Abstract
Objective
To evaluate whether 17-alpha hydroxyprogesterone caproate (17-OHP) reduces preterm birth (PTB) in nulliparous women with a midtrimester cervical length (CL) less than 30 mm.
Study Design
In this multicenter randomized controlled trial, nulliparous women with a singleton gestation between 16 and 22 3/7 weeks with an endovaginal CL < 30 mm (<10th percentile in this population) were randomized to weekly intramuscular 17-OHP (250 mg) or placebo through 36 weeks. The primary outcome was PTB < 37 weeks.
Results
The frequency of PTB did not differ between the 17-OHP (N = 327) and placebo (N = 330) groups (25.1% vs. 24.2%; relative risk (RR) 1.03, 95% confidence interval (CI), 0.79 – 1.35). There also was no difference in the composite adverse neonatal outcome (7.0% vs. 9.1%: RR 0.77, 95% CI 0.46 – 1.30).
Conclusion
Weekly 17-OHP does not reduce the frequency of PTB in nulliparous women with a midtrimester CL < 30 mm. (ClinicalTrials.gov number, NCT00439374).
Keywords: progestogen, progesterone, short cervix, nulliparous
Preterm birth (PTB) remains a major cause of morbidity and mortality worldwide. Not only is it responsible for approximately 50 percent of childhood blindness and one-third of cerebral palsy, but it increasingly has been implicated in adult morbidities, such as cardiovascular disease.1,2 Correspondingly, PTB reduction has been a prominent public health goal and a focus of perinatal research.
Progestogens, administered intramuscularly or vaginally, have been demonstrated to reduce the frequency of recurrent preterm birth.3,4 Although progestogens in this circumstance have been demonstrated to be efficacious, PTB epidemiology is such that their use for this indication will not markedly reduce the population’s frequency of PTB.5 Thus, investigators have evaluated whether progestogens also can reduce PTB in other at-risk groups. Although progestogen treatment does not reduce PTB in women with multiple gestations,6–8 it has been shown in two randomized trials to reduce PTB in women with a short cervix.9,10 The inclusion criterion for cervical length (CL) was less than 15mm in one trial, and 10–20mm in the other trial.
However, because the frequencies of a CL less than 15 mm or of 10–20 mm are approximately 1% and 2%, respectively, the restriction of progestogen to such women will not result in a substantial reduction in the overall PTB frequency and will leave many women who might benefit from its use untreated. In this study, we examined whether the use of 17-alpha hydroxyprogesterone caproate (17-OHP) administered to nulliparous women with a CL less than 30 mm (i.e., the 10th percentile at 16–22 weeks of gestation) would reduce PTB prior to 37 weeks.
Methods
From April 2007 through May 2011, the fourteen centers of the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development participated in this randomized double-blind placebo-controlled trial. The study was approved by the institutional review board at each clinical site and at the data coordinating center.
Women were eligible for participation if they were nulliparous with a viable singleton gestation and had a CL less than 30 mm between 16 weeks 0 days and 22 weeks 3 days. This gestational age range was chosen because it is during this period that women typically receive a sonographic examination for fetal evaluation. A woman was defined as nulliparous if she had no prior pregnancy that progressed beyond 19 weeks 6 days. Women were excluded from participation if they had undergone a selective fetal reduction to a singleton gestation; had sonographic evidence of an additional fetal pole/embryo at 12 weeks of gestation or later; had received progestogen treatment after 14 weeks 6 days; had experienced vaginal bleeding heavier than spotting after 15 weeks 6 days; had amniotic membranes prolapsing beyond the external os; had premature rupture of membranes; had a known major fetal anomaly or aneuploidy; had a current or planned cervical cerclage; had a known Mullerian abnormality; had a contraindication to intramuscular injections; had maternal medical conditions that increase the probability of preterm delivery (e.g. hypertension); had prior cervical surgery (i.e., cold knife conization, laser vaporization or loop electrosurgical excision procedure; or were planned to have an indicated preterm delivery.
For women who conceived spontaneously, the duration of gestation at the time of randomization was determined according to a previously described algorithm on the basis of the last menstrual period and the results of ultrasonography.11 For women who conceived by in vitro fertilization, the duration of gestation was calculated on the basis of the date of embryo transfer and the age of the embryo when transferred.
CL was measured endovaginally by trained and certified sonographers. Sonographer training consisted of a didactic program based on the method described by Iams et al. for endovaginal CL measurement.12 To become certified, a sonographer submitted three cervical images from each of five separate women, designating which of the three images per patient was the best image with the shortest measurement, and whether a funnel and/or debris (defined as echogenic material adjacent to the cervix within the amniotic cavity) were present. The images were reviewed centrally by an expert (JDI). Study personnel were certified only when the images of at least four of the five studies submitted were considered acceptable. Once certified, sonographers performed CL screening of women potentially eligible for the randomized trial in a standard fashion, by obtaining three cervical length images and measuring each cervix along the line of the cervical canal made by the interface of the mucosal surfaces, with calipers placed at the external and internal os. The shortest measurement was recorded and was the basis for eligibility for the trial.
