Abstract
Objective
To examine the neuropathological substrates of cognitive dysfunction and dementia in Parkinson’s disease (PD).
Methods
140 patients with a clinical diagnosis of PD and either normal cognition or onset of dementia two or more years after motor symptoms (PDD) were studied. Patients with a clinical diagnosis of dementia with Lewy bodies were excluded.
Autopsy records of genetic data and semi-quantitative scores for the burden of neurofibrillary tangles (NFTs), senile plaques (SPs), Lewy body (LB/LN) and other pathologies were used to develop a multivariate logistic regression model to determine the independent association of these variables with dementia. Correlates of co-morbid Alzheimer’s disease (PDD+AD) were also examined.
Results
92 PD patients developed dementia and 48 remained cognitively normal. Severity of cortical LB/LN (CLB/LN) pathology was positively associated with dementia (p<0.001), with an odds-ratio (OR) of 4.06 (CI95%1.87–8.81), as was Apolipoprotein E4 (APOE4) genotype (p=0.018,OR4.19 CI95% 1.28–13.75). 28.6% of all PD cases had sufficient pathology for co-morbid AD, of which 89.5% were demented. The neuropathological diagnosis of PDD+AD correlated with an older age of PD onset (p=0.001,OR1.12 CI95%1.04–1.21), higher CLB/LN burden (p=0.037,OR 2.48 CI95%1.06–5.82), and cerebral amyloid angiopathy severity (p=0.032, OR4.16 CI95%1.13–15.30).
Interpretation
CLB/LN pathology is the most significant correlate of dementia in PD. Additionally, APOE4 genotype may independently influence the risk of dementia in PD. AD pathology was abundant in a subset of patients, and may modify the clinical phenotype. Thus, therapies that target α-synuclein, tau, or Aβ could potentially improve cognitive performance in PD.
Introduction
Cognitive dysfunction and dementia are a significant non-motor manifestation of Parkinson’s disease (PD), with up to 80% of patients developing dementia.1 Cognitive dysfunction seriously compromises the ability to perform activities of daily living,2 resulting in reduced independence, quality of life, and survival.3, 4 Clinically, dementia in PDD is similar, and often identical to, dementia with Lewy bodies (DLB);5, 6 however these typical features may be masked by an Alzheimer’s disease (AD)-like amnestic syndrome.7
PDD is a heterogeneous neuropathological entity. Multiple clinicopathological correlation studies have addressed this issue with conflicting results. Our group,8 and others9–12 have reported that cortical (CLB) or limbic10, 13 Lewy bodies (LBs) and Lewy neurites (LNs) are the best correlate of dementia in PD, indicating a caudal to rostral spread of LB/LN pathology from the brainstem to cerebral cortex, as proposed by Braak and colleagues.14 However, others have found no correlations between cognitive function and the distribution of LBs in the brain.15–17
Co-morbid AD pathology is also common in PDD,11, 18, 19 and others have proposed that neurofibrillary tangle (NFT) and Aβ senile plaque (SP) pathology20, 21 or a combination of these and CLB/LNs form the neuropatholological basis for PDD.22 Furthermore, AD-specific neuroimaging23 and cerebrospinal fluid24 biomarkers are associated with cognitive impairment in PD. These overlapping features suggest a potential clinicopathological continuum between AD and PD.25 Advances in immunohistochemical (IHC) methods and diagnostic criteria, together with variability in case selection, cognitive assessments, and small sample sizes may all contribute to these discrepancies.9
Here we present a large, well characterized cohort of PD patients, followed longitudinally to autopsy at two major movement disorders centers in the US. Detailed analysis of neuropathological and genetic data enabled us to determine the strongest correlates of dementia in PD, and examine the relationship between CLB/LNs and AD pathology.
Patients and Methods
Patient Selection
140 patients with a clinical diagnosis of PD with and without dementia who had been treated at either the University of Pennsylvania’s Parkinson’s Disease and Movement Disorders Center, the Parkinson’s Disease Research, Education and Clinical Center at the Philadelphia VA Medical Center (Penn; n=121;40PD,81PDD), or the Udall Parkinson’s Disease Research Center at the University of Washington (n=19;8PD,11PDD) were selected for study. Forty two patients (20PD, 22PDD) from Penn were described in a previous report.8 Clinical diagnoses of PD and PDD were determined by the treating physician (JED,JBL,HIH) during life based on the United Kingdom Brain Bank26 and the Diagnostic and Statistical Manual of the American Psychiatric Association (4th edition)27 criteria. In most cases patients were seen in clinic or phone contact was made with the patient or his/her family during the last three months of life. In addition, phone contact with the next of kin immediately after death provided additional information on cognitive status prior to death. Patients diagnosed with mild cognitive impairment (MCI) were categorized in the non-demented group (n=4). All patients had either normal cognition or dementia starting two or more years after the onset of PD motor symptoms. Patients with a clinical diagnosis of DLB or onset of dementia within two years of PD motor symptom onset were excluded. Genotyping for hereditary forms of PD was performed only in cases with significant family history. All cases were sporadic, with the exception of one SCNA triplication case.28
Neuropathological assessment
Neuropathological examination was performed as previously described8, 29 with gross examination of fresh or fixed tissue. Informed consent was obtained in accordance with the rules of the respective institutional review boards at each university. Semi-quantitative scores (0–3) for the major histological signatures of AD (NFTs, SPs) and PD (LBs/LNs) were determined for each case using IHC with established monoclonal antibodies for tau (PHF-130 or AT-831) and alpha-synuclein (SYN30332). Mature SPs were evaluated by the amyloid-binding dye, Thioflavin-S (ThS) or tau IHC. Cerebral amyloid angiopathy (CAA) was evaluated in the midfrontal cortex using ThS. Scoring and post-mortem diagnosis were performed by experienced neuropathologists (JQT, TJM) and later extracted from the Penn33 and UW databases for use in the statistical analysis. Scoring of dystrophic LNs in the cornu-ammonis (CA) region 2 and 3 of the hippocampus (CA2-3 LN) 34 was based on the highest density in these regions. The diagnosis of hippocampal sclerosis (HpScl) was established using the criteria of selective neuronal loss and gliosis in CA-1 and subiculum as described.35 The diagnosis of argyrophilic grain disease (AGD) was made by review of hippocampal sections with IHC for tau (n=132) for the presence of dense tau-positive grains in the entorhinal cortex and mild to moderate involvement of the CA region, most consistent with a stage III36 or higher of AGD pathology, together with pretangles in the dentate gyrus, and variable glial white matter pathology in the entorhinal cortex, as described.36, 37 Cerebrovascular disease (CVD) was defined based on the presence of vascular brain injury (VBI) using modified criteria outlined in the latest NIA-Alzheimer’s Association guidelines.38 Briefly, gross evidence of ischemic or hemorrhagic infarction or 2 or more microvascular lesions (MVLs) in five hematoxylin and eosin stained sections (i.e. thalamus, basal ganglia, frontal, parietal and temporal cortex) were considered positive for CVD. MVLs were enumerated at the time of neuropathological diagnosis and retrospectively confirmed for all cases. Evaluation of CA2-3 LN, HpScl, AGD, CVD and missing database values were examined retrospectively at the time of this study. Staging of pathology was performed retrospectively using Braak39 (NFTs), CERAD40 (SPs) and McKeith41 (LBs/LNs) criteria on regional semi-quantitative data. Cases with an intermediate or high probability of AD42 were classified as having “co-morbid AD.” Seven cases with missing tissue/data precluded staging assessment in these cases. All retrospective analyses were performed blind to the clinical diagnosis.
