric intakes than recommended for healthy peers, presumably due to a diminished metabolic rate in light of their decreased muscle mass. In support of this hypothesis, we demonstrate that type I infants and children have substantially increased FM and lower FFM than healthy, age-matched controls. The lack of association between growth percentile categories and daily caloric intake indicates that standardized growth charts may not be adequate for determining healthy weight status in this population. Dietary and DEXA analyses indicate that calcium and magnesium intakes are strong predictors of increased BMD while intakes of alpha-linolenic fatty acid, linoleic fatty acid, vitamin A, vitamin D, vitamin E, vitamin K, folate, calcium, iron, and magnesium are frequently below DRI recommended ranges. Limitations of this study include a potential for bias among families willing to return a dietary record; they may be more attentive to their child’s dietary intake. Thus, the percentage of children receiving elemental-based diets and/or supplements in this study may be greater than in the general SMA type I population. There also was a limited number of subjects with DEXA analysis for age group comparison. However, such data provides a starting point to focus on questions regarding optimal nutritional interventions for this fragile population.
Caloric intake is less than the RDA for age in this cohort, and this becomes more apparent over time. As indicated in Table 2, there is little increase in caloric need with increasing age, and caloric intake is not associated with FTT, normal, or obese status based on growth chart evaluation. These data demonstrate that caloric needs may change very little for prolonged periods of time, and progressive weight gain across percentiles can occur even without any increase in calories in some children. In support of this, we have clinically observed dramatic weight gain after extremely modest caloric adjustments, on the order of 30 calories per day. It is important to note that children with other motor/neuromuscular disorders require lower caloric intakes as well. For example, intake guidelines for cerebral palsy, spina bifida, and nonambulatory motor disorders are 11–14 kcal/cm, 9–11 kcal/cm, and 7–11 kcal/cm, respectively (20,21). These intake guidelines for motor disorders are relatively close to the 7–11 kcal/cm caloric intakes that this study observed. Until indirect calorimetry and doubly labeled water studies in SMA are available, Table 2 could be a useful guide for estimating the initial caloric needs of the SMA type I child with adjustments based on individual growth and health parameters.
Some patients with SMA are proportionately small for both weight and length and may have a constitutional growth failure unrelated to inadequate nutrition. Therefore, it is important to consider weight for length and not just weight for age when assessing growth status. Although many patients may appear to have FTT, they may be growing normally for their disease state and even have excess FM. In a cohort of predominantly SMA type II and III children, Sproule et al demonstrated that those with SMA have significantly reduced total FFM as compared to healthy children (22). Our findings in this type I cohort are even more pronounced and are apparent by 5–7 months of age. This reduced FFM makes it difficult to determine ideal body mass index (BMI) using usual growth parameters since these children often plot as underweight for age in spite of a markedly increased body fat percentage (22). Percent fat mass increases significantly over time, due to increased FM with little increase in FFM. This results in grossly inadequate weight assessments based on standard growth curves. In other disease states where parameters deviate from normal growth curves, specific growth curves for the disease state have been developed (23). However, no such growth curves currently exist to facilitate proper growth assessment for children with SMA. Based on this data, standardized growth charts alone are insufficient to assess weight or growth status in this population. Thus, DEXA or other tools to determine body composition may be valuable for assessing healthy weight status in this population.
Paradoxically, a subset of patients grew beyond the 97th percentile length for age, and four subjects demonstrated absolute macrocephaly. The significance of these observations remains unclear. However, such features are commonly seen in genetic disorders associated with skeletal dysplasia or structural abnormalities in collagen.