Eligible women were offered participation into the randomized, double-blind, placebo-controlled trial. Women who signed informed consent received an intramuscular “compliance” injection of the placebo (1 ml inert oil), and were asked to return at least three days later, at which time randomization occurred. If a woman did not return for a randomization visit before 23 weeks 0 days of gestation, she was excluded from participation in the trial. Additionally, before randomization, all women were required to have a sonographic examination to exclude fetal anomalies and to estimate gestational age if no previous dating ultrasound had been performed. Women who met inclusion criteria and returned at the appropriate time after the “compliance” injection were assigned in a 1:1 ratio to receive identically appearing active (250 mg 17-OHP) or placebo (castor oil) intramuscular injections prepared according to CGMP guidelines by a research pharmacy (Eminent Services, Frederick, MD). The simple urn method of randomization, with stratification according to clinical center, was used by the data coordinating center to create the computer-generated randomization sequence.13 The active and placebo study medications were distributed according to this randomization sequence. The study was double masked; neither the patient nor medical staff (including research and clinical personnel) was aware of the treatment assignment. After randomization, participants received weekly intramuscular injections of 250 mg 17-OHP or placebo given by a study nurse, until 36 weeks 6 days of gestation or delivery, whichever occurred first. Compliance with the intervention was determined by the proportion of protocol-specified injections (one injection every 5–9 days from randomization to 36 weeks 6 days or delivery, whichever occurred first) that was received prior to delivery.
At each study visit, participants were asked whether they had experienced adverse symptoms since the last injection. In addition, they were asked whether they had consulted a physician for preterm labor symptoms, had undergone any medical procedures, or had been given any corticosteroids or tocolytic medications since their last visit. Participants also were queried about any activity restrictions that had been prescribed by their health provider. Prenatal, delivery, newborn, and postpartum records were abstracted by study personnel after delivery. Participating women and their infants were followed until hospital discharge.
PTB was defined as delivery prior to 37 weeks 0 days of gestation. Pre-specified secondary outcomes included PTB prior to 35 weeks 0 days and prior to 32 weeks 0 days gestation, a composite of serious adverse fetal or neonatal outcomes (i.e., respiratory distress syndrome, bronchopulmonary dysplasia, early-onset sepsis, grade II or III necrotizing enterocolitis, grade III or IV intraventricular hemorrhage, periventricular leukomalacia, grade III or IV retinopathy of prematurity, or fetal or neonatal death), and selected individual maternal and neonatal morbidities.
We estimated that 20 percent of the women in the placebo group would deliver before 37 weeks of gestation.12,14,15 Based on this estimate, a total sample size of 1000 women (500 in each group) was estimated to be sufficient to detect a reduction of 33 percent in the frequency of preterm delivery before 37 weeks, under the assumptions of a type I error (two-sided) of 5 percent and a power of at least 80 percent. This sample size also would yield more than 90% power to detect the same effect size if the frequency in the placebo group were as high as 25%. The analysis was performed according to the intention-to-treat principle. Continuous variables were compared with the use of the Wilcoxon rank-sum test, and categorical variables were compared with the use of the chi-square or Fisher’s exact test, as appropriate. The proportion of women in each study group remaining pregnant was compared using survival analysis with the log-rank test used to assess for the difference between the survival curves. A priori subgroup analyses were planned for the primary outcome with respect to the selected variables of gestational age at randomization (prior to 21 weeks 0 days of gestation versus at least 21 weeks 0 days of gestation), CL at screening (less than 15 mm versus at least 15 mm), and the presence or absence of a cervical funnel at screening. These analyses utilized Mantel-Haenszel stratification with the Breslow-Day test to assess for homogeneity. All tests were two-tailed and P < 0.05 was used to define statistical significance. Analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, NC).
An independent data and safety monitoring committee (DSMC) monitored the trial. A group sequential method was used to characterize the rate at which the type I error was spent; the chosen function was the Lan-DeMets characterization of the O’Brien-Fleming boundary.16 At the 3rd interim analysis, conducted when outcome data were available for 591 patients (59.1% percent of the planned sample), a conditional power analysis revealed that even if recruitment continued to the final sample size of 1000 women, and the primary outcome frequencies in the remaining women were as assumed for the alternative hypothesis (13.3% in the 17-OHP arm and 20% in the placebo arm) the probability of showing a benefit was extremely low (< 2.5%). Based on these data and the recommendation of the DSMC, enrollment in the trial was halted on May 9th, 2011.