Genetic studies
DNA was extracted from peripheral blood following the manufacturer’s protocols (Flexigene (Qiagen; Valencia, CA) or QuickGene DNA whole blood kit (Autogen; Holliston, MA). Genotyping was performed using real-time allelic discrimination with Applied Biosystem (ABI; Foster City, CA) TaqMan probes. The following SNPs were genotyped with the corresponding ABI assays: MAPT (rs1052553, C_7563736_10) and APOE (rs7412, C_904973_10 and rs429358, C_3084793_20). Genotyping was performed on an ABI 7500 real-time instrument using standard conditions. Data were analyzed using ABI 7500 Software v2.0.1.
Statistical analysis
The global cortical score for burden of CLB/LN, SP, and NFT was determined by averaging semi-quantitative scores in five cortical regions as described previously.8 Briefly, the regions studied include the mid-frontal, anterior cingulate, ventromedial-temporal (average of the amygdala, entrohinal cortex, and CA1-4), lateral-temporal and parietal cortex. Available tissue in Wernicke’s area or the superior/mid temporal cortex was used to evaluate the lateral-temporal lobe and post-central or angular cortex for the parietal lobe. A cortical distribution score was calculated based on the number of these five cortical regions with a score >0. These whole number scores were designated as ordinal variables, as were raw scores from individual regions. Categorical variables included presence of HpScl, CVD, AGD, CAA, APOE4 and H1/H1 genotyope. Continuous clinical variables included age of motor onset, age of dementia onset, age of death, disease duration, motor to dementia onset interval, and dementia onset to death interval. Demographic data were compared between groups using chi-square tests or Fisher’s exact tests for categorical variables and independent t-tests or Mann-Whitney U tests for continuous variables, as appropriate.
A stepwise-selection model building procedure was used to develop a logistic regression model to examine the association of these variables with the primary outcome of dementia in this cohort. Individual cortical region scores, AGD, CAA and HpScl were excluded from the selection procedure due to limited data for these features in some groups, but were examined in the univariate analysis (Supplementary Table 1, Figure 1). A receiver operating characteristic curve (ROC) was generated to assess the diagnostic accuracy of the model.
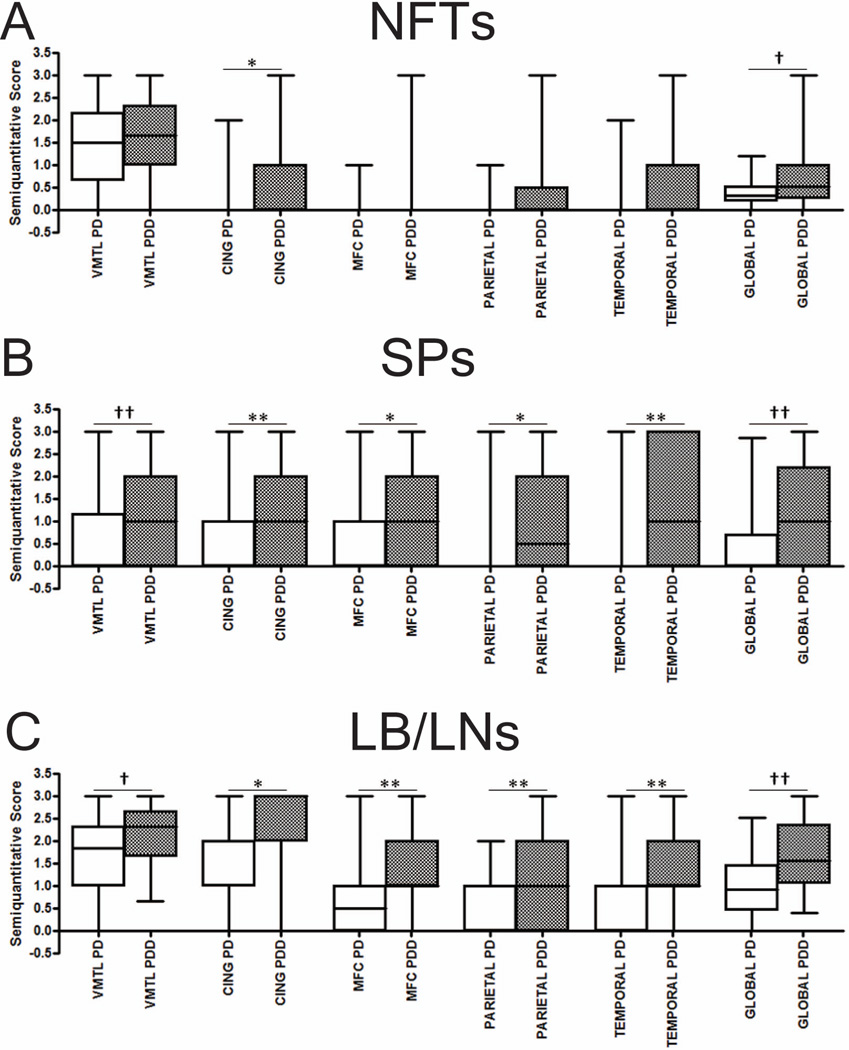
Figure 1.
