Abstract
Statins have anti-inflammatory and immunomodulatory properties in addition to lipid-lowering effects. The present study evaluated the effect of atorvastatin added to interferon beta-1b in multiple sclerosis (MS) in a multicenter, randomized, parallel-group, rater-blinded study performed in eight Swiss hospitals. Seventy-seven patients with relapsing-remitting MS started interferon beta-1b every other day. After 3 months, they were randomized 1:1 to receive atorvastatin 40 mg/day or not in addition to interferon beta-1b until month 15. The primary endpoint was the proportion of patients with new lesions on T2-weighted images at month 15 compared to baseline at month three. At study end, the proportion of patients with new lesions on T2-weighted images was equal in both groups (odds ratio 1.14; 95 % CI 0.36–3.56; p = 0.81). All predefined secondary endpoints including number of new lesions and total lesion volume on T2-weighted images, total number of new Gd-enhancing lesions on T1-weighted images, total brain volume, volume of grey matter, volume of white matter, EDSS, MSFC, relapse rate, time to first relapse, number of relapse-free patients and neutralizing antibodies did not show any significant differences (all p values >0.1). Transient elevations of liver enzymes were more frequent with atorvastatin (p = 0.02). In conclusion, atorvastatin 40 mg/day in addition to interferon beta-1b did not have a beneficial effect on relapsing-remitting MS compared to interferon beta-1b monotherapy over a 12-month period.
Electronic supplementary material
The online version of this article (doi:10.1007/s00415-012-6513-7) contains supplementary material, which is available to authorized users.
Keywords: Multiple sclerosis, Atorvastatin, Interferon beta, Randomized clinical trial
Introduction
Statins are lipid-lowering drugs inhibiting the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA-) reductase, the main regulatory enzyme of cholesterol biosynthesis. In addition, statins have anti-inflammatory and immunomodulatory properties independent of their cholesterol-lowering effects [1].
Multiple sclerosis (MS) is a chronic inflammatory disorder of the central nervous system involving autoimmune mechanisms [2]. Some years ago, interest into statins for treatment of MS arose. Statins improve the course of experimental allergic encephalomyelitis (EAE), an animal model of MS [3–6]. Other experimental studies suggest a negative impact of statins on oligodendrocytes and myelin formation with impaired remyelination [7, 8]. Several clinical studies of different statins in different dosages given alone or in combination with interferon beta (IFNB) for relapsing-remitting MS (RRMS) yielded beneficial, harmful, or no effects as summarized in Table 1, whereas the largest trial of simvastatin as add-on therapy to interferon beta-1a (SIMCOMBIN) showed no beneficial effect [9–15]. However, to date, it is not clear whether statins have a class effect in MS or other statins in addition to disease modifying drugs might be beneficial or even harmful.
Table 1.
Overview of clinical studies evaluating the combination of IFNB and statins in RRMS
| Study type | Patients | Allocation | IFNB | Statin | Primary endpoint/results | |
|---|---|---|---|---|---|---|
| Paul et al. [10] | Open-label baseline-to-treatment trial | RRMS (n = 41) |
IFNB + statin (n = 16) Statin (n = 25) |
IFNB-1a 22 μg t.i.w. or IFNB-1b e.o.d. | Atorvastatin 80 mg/day | Trend towards reduction of Gd-enhancing lesions with IFNB + atorvastatin (p = 0.060) |
| Birnbaum et al. [12] | Double-blind, placebo-controlled trial | RRMS (n = 26) |
IFNB + placebo (n = 9) IFNB + statin (n = 17) |
IFNB-1a 44 μg t.i.w. | Atorvastatin 80 mg/day (n = 10) or 40 mg (n = 7) | Increased MRI and clinical disease activity with atorvastatin (p = 0.019) |
| Rudick et al. [13] | Post hoc analysis | RRMS (n = 582) |
IFNB (n = 542) IFNB + statin (n = 40) |
IFNB-1a 30 μg once weekly | Atorvastatin or simvastatin | No difference in annualized relapse rate and secondary endpoints |
| Lanzillo et al. [14] | Longitudinal controlled trial | RRMS (n = 45) |
IFNB (n = 24) IFNB + statin (n = 21) |
IFNB-1a 44 μg s.c. t.i.w. | Atorvastatin 20 mg/day | Fewer Gd-enhancing lesions versus baseline (p = 0.007) and fewer relapses versus the two pre-randomization years (p < 0.001) with atorvastatin |
| Togha et al. [11] | Double-blind, randomized controlled trial | RRMS (n = 80) |
IFNB + placebo (n = 38) IFNB + simvastatin (n = 42) |
IFNB-1a 30 μg once weekly | Simvastatin 40 mg/day | Lower relapse rate with simvastatin (p = 0.01) |
| Sörensen et al. [15] | Placebo-controlled randomized trial | RRMS (n = 307) |
IFNB + statin (n = 151) IFNB + placebo (n = 156) |
IFNB-1a 30 μg once weekly | Simvastatin 80 mg/day | No difference in annualized relapse rate and secondary endpoints |
| SWABIMS | Randomized controlled trial | RRMS (n = 76) |
IFNB + statin (n = 38) IFNB (n = 38) |
IFNB-1b e.o.d. | Atorvastatin 40 mg/day | No difference of patients with new T2-lesions and in secondary endpoints |
n number, IFNB interferon beta, t.i.w. three times per week, e.o.d. every other day
In the SWiss Atorvastatin and Interferon Beta-1b trial in MS (SWABIMS) we evaluated the efficacy, safety, and tolerability of atorvastatin 40 mg per os (p.o.) daily and subcutaneous (s.c.) interferon beta-1b (IFNB-1b) every other day (e.o.d) compared to monotherapy with s.c. IFNB-1b e.o.d., an established therapy for RRMS [16].