Results
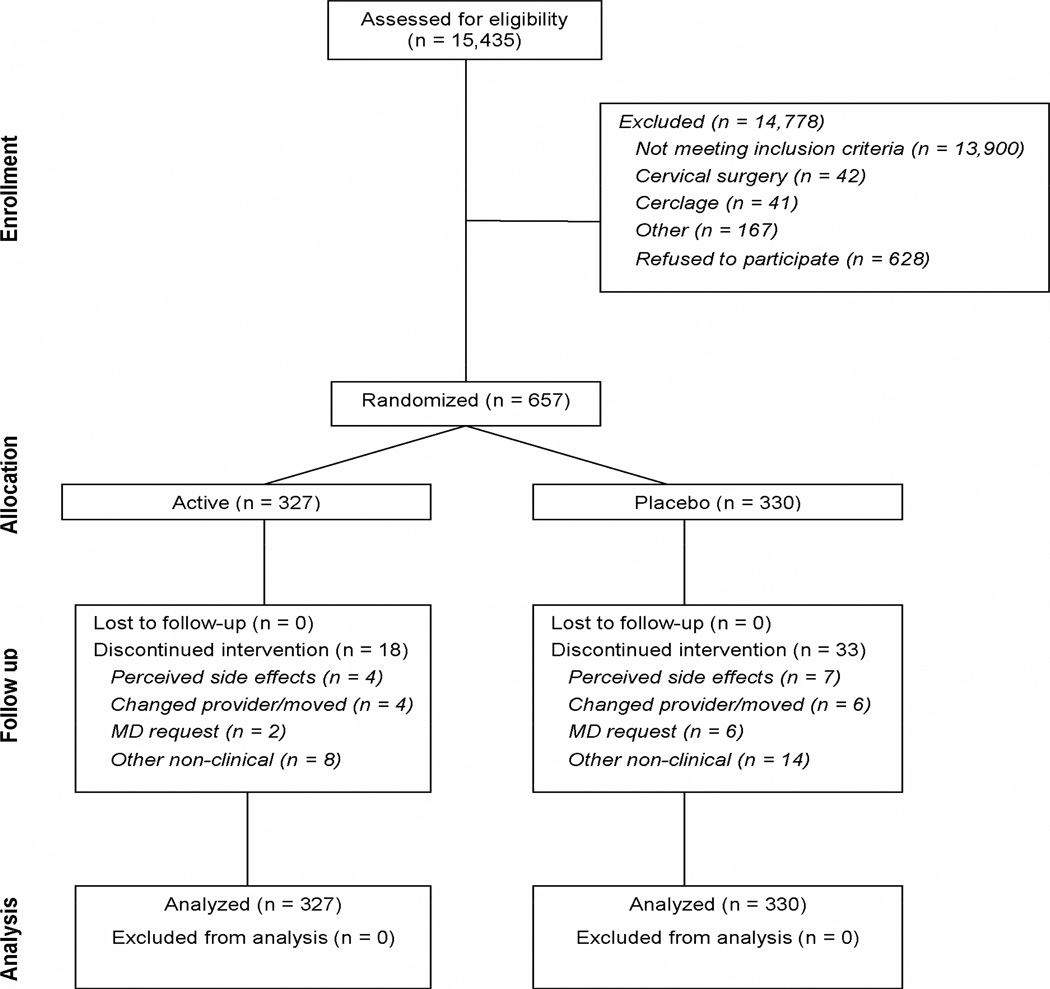
A total of 15,435 women were screened, of whom 1,588 (10.3%) had a CL less than 30 mm. At the time the study was halted, 657 women had been randomized. Outcome data were available for all randomized participants and their neonates (Figure 1). Baseline characteristics of the two study groups were largely similar, although women randomized to 17-OHP treatment were slightly older (Table 1). The mean compliance rate was 88.8% in the 17-OHP group and 89.1% in the placebo group (P = 0.89). Seven women (1.1%) also were prescribed vaginal progesterone by their health care provider (2 in the 17-OHP group and 5 in the placebo group, P = 0.45).
Figure 1.
Flow diagram detailing patient enrollment
Table 1.
Baseline characteristics of the study population stratified by treatment group
| 17-OHP (N = 327) |
Placebo (N = 330) |
|
|---|---|---|
| Maternal age at screening (years) | 22.8 ± 5.3 | 21.6 ± 4.4 |
| Pre-pregnancy body mass index (kg/m2)† | 26.1 ± 6.9 | 25.4 ± 6.5 |
| Race/ethnicity‡ | ||
| Non-Hispanic White | 76 (23.2) | 74 (22.4) |
| Non-Hispanic Black | 179 (54.7) | 161 (48.8) |
| Hispanic White | 19 (5.8) | 38 (11.5) |
| Hispanic Black | 2 (0.6) | 0 |
| Asian | 4 (1.2) | 3 (0.9) |
| Other | 47 (14.4) | 54 (16.4) |
| Prior pregnancy | ||
| < 13 weeks of gestation | 92 (28.1) | 82 (24.8) |
| 13–19 weeks of gestation | 13 (4.0) | 10 (3.0) |
| Payment for obstetric care | ||
| Uninsured/self-pay | 17 (5.2) | 30 (9.1) |
| Private insurance | 74 (22.6) | 62 (18.8) |
| Government-assisted insurance | 236 (72.2) | 238 (72.1) |
| Married or living with partner | 125 (38.2) | 110 (33.3) |
| Alcohol use during pregnancy | 33 (10.1) | 20 (6.1) |
| Smoking during pregnancy | 48 (14.7) | 62 (18.8) |
| Illicit substance use during pregnancy | 15 (4.6) | 24 (7.3) |
| Gestational age at randomization (weeks) | 21.4 ± 1.2 | 21.3 ± 1.3 |
| Cervical length at screening (mm) | 23.9 ± 5.6 | 23.8 ± 5.7 |
| Cervical length at screening < 15 mm | 25 (7.6) | 31 (9.4) |
| Cervical funnel present□ | 88 (26.9) | 69 (20.9) |
| Cervical funnel length (mm)□ | 14.8 ± 8.0 | 16.7 ± 8.7 |
| Debris present | 39 (11.9) | 39 (11.8) |
Data are presented as mean ± standard deviation or n (%).