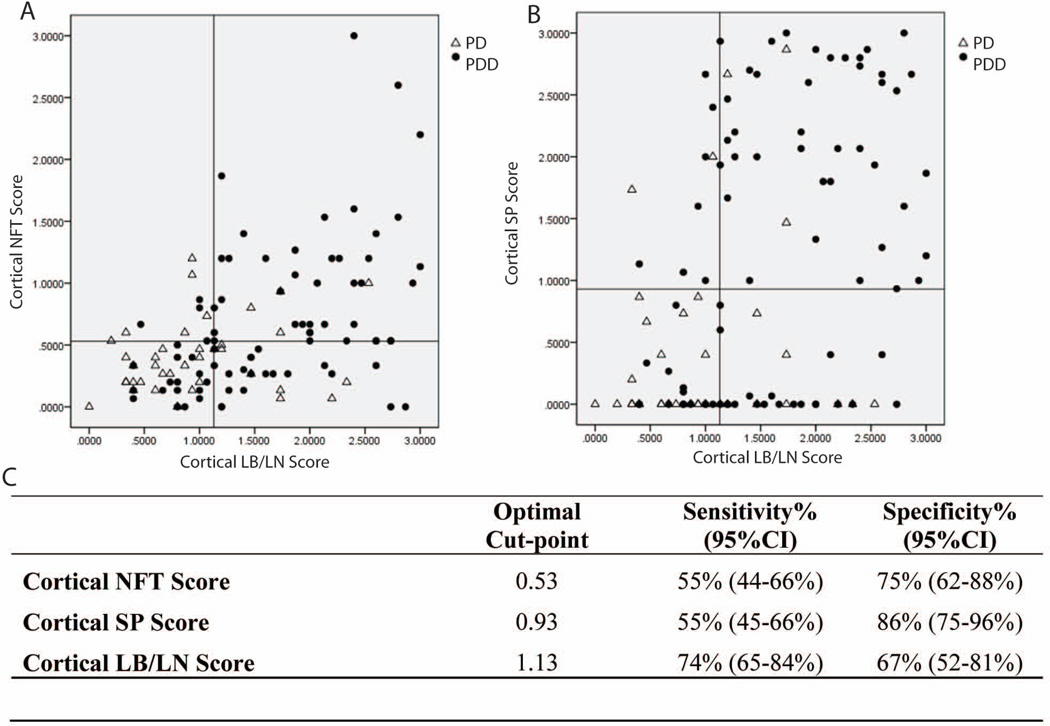
Multiple logistic regressions were applied to the baseline model of dementia, controlling for age of death, gender, and APOE genotype to measure the independent effects of each cortical pathology type (Table 2). Categories with too few subjects were collapsed for analysis (i.e. Braak≥ III-IV, CERAD≥A, NFT distribution score≥2, SP distribution score≥1). Finally, estimates of sensitivity and specificity for global cortical pathology scores were obtained at an optimal cut-point, defined as the point which maximizes the sum of the specificity and sensitivity.
Stepwise-selection procedures incorporating all variables from the previous multivariate model were performed to determine correlates of the presence of co-morbid AD and CLB/LN burden. All statistical tests were two sided, and significance set at the 0.05 level. Analyses were performed using SPSS 19.0 (SPSS, Chicago, Ill) and R version 2.13.43
Results
Demographic information
One hundred-forty patients were included in the study (Table 1). Ninety-two developed dementia during the course of their illness while 48 were judged by the clinician non-demented at the time of death. The two groups had similar age of motor onset and disease duration. The APOE4 allele was more prevalent in the PDD group (p<0.001) while the proportion of H1/H1 haplotype carriers was similar between groups (p= 0.223).
Table 1.
Demographic information for patient groups.
| PD N=48 |
PDD N=92 |
p-value | |
|---|---|---|---|
| Gender N (%M) | 48 (70.8%) | 92 (78.3%) | 0.3311 |
|
Age of Motor Onset (years) Median (IQR) |
61.00 (48.75, 70.00)* | 63.50 (57.25, 71.75) | 0.2572 |
|
Age of Dementia Onset (years) Median (IQR) |
NA | 74.00 (69.25, 79.75) | NA |
|
Age of Death (years) Median (IQR) |
80.00 (72.00, 83.50) | 79.00 (74.00, 82.00) | 0.7652 |
|
Disease Duration (years) Median (IQR) |
14.50 (9.75, 23.50)* | 13.00 (9.00, 19.00) | 0.2532 |
|
Motor-Dementia Interval (years) Median (IQR) |
NA | 8.00 (5.00, 14.00) | NA |
|
Dementia-Death Interval (years) Median (IQR) |
NA | 4.00 (2.00, 6.00) | NA |
|
Brain Weight (grams) Median (IQR) |
1320.5 (1177.8, 1395.8) | 1300.0 (1204.0, 1423.0)** | 0.2873 |
| APOE4 N (% carriers) | 4/42 (9.5%) | 40/89 (44.9%) | <0.0011 |
| H1/H1 Haplotype N (% carriers) | 20/37 (54.1%) | 40/89 (44.9%) | 0.2231 |
Chi-Square,
Mann Whitney-U,
Independent T-test.
Missing Data for 2* and 1** cases.
Neuropathological analysis
PDD patients had a significantly higher severity and wider distribution of cortical neuropathology for the three main lesion types studied (Supplementary Table 1). In addition to semi-quantitative measures, classification of disease burden differed significantly between the two groups, most notably with the PDD subgroup composed of exclusively limbic or neocortical LB/LN stage cases. Both Braak stages (p=0.009) and CERAD scores (p=0.001) were overall more advanced in the demented group; however, 9.1% of PD cases without dementia had significant pathology for a histiologic diagnosis of co-morbid AD. Conversely, 41.6% of the PDD group had no significant cortical SP pathology (CERAD 0) and 49.4% had minimal NFTs (Braak 0–II). Thus, co-morbid AD was common, affecting a subgroup of PDD (38.2%). CAA was also more prevalent in PDD (p=0.003). HpScl, CVD, AGD, CA2-3 LN and striatal NFTs, SP, and LB/LNs were not significantly different between groups (Supplementary Table 1).
Regional analysis showed a significantly increased burden of SP and LB/LN pathology in the PDD group for all regions studied (Figure 1). NFT density was significantly higher in the anterior cingulate gyrus and global cortical score only.