Children with SMA have demonstrated metabolic abnormalities attributed to a generalized defect in fatty acid metabolism (9, 24, 25). This literature and intolerance of fat in some patients has led to an increasing number of children with SMA on low fat diets similar to those used in children with inborn errors of fatty acid oxidation (fat intake less than 30% of energy intake). In our cohort, use of elemental formulas increased from 38 % at the first visit to 68% at the last visit over an average span of 18 months. Unfortunately, families of children with SMA rarely have the benefit of a metabolic dietitian to monitor them prospectively and ensure that intake of essential fatty acids and other nutrients are adequate. Children in this study using elemental formulas had a substantially lower percentage of fat intake and thus, lower intake of essential fatty acids. This may increase risk for essential fatty acid deficiency that may affect their growth. A relatively small proportion of children were on supplemental oils, and the majority had intakes below the DRIs for linoleic and alpha-linolenic fatty acids. Some of the children with fatty acid intake far below DRI exhibit essential fatty acid deficiency as confirmed by plasma essential fatty acid profiles. Supplementation with oils high in these essential fatty acids, such as small amounts of safflower or flaxseed oils (for linoleic and alpha linolenic, respectively) may be appropriate in some cases. However, in an SMA animal model, one supplemental formula that enhanced survival was a standard formula with a relatively high fat content (12). Thus, the type and content of fat in the diet of SMA patients, and the potential benefit of elemental formulas, if any, and their appropriate use, deserves further study. Pending further studies, we suggest routine monitoring of plasma essential fatty acid profiles and/or supplementation for those children on low fat diets.
Children with SMA type I have significantly diminished BMD resulting in fragile bone health (26). Hypercalcemia has also been observed, and its prevalence and etiology are not well understood (27). Thus, it is of particular interest that high serum calcium levels were related to significantly higher BMD compared to normal serum levels. This may be related to the low bone mineral status in children with SMA and compensatory increased calcium absorption or altered bone remodeling; clearly, more study is needed (28). In this study, we demonstrate that intake of both calcium and magnesium is independently and significantly predictive of increased BMD. Further, a substantial proportion of this cohort had potentially inadequate intakes for bone-related nutrients such as vitamin D, vitamin K, calcium, and magnesium. Additional vitamins and minerals for which intake is commonly insufficient as listed in Table 4 are primarily observed in patients not on an additional multivitamin/mineral supplement. Thus, multivitamin and mineral supplementation to meet DRIs should be considered in children with SMA type I, especially for bone-related nutrients. Ideally, given that all children with SMA type I ultimately manifest bulbar dysfunction and feeding problems, they should be referred for dietary consultation as early as possible following their diagnosis and assessed by a dietitian on a regular basis to help monitor and guide appropriate nutrient intake (19, 29).
This study highlights a number of issues to consider when assessing dietary intake and nutritional status in infants and children with SMA type I. However, many questions remain. An increasing percentage of SMA type I children in this cohort with long term survival (exceeding an average of 18 months) were using elemental formulas; clinical research is needed to determine the potential benefits of elemental formula use, if any, before gastrointestinal and malabsorptive symptoms indicate. Clinically reported difficulties with fat and/or formula intolerance are increasingly prevalent with disease progression, and may or may not be related to metabolic status and/or gastrointestinal dysfunction. The optimal percentage and type of fat calories required for proper growth and optimal motor development needs to be determined. Indirect calorimetry or doubly labeled water techniques may be necessary to determine optimal caloric intake needs. A specific growth chart for children with SMA type I needs to be developed. A core set of evidence-based guidelines to enhance nutritional assessment and monitoring would help further improve survival and quality of life outcomes in children with SMA.
Acknowledgments
Funding sources: This research was supported by grants from SMA Angels Charity, Inc, and NIH grant R01-HD054599 from the National Institute of Child Health and Human Development, with additional support from Award Number UL1RR025764 from the National Center for Research Resources. Additional support was provided by Families of SMA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the national Center for Research Resources or the National Institutes of Health. We would like to acknowledge Charles Scott of CBS Squared, Inc. for statistical analysis.