The only between-group difference that was significant was maternal age at screening (P = 0.003).
Percentages may not total 100 percent due to rounding.
Values were unavailable for 4 mothers in the 17-OHP group and 9 mothers in the placebo group.
Race or ethnicity was self-reported.
Values were available for all mothers with a funnel present; 88 in the 17-OHP group and 69 in the placebo group.
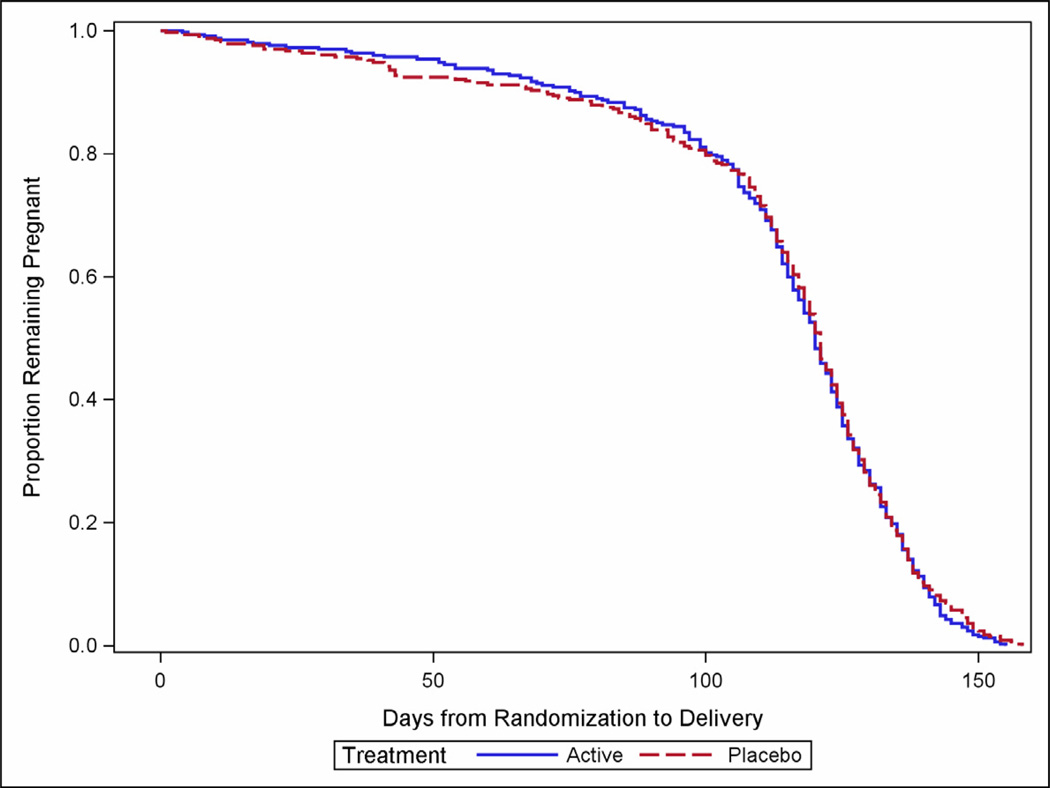
The frequency of the primary outcome did not differ significantly between groups (Table 2). Delivery prior to 37 weeks of gestation occurred in 25.1% of women in the 17-OHP group and 24.2% of women in the placebo group (relative risk (RR), 1.03; 95% confidence interval (CI), 0.79 to 1.35). The proportion of spontaneous and medically-indicated deliveries was similar between the groups (Table 2). The proportion of births prior to 35 weeks and prior to 32 weeks also was similar between the groups, as were the survival curves from randomization to delivery (P = 0.55) (Figure 2). The median gestational ages at delivery were similar as well (38.9 weeks, interquartile range 36.9 – 40.0 in the 17-OHP group vs. 38.9, interquartile range 37.1–40.0 in the placebo group; P = 0.93). There were no differences in other selected maternal outcomes. A majority of women in both groups noted side effects from the injections, the majority of which were related to irritation at the injection site.
Table 2.