The associations between dementia and NFTS, SPs, and CLB/LNs were assessed using logistic regression models. The likelihood-ratio test was used in each model to determine whether the neuropathological variable contributed significantly to the fit of the model after adjusting for age at death, gender, and APOE status. All else equal, an increased CLB/LN global score, distribution score, and neocortical stage were associated with increased odds of dementia, as was advanced SP and NFT distribution and global cortical SP scores (Table 2).
Table 2.
Correlation of independent neuropathologic variables with dementia in PDD.
| Measure† | OR (95% CI)†† | P Value††† |
|---|---|---|
| Staging | ||
| Braak I–II | 0.68 (0.12–3.94) | 0.0151 |
| Braak ≥III–IV | 2.58 (0.38–17.41) | |
| CERAD ≥ A | 1.99 (0.85–4.68) | 0.1117 |
| Neocortical LB/LN Stage | 5.80 (2.38–14.16) | 0.0001 |
| Cortical Severity | ||
| Cortical NFT Score | 3.08 (0.95–9.99) | 0.0316 |
| Cortical SP Score | 1.84 (1.14–2.97) | 0.0082 |
| Cortical LB/LN Score | 4.15 (1.88–9.18) | 0.0001 |
| Cortical Distribution | ||
| NFT Distribution Score ≥2 | 2.58 (1.03–6.44) | 0.0384 |
| SP Distribution Score ≥1 | 2.43 (1.03–5.75) | 0.0419 |
| LB/LN Distribution Score =3 | 2.33 (0.62–8.78) | 0.0012 |
| LB/LN Distribution Score =4 | 5.50 (1.47–20.64) | |
| LB/LN Distribution Score =5 | 11.27 (2.84–44.7) |
Categories with fewer than three individuals were combined for analysis (i.e. Braak stage, CERAD score, NFT distribution score, and SP distribution score).
ORs and 95% CIs were generated from logistic regression models where the dependent (outcome) variable was presence of dementia. Age at death, gender and APOE were included as covariates and each neuropathological measure was analyzed in a separate model.
P-values were obtained from a likelihood ratio test comparing a model including age at death, gender, APOE and the indicated neurpathological measure versus a model including age at death, gender and APOE only.
At the optimal cut points for the global cortical scores, CLBs/LNs have the highest sensitivity (74%; specificity 67%) for dementia, while NFTs and SPs have a higher specificity (75% and 86%, respectively; sensitivity 55% for both) (Figure 2).
Figure 2.
Neuropathological correlates of PDD
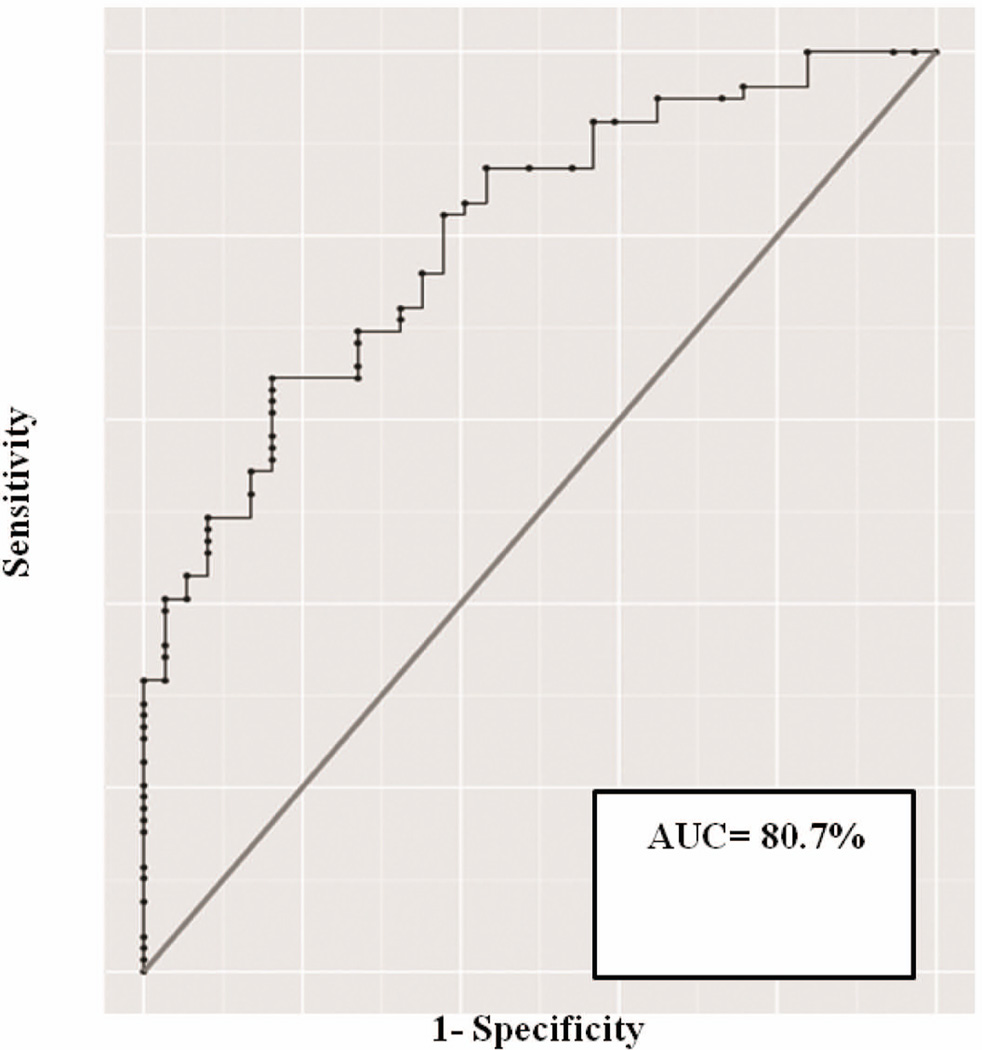
The stepwise-selection model building procedure identified two significant correlates of dementia: CLB/LN score (p<0.001, OR 4. 06, 95%CI 1.87–8.81) and APOE4 genotype carrier status (p= 0.018, OR 4.19, 95%CI 1.28–13.75; Table 3). We found no significant interaction between these variables, and also between APOE4 genotype and measures of AD pathology or gender. The ROC curve obtained using this model (Figure 3) shows high diagnostic performance of the model (area under the curve= 80.7%).