Footnotes
Disclosure Statement
The authors have no known conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsirikos AI, Baker ADL. Spinal Muscular Atrophy: Classification, aetology, and treatment of spinal deformity in children and adolescents. Current Orthopaedics. 2006;20:430–445. [Google Scholar]
- 2.Zerres K, Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52(5):518–523. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 4.Feldkotter M, Schwarzer V, Wirth R, Weinker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time light Cycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearn J. Classification of spinal muscular atrophies. Lancet. 1980;1(8174):919–922. doi: 10.1016/s0140-6736(80)90847-8. [DOI] [PubMed] [Google Scholar]
- 6.Oskoui M, Kaufmann P. Spinal Muscular Atrophy. Neurotherapeutics. 2008;5(4):499–506. doi: 10.1016/j.nurt.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sy K, Mahant S, Taback N, Vajsar J, Chait PG, Friedman JN. Enterostomy tube placement in children with spinal muscular atrophy type 1. J Pediatr. 2006;149(6):837–839. doi: 10.1016/j.jpeds.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Bruce AK, Jacobsen E, Dossing H, Kondrup J. Hypoglycaemia in spinal muscular atrophy. Lancet. 1995;346(8975):609–610. doi: 10.1016/s0140-6736(95)91439-0. [DOI] [PubMed] [Google Scholar]
- 9.Crawford TO, Sladky JT, Hurko O, Besner-Johnston A, Kelley RI. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann Neurol. 1999;45(3):337–343. doi: 10.1002/1531-8249(199903)45:3<337::aid-ana9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22:1027–1049. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 11.Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69(20):1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 12.Narver HL, Kong L, Burnett BG, et al. Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Ann Neurol. 2008;64(4):465–470. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butchbach ME, Rose FF, Jr, Rhoades S, Marston J, et al. Effect of diet on the survival and phenotype of a mouse model for spinal muscular atrophy. Biochem Biophys Res Commun. 2010;391(1):835–840. doi: 10.1016/j.bbrc.2009.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 15.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, Amino Acids (Macronutrients) Institute of Medicine of the National Academy of Science. Food and Nutrition Board; 2005. [Google Scholar]
- 16.Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine of the National Academy of Science; 2011. [Google Scholar]
- 17.Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Institute of Medicine of the National Academy of Science. Food and Nutrition Board; 1998. [PubMed] [Google Scholar]
- 18.Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, vanadium, and Zinc. Institute of Medicine of the National Academy of Science. Food and Nutrition Board; 2001. [Google Scholar]
- 19.Dietary Reference Intakes for Vitamin C, Vitamin EW, Selenium, and Carotenoids. Institute of Medicine of the National Academy of Science. Food and Nutrition Board; 2000. [Google Scholar]
- 20.Heinricks E, Rokusek C. Nutrition and Feeding for the Developmentally Disabled. Vermillion: South Dakota Department of Education and Cultural Affairs; 1992. [Google Scholar]
- 21.Samour PQ, King K. Handbook of Pediatric Nutrition. 3. Sudbury, MA: Jones and Bartlett Publishers; 2005. pp. 290–291. [Google Scholar]
- 22.Sproule DM, Montes J, Montgomery M, et al. Increased fat mass and high incidence of overweight despite low body mass index in patients with spinal muscular atrophy. Neuromuscul Disord. 2009;19(6):391–396. doi: 10.1016/j.nmd.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krick J, Murphy-Miller P, Zeger S, Wright E. Pattern of growth in children with cerebral palsy. J Am Diet Assoc. 1996;96(7):680–685. doi: 10.1016/s0002-8223(96)00188-5. [DOI] [PubMed] [Google Scholar]
- 24.Kelley RI, Sladky JT. Dicarboxylic aciduria in an infant with spinal muscular atrophy. Ann Neurol. 1986;20(6):734–736. doi: 10.1002/ana.410200615. [DOI] [PubMed] [Google Scholar]
- 25.Tein I, Sloane AE, Donner EJ, Lehotay DC, Millington DS, Kelley RI. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatr Neurol. 1995;12(1):21–30. doi: 10.1016/0887-8994(94)00100-g. [DOI] [PubMed] [Google Scholar]
- 26.Khatri IA, Chaudhry US, Seikaly MG, Browne RH, Iannaccone ST. Low bone mineral density in spinal muscular atrophy. J Clin Neuromuscul Dis. 2008;10(1):11–7. doi: 10.1097/CND.0b013e318183e0fa. [DOI] [PubMed] [Google Scholar]
- 27.Khawaja K, Houlsby WT, Watson S, Bushby K, Cheetham T. Hypercalcaemia in infancy; a presenting feature of spinal muscular atrophy. Arch Dis Child. 2004;89(4):384–385. doi: 10.1136/adc.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanmugarajan S, Tsuruga E, Swoboda KJ, Maria BL, Ries WL, Reddy SV. Bone loss in survival motor neuron (Smn-/-SMN2) genetic mouse model of spinal muscular atrophy. J Pathol. 2009;219(1):52–60. doi: 10.1002/path.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iannacone ST. Spinal muscular atrophy. Semin Neuro. 1998;18:19–26. doi: 10.1055/s-2008-1040858. [DOI] [PubMed] [Google Scholar]