Maternal outcomes and interventions stratified by treatment group
| 17-OHP (N = 327) |
Placebo (N = 330) |
RR (95% CI) | |
|---|---|---|---|
| Delivery < 37 weeks | 82/327 (25.1) | 80/330 (24.2) | 1.03 (0.79 – 1.35) |
| Spontaneous | 54/327 (16.5) | 55/330 (16.7) | 0.99 (0.70 – 1.40) |
| Medically indicated | 27/327 (8.3) | 25/330 (7.6) | 1.09 (0.65 – 1.84) |
| Fetal loss/abortion < 20 weeks | 1/327 (0.3) | 0/330 | -- |
| Gestational age at delivery (weeks) | 37.6 ± 3.9 | 37.4 ± 4.3 | P = 0.93 |
| Preterm premature rupture of membranes | 25/327 (7.6) | 24/330 (7.3) | 1.05 (0.61 – 1.80) |
| Delivery < 35 weeks | 44/327 (13.5) | 53/330 (16.1) | 0.84 (0.58 – 1.21) |
| Delivery < 32 weeks | 28/327 (8.6) | 32/330 (9.7) | 0.88 (0.54 – 1.43) |
| Delivery < 28 weeks | 15/327 (4.6) | 22/330 (6.7) | 0.69 (0.36 – 1.30) |
| Hospital visit for preterm labor | 145/327 (44.3) | 151/330 (45.8) | 0.97 (0.82 – 1.15) |
| Tocolytic therapy | 35/321 (10.9) | 42/325 (12.9) | 0.84 (0.55 – 1.29) |
| Corticosteroid therapy | 55/321 (17.1) | 51/325 (15.7) | 1.09 (0.77 – 1.55) |
| Cerclage placement | 6/321 (1.9) | 4/325 (1.2) | 1.52 (0.43 – 5.33) |
| Gestational hypertension or preeclampsia | 46/327 (14.1) | 40/329 (12.2) | 1.16 (0.78 – 1.72) |
| Gestational diabetes mellitus | 15/327 (4.6) | 13/330 (3.9) | 1.16 (0.56 – 2.41) |
| Cholestasis | 1/327 (0.3) | 0/329 | -- |
| Placental abruption | 11/327 (3.4) | 15/328 (4.6) | 0.74 (0.34 – 1.58) |
| Chorioamnionitis | 29/327 (8.9) | 20/328 (6.1) | 1.45 (0.84 – 2.52) |
| Cesarean delivery | 67/327 (20.5) | 63/329 (19.1) | 1.07 (0.79 – 1.46) |
| Side effects | |||
| Any | 223/326 (68.4) | 220/328 (67.1) | 1.02 (0.92 – 1.13) |
| Injection site | 217/326 (66.6) | 209/328 (63.7) | 1.04 (0.93 – 1.17) |
| Urticaria | 10/326 (3.1) | 2/328 (0.6) | 5.03 (1.11 – 22.78) |
| Nausea | 7/326 (2.1) | 10/328 (3.0) | 0.70 (0.27 – 1.83) |
Data are presented as mean ± standard deviation or n/N (%)
Figure 2.
Survival curve illustrating the proportion of participants remaining pregnant after randomization.
Table 3 presents selected perinatal outcomes. There were no differences between groups in the composite adverse perinatal outcome or in most individual outcomes. The exception was that early-onset sepsis was less frequent in the 17-OHP group (0.9% versus 3.4%; RR 0.27, 95% CI 0.08 – 0.97). There were no differences between groups with regard to the frequency of low birth weight, small-for-gestational-age birth weight, or admission to the neonatal intensive care unit.
Table 3.
Perinatal outcomes stratified by treatment group*
| 17-OHP (N = 327) |
Placebo (N = 330) |
RR (95% CI) | |
|---|---|---|---|
| Composite adverse outcome | 23/327 (7.0) | 30/330 (9.1) | 0.77 (0.46 – 1.30) |
| Fetal death | 4/327 (1.2) | 1/330 (0.3) | 4.04 (0.45 – 35.92) |
| Neonatal death | 6/327 (1.8) | 8/330 (2.4) | 0.76 (0.27 – 2.16) |
| Respiratory distress syndrome | 13/320 (4.1) | 16/323 (5.0) | 0.82 (0.40 – 1.68) |
| Bronchopulmonary dysplasia | 3/320 (0.9) | 5/322 (1.6) | 0.60 (0.15 – 2.51) |
| Necrotizing enterocolitis, grade II or III | 2/320 (0.6) | 5/322 (1.6) | 0.40 (0.08 – 2.06) |
| Intraventricular hemorrhage, grade III or IV | 2/320 (0.6) | 1/322 (0.3) | 2.01 (0.18 – 22.08) |
| Periventricular leukomalacia | 4/320 (1.3) | 1/322 (0.3) | 4.03 (0.45 – 35.81) |
| Early-onset sepsis | 3/320 (0.9) | 11/322 (3.4) | 0.27 (0.08 – 0.97) |
| Retinopathy of prematurity, grade III or IV | 1/320 (0.3) | 3/322 (0.9) | 0.34 (0.04 – 3.21) |
| Birth weight (g) | 2855 ± 747 | 2824 ± 807 | P = 0.82 |
| < 2500 g | 72/323 (22.3) | 75/328 (22.9) | 0.97 (0.73 – 1.30) |
| < 1500 g | 23/323 (7.1) | 29/328 (8.8) | 0.81 (0.48 – 1.36) |