Table 3.
Stepwise selection logistic regression model to predict PDD.
| Variable | Estimate | Std. Error |
z value |
P- value |
Odds- Ratio |
95% Confidence- Interval |
|---|---|---|---|---|---|---|
| (Intercept) | −1.37 | 0.50 | −2.73 | 0.0063 | 0.25 | 0.10–0.63 |
|
Global Cortical LB/LN Score |
1.40 | 0.40 | 3.53 | 0.0004 | 4.06 | 1.87–8.81 |
| APOE4 carrier | 1.43 | 0.61 | 2.44 | 0.0182 | 4.19 | 1.28–13.75 |
based on 116 observations
Figure 3.
PDD subgroup analysis by Motor-Dementia Interval (MDI)
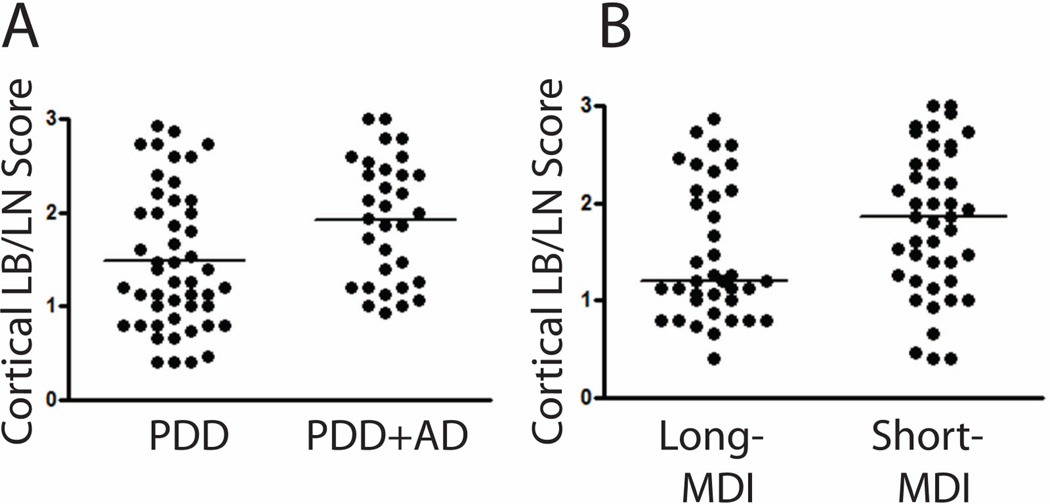
Some studies have suggested an exponential rate of clinical progression in PD,44 with older age of motor onset associated with a shorter MDI and higher burden of CLB/LN, SP and NFT pathology.22, 45 Due to the large range in MDI in our cohort (2–30 years), we chose a similar stratification of the PDD group into short-(MDI <10 years) and long-MDI (MDI ≥10 years) groups to explore this phenomenon (Table 4). The short-MDI cases were mostly male (88%, p=0.013), older at PD onset (p<0.001) and had a shorter overall disease duration (p<0.001). Furthermore, they had higher levels of cortical NFTs (p=0.003) and CLBs/LNs (p=0.028) (Figure 4), with a higher percentage (47.8%) of co-morbid AD (p=0.027).
Table 4.
Comparison of long- and short-MDI for PDD patients
| PDD- Short MDI N=50 |
PDD- Long MDI N=42 |
P-value | |
|---|---|---|---|
| Number of Patients (%M) | 44/50 (88.0%) | 28/42 (66.7%) | 0.0131 |
|
Age of Motor Onset (years) Median (IQR) |
69.50 (64.75, 75.25) | 58.50 (50.00, 63.00) | <0.0012 |
|
Age of Dementia Onset (years) Median (IQR) |
74.00 (71.25, 80.00) | 74.50 (68.75, 77.75) | 0.5402 |
|
Age of Death (years) Median (IQR) |
79.00 (73.00, 83.25) | 78.00 (74.00, 81.25) | 0.7182 |
|
Disease Duration (years) Median (IQR) |
9.00 (8.00, 11.25) | 19.50 (15.75, 23.00) | <0.0012 |
|
Motor-Dementia Interval (years) Median (IQR) |
5.00 (4.00, 8.00) | 14.00 (12.00, 19.00) | NA |
|
Dementia-Death Interval (years) Median (IQR) |
4.00 (2.00, 6.00) | 3.50 (1.00, 6.00) | 0.8892 |
|
Brain Weight (grams) Median (IQR) |
1308.00 (1220.0, 1486.0)* | 1285.5 (1185.25, 1400.0) | 0.1253 |
| APOE4 N (% carriers) | 23/48 (47.9%) | 17/41 (41.5%) | 0.5421 |
| H1/H1 Haplotype (% carriers) | 25/37 (67.6%) | 26/40 (65.0%) | 0.6391 |
|
Braak Stage N (% cases) 0 I–II III–IV V–VI |
1/46 (2.2%) 15/46 (32.6%) 15/46 (32.6%) 15/46 (32.6%) |
6/40 (15.0%) 20/40 (50.0%) 8/40 (20.0%) 6/40 (15.0%) |
0.0101 |
|
CERAD Stage N (% cases) 0 A B C |
14/46 (30.4%) 3/46 (6.5%) 11/46 (23.9%) 18/46 (39.1%) |
23/42 (54.8%) 2/42 (4.8%) 7/42 (16.7%) 10/42 (23.8%) |
0.1211 |
| AD diagnosis N (% cases) | 22/46 (47.8%) | 11/40 (27.5%) | 0.0271 |
|
LB/LN Stage N (% cases) Brainstem Limbic Neocortical |
0/48 (0.0%) 4/48 (8.3%) 44/48 (91.7%) |
0/39 (0.0%) 11/39 (28.2%) 28/39 (74.4%) |
0.0191 |
|
Global Cortical NFT N Median (IQR) |
42 0.67 (0.33, 1.2) |
38 0.43 (0.13, 0.67) |
0.0032 |
|
Global Cortical SP N Median (IQR) |
45 1.6 (0.03, 2.50) |
40 0.33 (0.00, 2.10) |
0.1122 |
|
Global Cortical LB/LN N Median (IQR) |
44 1.87 (1.22, 2.50) |
38 1.20 (1.00, 2.13) |
0.0282 |
Chi-Square,
Mann Whitney-U,
Independent T-test.
Missing data from 1 case.
Figure 4.
Relationship between AD and CLB pathology in PDD
Using a stepwise-selection model, older age of PD onset (p=0.001, OR 1.12 95%CI 1.04–1.21), higher CLB/LN score (p=0.037, OR 2.48 95%CI 1.06–5.82), and increased severity of CAA (p=0.032, OR4.16 CI95%1.13–15.30) were found to be independently associated with PDD+AD (Table 5). Univariate analysis showed higher CLB/LN and CAA severity in the PDD+AD subgroup as well (Figure 4, Supplementary Table 2)
Table 5.
Step-wise selection logistic regression model to predict a secondary diagnosis of co-morbid AD in PDD patients.
| Variable | Estimate | Std. Error |
z value |
P- value |
Odds- Ratio |
95% Confidence- Interval |
|---|---|---|---|---|---|---|
| (Intercept) | −9.82 | 2.66 | −3.70 | 0.0002 | <0.01 | <0.01–0.01 |
| Age of Motor Onset | 0.12 | 0.04 | 3.19 | 0.0014 | 1.12 | 1.04–1.21 |
| Global Cortical LB/LN Score | 0.91 | 0.44 | 2.08 | 0.0372 | 2.48 | 1.06–5.82 |
| CAA score | 1.43 | 0.66 | 2.15 | 0.0319 | 4.16 | 1.13–15.30 |
based on 81 observations
To examine correlates of CLB/LN burden, a stepwise linear regression model showed increased global cortical NFT score (p<0.001), presence of dementia (p=0.002), CA2-3 LN score >1 (p=0.001) and APOE 4 carrier status (p=0.014) to be significant (Table 6). There was also a significant interaction between APOE4 genotype and age of motor onset (p=0.048); for APOE4 carriers, an earlier age of motor onset was associated with a higher CLB/LN burden. Age of motor onset was not significantly associated with CLB/LN burden in APOE4 negative patients (p=0.542).
Table 6.
Step-wise selection linear regression model to predict global cortical LB/LN score.
| Variable | Estimate | Std. Error | t value | P-value |
|---|---|---|---|---|
| (Intercept) | 0.40 | 0.37 | 1.07 | 0.2885 |
| Global Cortical NFT Score | 0.75 | 0.13 | 5.73 | <0.0001 |
| Clinical Dementia | 0.39 | 0.12 | 3.16 | 0.0021 |
| LN CA2-3 Score ≥1 | 0.45 | 0.13 | 3.45 | 0.0008 |
| APOE4 Carrier | 1.82 | 0.73 | 2.49 | 0.0144 |
| Age of Motor Onset | −0.003 | 0.01 | −0.61 | 0.5424 |
| APOE4/Age of Motor Onset Interaction | −0.02 | 0.01 | −2.01 | 0.0476 |
based on 104 observations
Discussion
Our detailed analysis of a large cohort of PD patients from two university-based PD movement disorders centers shows that the most robust correlate of dementia in PD is the severity of CLB/LNs and APOE4 genotype. This combination of pathologies and genetic factors account for the majority of variability in our model. There was an independent contribution of NFTs and SPs for increased odds ratio for dementia in PD, but these effects did not reach significance in the multivariate model; however, a thorough and comprehensive sub-analysis of the PDD group which was designed to examine variables predictive of a co-morbid AD diagnosis and demographics of PDD+AD patients, suggests that plaque and tangle pathology may influence cognitive status and the course of disease progression in a subset of PDD patients.
These data confirm our previous report8 of the importance of CLB/LNs in the development of dementia in PD. Others have suggested cognitive impairment in PD is due to a generalized process rather than involvement in specific regions.9 Indeed, we show here that CLB/LN density was greater in all cortical regions examined for PDD.
Subcortical basal ganglia SP46 and LB/LN32, 46 pathology is often more robust in DLB than in PDD cases and some studies also reported higher levels of SP22, 47 and LB/LN pathology48 in PDD compared to PD. In this study, we examined a larger number of cases and found modest levels of SPs, NFTs, and LB/LNs in the striatum for both demented and non-demented patients. Since we measured mature plaques only, the effect of other types of Aβ plaques or deposits in this study could be understated; however, other investigators have found a similar burden of diffuse plaques in PD and PDD groups.22
While the optimal CLB/LN cut-point was sensitive to detect the majority of PDD, it was less specific, mirroring previous data showing CLB/LN pathology in non-demented cases.15, 16 Thus, CLB/LNs do correlate significantly with cognitive impairment in the majority of PDD patients; however, differing thresholds resulting in the emergence of cognitive impairment during life may exist due to other factors, including APOE genotype as well as co-morbid CVD and AD. Indeed, all PDD cases in our series with minimal (<0.5) CLB/LN scores had co-morbid CVD or significant subcortical pathology as a possible contributor to dementia (Supplementary Table 3).
The significant association of the APOE4 genotype with PDD in our cohort is intriguing and suggests an independent contribution to cognitive decline in PD, as there was no significant interaction between APOE4 carrier status and the global CLB/LN score or measures of AD neuropathology in our dementia model (Table 3). Despite the lack of significance of this interaction, the APOE4 genotype was a significant correlate in the multivariate regression model to predict a greater CLB/LN severity (Table 6). Thus, the APOE4 genotype may contribute to cognitive decline in PD through both shared and independent neurodegenerative pathways to those associated with Lewy pathology. Interestingly, in contrast to AD,49 there was no interaction between APOE4 genotype and gender in our PD cohort, which may be due to the predominant number of male patients in our study. Others have also shown an effect of APOE genotype on PDD50, 51 and CLB/LN severity,11 as well as a potential involvement of the APOE protein in cell52 and animal models53 of α-synuclein-mediated neurodegeneration, but further research is needed to elucidate the molecular mechanisms underlying these connections. Diagnostic accuracy was enhanced by incorporating both CLBs/LNs and APOE in the multivariate model for PDD (Figure 3); implying that these factors influence cognitive impairment in the majority of PDD cases and that APOE genotype may be important to examine in clinical trials of PD involving cognitive outcomes.