| Small-for-gestational age | |||
| < 10th percentile | 54/323 (16.7) | 47/328 (14.3) | 1.17 (0.81 – 1.67) |
| < 3rd percentile | 15/323 (4.6) | 14/328 (4.3) | 1.09 (0.53 – 2.22) |
| 5-minute Apgar < 7 | 15/323 (4.6) | 19/328 (5.8) | 0.80 (0.41 – 1.55) |
| Major congenital anomaly | 6/326 (1.8) | 2/328 (0.6) | 3.02 (0.61 – 14.85) |
| Patent ductus arteriosus | 2/320 (0.6) | 8/322 (2.5) | 0.25 (0.05 – 1.18) |
| Seizures | 1/320 (0.3) | 2/322 (0.6) | 0.50 (0.05 – 5.52) |
| NICU admission | 63/322 (19.6) | 69/329 (21.0) | 0.93 (0.69 – 1.27) |
| Length of NICU stay | 17 (6.0 – 43.0) | 15.5 (6.0 – 57.5) | P = 0.61 |
Data are presented as mean ± standard deviation, n/N (%), or median (interquartile range)
Planned subgroup analyses were performed to determine whether any interaction existed between selected variables and treatment group (Table 4). Stratification based on gestational age at randomization less than 21 weeks of gestation, CL less than 15 mm at screening, or the presence of a funnel at screening did not reveal any interaction with treatment group. Additional subgroup analyses, not defined a priori, were performed to examine the effect of 17-OHP on preterm birth < 37 weeks and < 34 weeks among women with a CL of 10–20 mm, as well on preterm birth < 34 weeks among women with a CL < 15mm (Table 5). Stratification by these cervical lengths also failed to demonstrate any interaction with treatment group.
Table 4.
A priori subgroup analyses for preterm birth prior to 37 weeks*
| 17-OHP (N = 327) |
Placebo (N = 330) |
RR (95% CI) | P** | |
|---|---|---|---|---|
| Gestational age at randomization |
0.20 | |||
| < 21 weeks | 21/104 (20.2) | 26/101 (25.7) | 0.78 (0.47 – 1.30) | |
| ≥21 weeks | 61/223 (27.4) | 54/229 (23.6) | 1.16 (0.85 – 1.59) | |
| Cervical length | 0.70 | |||
| < 15 mm | 13/25 (52.0) | 17/ 31(54.8) | 0.95 (0.58 – 1.55) | |
| ≥15 mm | 69/302 (22.8) | 63/299 (21.1) | 1.08 (0.80 – 1.47) | |
| Cervical funnel | 0.08 | |||
| Absent | 54/239 (22.6) | 50/261 (19.2) | 1.18 (0.84 – 1.66) | |
| Present | 28/88 (31.8) | 30/69 (43.5) | 0.73 (0.49 – 1.10) |
Data presented as n/N (%)
P value for Breslow-Day interaction term
Table 5.
Additional subgroup analyses for preterm birth prior to 37 and 34 weeks*
| 17-OHP (N=327) |
Placebo (N=330) |
RR (95% CI) | P** | |
|---|---|---|---|---|
| Preterm birth < 37 weeks | ||||
| Cervical length | 0.59 | |||
| < 10 mm | 5/9 (55.6) | 10/16 (62.5) | 0.89 (0.44 – 1.78) | |
| 10–20 mm | 19/50 (38.0) | 18/40 (45.0) | 0.84 (0.52 – 1.38) | |
| > 20 mm | 58/268 (21.6) | 52/274 (19.0) | 1.14 (0.82 – 1.59) | |
| Preterm birth < 34 weeks | ||||
| Cervical length | 0·82 | |||
| < 15 mm | 9/25 (36·0) | 13/31(41.9) | 0·86 (0·44–1·67) | |
| ≥15 mm | 32/302 (10.6) | 35/299 (11.7) | 0.91 (0·58–1·42) | |
| Cervical length | 0·49 | |||
| < 10 mm | 5/9 (55·6) | 6/16 (37·5) | 1.48 (0·63–3.51) | |
| 10–20 mm | 11/50 (22·0) | 12/40 (30·0) | 0·73 (0·36–1·48) | |
| > 20 mm | 25/268 (9.3) | 30/274 (10.9) | 0·85 (0·52–1·41) |
Data presented as n/N (%)
P value for Breslow-Day interaction term
Comment
In this randomized trial conducted among nulliparous women with singleton gestations and midtrimester CLs less than 30 mm, weekly intramuscular injections of 250 mg 17-OHP neither altered the frequency of PTB prior to 37 weeks nor had a discernable effect on PTB at lower gestational age thresholds, maternal outcomes, or most neonatal complications. While there was a lower frequency of early-onset sepsis among the neonates of mothers exposed to 17-OHP, the lack of difference between the two groups in either gestational age at delivery or other neonatal outcomes suggests that this may be a chance finding.