Variability among previous studies may partly reflect the effects of CVD, since most clinicopathological studies of PDD did not evaluate CVD; however, one study found an association between advanced Braak NFT stage and CVD in PDD.54 Thus, AD pathology may be additive in causing CVD in PDD. A reported inverse relationship between CVD and CLB/LN scores and direct correlation with CAA55 suggests that AD-type pathology may accelerate CVD through associated CAA, independent of atherosclerosis and lipohyalinosis, and CVD may potentiate CLB/LN associated cognitive impairment. Furthermore, APOE4 genotype confers a risk for vascular dementia both with and without co-morbid AD.56 We found that CAA was more common in PDD (Supplementary Table 1), especially in those with co-morbid AD; however, there was no unequivocal increased presence of CVD in the PDD and PDD+AD subgroups (Supplementary Tables 1,2) and CVD did not reach significance in our multivariate model of dementia. Our characterization of CVD was based on the neuropathological assessment in the recently revised NIA-AA AD guidelines,38 and therefore our study was limited to measures of VBI. Further study and validation of the neuropathological correlates of VBI are needed; however, using the most recent criteria available we do not show a significant influence of CVD on cognitive outcomes in PD.
Neither NFTs nor SPs were significant in our overall multivariate model of PDD. This notwithstanding, cortical NFT and SP severity scores were more specific for dementia than CLB/LNs (Figure 2), reflecting the high frequency of dementia in patients with sufficient pathology for a diagnosis of co-morbid AD (89.5%). This finding suggests that PD patients, especially those with an older age of onset, may be at increased risk for developing AD. Hence, we speculate that this may reflect a “double hit” model of cognitive impairment in PD wherein AD and CLB/LN pathologies converge to cause distinct forms of cognitive impairment in PD/PDD. There were also independent associations of NFTs and SPs in the univariate analysis of dementia (Table 2), indicating that these pathologies contribute to dementia in the subset of PDD+AD patients. The presence of a large proportion of PDD cases without significant AD pathology most likely explains why these measures may not be significant in the multivariate model. Furthermore, patients with co-morbid AD had a shorter MDI (Supplementary Table 2), suggesting an accelerated disease course. Thus, the presence of AD appears to be a relatively specific, although not exclusive,19 finding in PDD that potentially modifies the clinical phenotype.54 Indeed, others have shown a poorer prognosis for PDD cases with co-morbid AD.20
Our finding that patients with a shorter MDI (<10 years) also have an older age of PD onset, shorter disease duration and higher burden of co-morbid AD pathology (Table 4, Figure 4), agrees with previous reports;20, 22, 44, 45, 57 although age of PD onset itself was not a significant correlate of dementia. Additionally, we show a similar dementia-death interval between PDD short- and long-MDI groups. This is in agreement with previous studies that have dissociated the effects of aging from the age of PD onset,44, 58 showing a stereotyped disease progression after the onset of dementia.
We further demonstrate a link between PD and AD by showing a correlation of NFT severity with increasing CLB/LN burden. Other investigators have also found correlations of SPs9, 11, 22, 59, 60 and NFTs9, 22 with CLB/LN burden in PDD. In vivo animal studies, 61 in vitro cross-seeding experiments62–64 and significant co-morbid tau pathology in hereditary PD patients with the A53T SCNA gene mutation65 suggest there are synergistic interactions between tau and α-synuclein that may contribute to a clinicopathological spectrum between PD and AD.
In summary, our work here provides fresh insight into the complex pathogenesis of dementia in PD, and further emphasizes the importance of CLB, aging, co-morbid AD pathology and genetic susceptibility as pathological substrates of cognitive impairment and dementia in PD. Further research will be necessary to clarify the relative contribution of each of these strands before effective treatment can emerge.
Supplementary Material
Acknowledgements
This study was supported by NIH grant: AG05136, and Morris K Udall Center for PD Research Core grants P50 NS053488 and NS062684. DJI is supported by T32-AG000255 Training in Age-Related Neurodegenerative Diseases. JBT is supported by a grant of the Alfonso Martín Escudero Foundation.
References
- 1.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Archives of neurology. 2003 Mar;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal E, Brennan L, Xie S, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010 Jul 15;25(9):1170–1176. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008 Mar 25;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 4.Lo RY, Tanner CM, Albers KB, et al. Clinical features in early Parkinson disease and survival. Archives of neurology. 2009 Nov;66(11):1353–1358. doi: 10.1001/archneurol.2009.221. [DOI] [PubMed] [Google Scholar]
- 5.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007 Sep 15;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 837. [DOI] [PubMed] [Google Scholar]
- 6.Galvin JE. Cognitive change in Parkinson disease. Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4):302–310. doi: 10.1097/01.wad.0000213858.27731.f8. [DOI] [PubMed] [Google Scholar]
- 7.Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007 Mar 13;68(11):812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 8.Hurtig HI, Trojanowski JQ, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000 May 23;54(10):1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 9.Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Archives of neurology. 2002 Jan;59(1):102–112. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- 10.Kovari E, Gold G, Herrmann FR, et al. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathol. 2003 Jul;106(1):83–88. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- 11.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol. 2000 Sep;100(3):285–290. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- 12.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol. 2005 Nov;58(5):773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 13.Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001 Oct;102(4):355–363. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003 Mar-Apr;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 15.Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005 Jan;57(1):82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 16.Colosimo C, Hughes AJ, Kilford L, Lees AJ. Lewy body cortical involvement may not always predict dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2003 Jul;74(7):852–856. doi: 10.1136/jnnp.74.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xuereb JH, Tomlinson BE, Irving D, Perry RH, Blessed G, Perry EK. Cortical and subcortical pathology in Parkinson's disease: relationship to parkinsonian dementia. Adv Neurol. 1990;53:35–40. [PubMed] [Google Scholar]
- 18.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006 Nov 14;67(9):1605–1611. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- 19.Sabbagh MN, Adler CH, Lahti TJ, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009 Jul-Sep;23(3):295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J Neural Transm. 2002 Mar;109(3):329–339. doi: 10.1007/s007020200027. [DOI] [PubMed] [Google Scholar]