Unlike women with a prior PTB, nulliparous women with a singleton gestation have no risk factor that can be derived from their obstetric history that allows for targeted preterm birth prevention. A short CL is useful in risk stratification for nulliparous women, as it has been shown in this population to have the greatest population attributable risk associated with PTB.14 A threshold of 30 mm during the gestational age range that we studied not only would allow approximately 10% of nulliparous women to be eligible for treatment aimed at PTB prevention but would also identify women at clinically significant risk of PTB.14,15,17 Indeed, the probability of PTB in the present cohort was 25%, a frequency similar to that cited for a general population of women who have had a prior PTB.14 The primary outcome of our study, PTB prior to 37 weeks gestation, was chosen given the recognition that prevention of all PTBs, even those in the late preterm period between 34 weeks 0 days and 36 weeks 6 days, is desirable given the health and economic burden associated with these births.18–20
The results of this study contrast with the results of two prior randomized trials that have shown that vaginal progesterone treatment reduces the frequency of PTB in women with a short cervix. One potential explanation is that those trials enrolled women with notably shorter cervical lengths (less than 15 mm and 10–20 mm) that are present in only 1% and 2% of women, respectively.9,10 The presence of a “short” cervix, defined according to a population standard and not underlying pathophysiology, may not reflect a single parturitional process that is uniformly amenable to progestogen therapy. Indeed, PTB is best understood as a complex condition with multiple potential etiologies.21,22 Investigators of prior trials have recognized this possibility; one reason that Hassan et al chose 10–20 mm for progesterone treatment was their concern that women with a cervix less than 10 mm “have a higher rate of intra-amniotic infection/inflammation” and would be “less likely to benefit from progesterone administration than are patients with a longer cervix.”10 Although the present study was unable to demonstrate benefit from 17-OHP at shorter cervical lengths, it was underpowered to do so, and thus cannot be used to make conclusions about progestogen treatment for women with such cervical lengths. Conversely, the current study is the largest trial to date of women treated with a progestogen for a short cervix, the substantial majority of whom had a CL greater than 20 mm. While one meta-analysis did suggest that progesterone was effective for women with a CL up to 25 mm, more than 90% of women in that analysis were from studies that restricted enrollment to women with a CL ≤ 20 mm, and all women with a CL > 20 mm had high-risk conditions, such as prior preterm birth.23 Accordingly, in light of the negative findings, there is at present no evidence from any randomized trial that progestogens benefits nulliparous women with a CL greater than 20 mm.
There are other possible reasons for the discrepant findings as well. Both Fonseca et al and Hassan et al used vaginal progesterone, albeit different formulations.9,10 Although both 17-OHP and vaginal progesterone have been shown to be efficacious at reducing recurrent PTB, it is possible that a vaginally administered progesterone may be of particular benefit for women with a short cervix. However, this possibility remains purely speculative, as 17-OHP has not been evaluated in an adequately powered trial of women with a CL less than 20 mm, and vaginal progesterone has not been evaluated in an adequately powered trial of women with singleton gestations and no prior preterm birth with a CL greater than 20mm. Additional comparative effectiveness or placebo-controlled trials may provide further insight. The previously published two trials also differed from the present study with regard to their populations. These trials included women with other risk factors for PTB, including multiple gestations, prior PTBs, and prior cervical surgeries. The present study, in contrast, excluded women with such conditions, and focused instead on an otherwise low-risk population of women who were nulliparous and had no other indication for either progestogen treatment or PTB surveillance. Such differences in the study population also may explain the different results.
The population that was selected should be generalizable to the general population of nulliparous women with a singleton gestation. Women were enrolled from across the United States in fourteen different centers, and were socioeconomically diverse. Approximately 10% had a CL less than the 10th percentile, as predicted from prior observational studies.14,15,17 The frequency of PTB, moreover, was 24.7%, which similarly is consistent with the frequency of PTB for nulliparous women with a cervix less than the 10th percentile that has been cited in other studies.14,15
In summary, the results of our trial show no demonstrable benefit from the use of weekly intramuscular 17-OHP to reduce the frequency of PTB in nulliparous women with a midtrimester CL less than 30 mm. Further investigation is needed to determine whether 17-OHP may benefit women with a CL below a lower percentile threshold, or whether other forms of progestogens provide benefit for women other than the 1–2% of the population identified by the CL criteria used in prior trials.
Acknowledgements
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
Northwestern University, Chicago, IL – A. Peaceman, M. Dinsmoor (NorthShore University HealthSystem), G. Mallett, J. Senka (NorthShore University HealthSystem)
The Ohio State University, Columbus, OH – F. Johnson, D. Cline, C. Latimer, S. Frantz, S. Fyffe, P. Shubert (St. Ann’s), L. Gerwig (St. Ann’s)
University of Texas Medical Branch, Galveston, TX – J. Moss, A. Salazar, G.D.V. Hankins, G. Olson, A. Jackson, C. Sutherland
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH – C. Milluzzi, W. Dalton, J. Russo, S. Myers, T. Waters, T. Dotson
University of Alabama at Birmingham, Birmingham, AL – W. Andrews, A. Northen, J. Sheppard, J. Grant, D.J. Rouse
Brown University, Providence, RI – D. Allard, J. Hunt, J. Tillinghast, M. Barthelemy, D. Gardner, C. Duquette
Wayne State University, Detroit, MI – N. Hauff, G. Norman, M. King (RDMS), D. Allen (RDMS), T. Smith
Columbia University, New York, NY – R. Miller, S. Bousleiman, L. Plante (Drexel), C. Tocci (Drexel), A. Ranzini (St. Peter's), M. Lake (St. Peter's), M. Hoffman (Christiana), S. Lynch (Christiana)
University of Texas Southwestern Medical Center, Dallas, TX – J. Dashe, L. Moseley, J. Kingsbery, V. Bludau, R. Benezue
The University of Texas Health Science Center at Houston, Houston, TX – F. Ortiz, P. Givens, B. Rech, C. Moran
University of Utah Health Sciences Center, Salt Lake City, UT – P. Reed, K. Hill, M.W. Varner, A. Weaver (McKay-Dee), S. Alexander (LDS Hospital), D. Thompson-Garbrecht (Intermountain Medical Center), J. Miller (UVRMC)
Oregon Health & Science University, Portland, OR – R. Acosta (Providence Sacred Heart Medical Center), C. Flores (Providence Sacred Heart Medical Center), M. Ricon, W. Smith (Kaiser Permanente), S. Butcher (Kaiser Permanente), S. Segel, L. Pereira
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Dorman, K. Pena-Centeno, K. Clark, S. Timlin
University of Pittsburgh, Pittsburgh, PA – M.H. Birkland, H. Simhan, P. Cotroneo, R. Zubic, D. Nowinski
The George Washington University Biostatistics Center, Washington, DC – S. Gilbert, A. Lozitska
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – S. Tolivaisa
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD21410, UL1 RR024153; UL1 TR000005; HD27869, HD27915, HD27917, HD34116, HD34208, 5UL1RR025764, HD36801, HD40500, HD40512, HD40544, M01 RR00080, UL1 RR024989 (NCRR), HD40545, HD40560, HD40485, HD53097, HD53118] does not necessarily represent the official views of the NCRR, NICHD, or NIH.
The authors thank the following subcommittee members who participated in protocol/data management and statistical analysis (Sharon Gilbert, MS, MBA) and protocol development and coordination between clinical research centers (Gail Mallett, RN, BSN, CCRC and Cynthia Milluzzi, RN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE: The authors report no conflict of interest
This work was presented at the Society for Maternal-Fetal Medicine, February 2012
References
- 1.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 2.Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89:417–421. doi: 10.1016/j.lfs.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 3.DaFonseca EB, Bittar RE, Carvalho MHB, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 4.Meis PJ, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 5.Petrini JR, Callaghan WM, Klebanoff M, et al. Estimated effect of 17 alphahydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. doi: 10.1097/01.AOG.0000150560.24297.4f. [DOI] [PubMed] [Google Scholar]
- 6.Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 7.Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, doubleblind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–2040. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 8.Combs CA, Garite T, Maurel K, Das A, Porto M, et al. 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2011;204:221, e1–e8. doi: 10.1016/j.ajog.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 10.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 12.Iams J, Goldenberg R, Meis P, Mercer B, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 13.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [Erratum, Control Clin Trials 1989; 10:following 126.] [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg RL, et al. The preterm prediction study: The value of new vs. standard risk factors in predicting early and all spontaneous preterm births. Am J Pub Health. 1998;88:233–238. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbard JU, et al. Cervical length at 16–22 weeks’ gestation and risk for preterm delivery. Obstet Gynecol. 2000;96:972–978. doi: 10.1016/s0029-7844(00)01074-7. [DOI] [PubMed] [Google Scholar]
- 16.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 17.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 18.Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. JAMA. 2000;284:843–849. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- 19.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 20.Lindström K, Winbladh B, Haglund B, Hjern A. Preterm infants as young adults: a Swedish national cohort study. Pediatrics. 2007;120:70–77. doi: 10.1542/peds.2006-3260. [DOI] [PubMed] [Google Scholar]
- 21.Pennell CE, Jacobsson B, Williams SM, Buus RM, Muglia LJ, Dolan SM, et al. Genetic epidemiologic studies of preterm birth: guidelines for research. Am J Obstet Gynecol. 2007;196:107–118. doi: 10.1016/j.ajog.2006.03.109. [DOI] [PubMed] [Google Scholar]
- 22.Kramer MS, Papageorghiou A, Culhane J, Bhutta Z, Goldenberg RL, Gravett M, et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;206:108–112. doi: 10.1016/j.ajog.2011.10.864. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O'Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124, e1–e19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]