- 21.Boller F, Mizutani T, Roessmann U, Gambetti P. Parkinson disease, dementia, and Alzheimer disease: clinicopathological correlations. Ann Neurol. 1980 Apr;7(4):329–335. doi: 10.1002/ana.410070408. [DOI] [PubMed] [Google Scholar]
- 22.Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain : a journal of neurology. 2011 May;134(Pt 5):1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weintraub D, Dietz N, Duda JE, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain : a journal of neurology. 2011 Nov 21; doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010 Sep 21;75(12):1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perl DP, Olanow CW, Calne D. Alzheimer's disease and Parkinson's disease: distinct entities or extremes of a spectrum of neurodegeneration? Ann Neurol. 1998 Sep;44(3 Suppl 1):S19–S31. doi: 10.1002/ana.410440705. [DOI] [PubMed] [Google Scholar]
- 26.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson's disease. Adv Neurol. 1990;53:245–249. [PubMed] [Google Scholar]
- 27.Association AP, editor. Diagnostic and statistical manual of mental disorders IV ed. Washington, DC: 2000. [Google Scholar]
- 28.Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003 Oct 31;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 29.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006 Jun;59(6):952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994 Dec 15;39(6):669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- 31.Mercken M, Vandermeeren M, Lubke U, et al. Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol. 1992;84(3):265–272. doi: 10.1007/BF00227819. [DOI] [PubMed] [Google Scholar]
- 32.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002 Aug;52(2):205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 33.Xie SX, Baek Y, Grossman M, et al. Building an integrated neurodegenerative disease database at an academic health center. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 Jul;7(4):e84–e93. doi: 10.1016/j.jalz.2010.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickson DW, Schmidt ML, Lee VM, Zhao ML, Yen SH, Trojanowski JQ. Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol. 1994;87(3):269–276. doi: 10.1007/BF00296742. [DOI] [PubMed] [Google Scholar]
- 35.Amador-Ortiz C, Dickson DW. Neuropathology of hippocampal sclerosis. Handb Clin Neurol. 2008;89:569–572. doi: 10.1016/S0072-9752(07)01253-5. [DOI] [PubMed] [Google Scholar]
- 36.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain : a journal of neurology. 2008 Jun;131(Pt 6):1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 37.Tolnay M, Spillantini MG, Goedert M, Ulrich J, Langui D, Probst A. Argyrophilic grain disease: widespread hyperphosphorylation of tau protein in limbic neurons. Acta Neuropathol. 1997 May;93(5):477–484. doi: 10.1007/s004010050642. [DOI] [PubMed] [Google Scholar]
- 38.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012 Jan;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 40.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 41.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996 Nov;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 42.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997 Jul-Aug;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 43.RDC T. A language and Environment for Statistical Computing R Foundation for Statistical Computing. 2011 [serial on the Internet]: Available from: http://www.R-Project.org.
- 44.Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain : a journal of neurology. 2010 Jun;133(Pt 6):1755–1762. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- 45.Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006 Dec 12;67(11):1931–1934. doi: 10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- 46.Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006 Sep;112(3):253–260. doi: 10.1007/s00401-006-0088-2. [DOI] [PubMed] [Google Scholar]
- 47.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. Striatal beta-amyloid deposition in Parkinson disease with dementia. J Neuropathol Exp Neurol. 2008 Feb;67(2):155–161. doi: 10.1097/NEN.0b013e31816362aa. [DOI] [PubMed] [Google Scholar]
- 48.Tsuboi Y, Uchikado H, Dickson DW. Neuropathology of Parkinson's disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord. 2007;13(Suppl 3):S221–S224. doi: 10.1016/S1353-8020(08)70005-1. [DOI] [PubMed] [Google Scholar]
- 49.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA : the journal of the American Medical Association. 1997 Oct 22–29;278(16):1349–1356. [PubMed] [Google Scholar]
- 50.Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson's disease. J Neurol. 2009 Mar;256(3):493–498. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- 51.Huang X, Chen P, Kaufer DI, Troster AI, Poole C. Apolipoprotein E and dementia in Parkinson disease: a meta-analysis. Arch Neurol. 2006 Feb;63(2):189–193. doi: 10.1001/archneur.63.2.189. [DOI] [PubMed] [Google Scholar]
- 52.Koob AO, Paulino AD, Masliah E. GFAP reactivity, apolipoprotein E redistribution and cholesterol reduction in human astrocytes treated with alpha-synuclein. Neuroscience letters. 2010 Jan 18;469(1):11–14. doi: 10.1016/j.neulet.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallardo G, Schluter OM, Sudhof TC. A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nature neuroscience. 2008 Mar;11(3):301–308. doi: 10.1038/nn2058. [DOI] [PubMed] [Google Scholar]
- 54.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008 Apr;115(4):427–436. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- 55.Ghebremedhin E, Rosenberger A, Rub U, et al. Inverse relationship between cerebrovascular lesions and severity of lewy body pathology in patients with lewy body diseases. J Neuropathol Exp Neurol. 2010 May;69(5):442–448. doi: 10.1097/NEN.0b013e3181d88e63. [DOI] [PubMed] [Google Scholar]
- 56.Slooter AJ, Tang MX, van Duijn CM, et al. Apolipoprotein E epsilon4 and the risk of dementia with stroke. A population-based investigation. JAMA : the journal of the American Medical Association. 1997 Mar 12;277(10):818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- 57.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol. 2008 Apr;115(4):409–415. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- 58.Aarsland D, Kvaloy JT, Andersen K, et al. The effect of age of onset of PD on risk of dementia. J Neurol. 2007 Jan;254(1):38–45. doi: 10.1007/s00415-006-0234-8. [DOI] [PubMed] [Google Scholar]
- 59.Lashley T, Holton JL, Gray E, et al. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol. 2008 Apr;115(4):417–425. doi: 10.1007/s00401-007-0336-0. [DOI] [PubMed] [Google Scholar]
- 60.Pletnikova O, West N, Lee MK, et al. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005 Aug-Sep;26(8):1183–1192. doi: 10.1016/j.neurobiolaging.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010 May 26;30(21):7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee VM, Giasson BI, Trojanowski JQ. More than just two peas in a pod: common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases. Trends Neurosci. 2004 Mar;27(3):129–134. doi: 10.1016/j.tins.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003 Apr 25;300(5619):636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 64.Waxman EA, Giasson BI. Induction of intracellular tau aggregation is promoted by alpha-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J Neurosci. 2011 May 25;31(21):7604–7618. doi: 10.1523/JNEUROSCI.0297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duda JE, Giasson BI, Mabon ME, et al. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002 Jul;104(1):7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.