Materials and methods
Study design
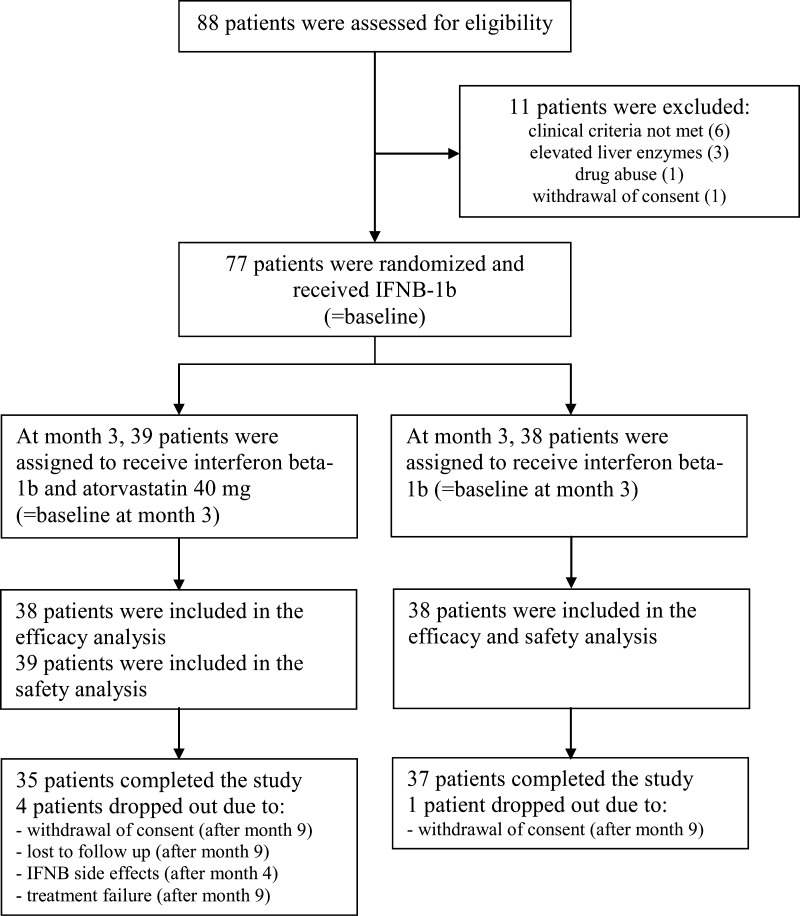
SWABIMS was a multi-center, randomized, parallel-group, rater-blinded study in eight Swiss hospitals [17]. At the beginning of the study (termed “baseline”), all patients started IFNB-1b (Betaferon®/Betaseron®, Bayer Schering Pharma) for 3 months (termed “monotherapy phase”). At month three (termed “baseline at month three”), they were randomized 1:1 to receive atorvastatin 40 mg/day or not in addition to IFNB-1b for another 12 months (termed “randomized phase”) (Fig. 1).
Fig. 1.
Enrollment, allocation, and follow-up of patients
For the primary endpoint and all clinical and radiological secondary endpoints, data at month 15 were compared to data at baseline at month three before randomization to atorvastatin or not.
Randomization was performed centrally by the clinical research organization (CRO) after baseline visit in four-block size, according to the randomization list (atorvastatin “yes” or “no”) generated with “RANCODE Professional 3.6” [18].
Patients and treating physicians were aware, whether atorvastatin was added. Placebo was not dispensed. Examining physicians scoring disability [Expanded Disability Status Scale (EDSS); Multiple Sclerosis Functional Composite (MSFC)] and neuroradiologists evaluating magnetic resonance images (MR) were blinded to treatment assignments [19, 20].
Atorvastatin was chosen because of its potent anti-inflammatory and immunomodulatory properties and favorable safety and pharmacokinetic profile [21–23]. Statins may cause dose-dependent elevation of hepatic enzymes [24]. Therefore, the use of atorvastatin 40 mg/day in combination with the potentially also hepatotoxic IFNB-1b was reasonable, especially since the optimal immunomodulatory dose of statins in MS is unknown.
Each patient had to provide written informed consent prior to study entry. The study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice (1996) and the Declaration of Helsinki (2006), and was approved by the local ethics committees and Swissmedic [25, 26]. The trial Registration Identifier is 2005DR2119 (Swissmedic) and NCT00942591 (clinicaltrials.gov).
Patients
Patients with RRMS according to the 2005 McDonald’s criteria and disease duration >3 months, at least one relapse in the past 2 years, ≥ three lesions on spinal or brain-MR or both, baseline EDSS score from 0 to 3.5 (inclusive), and age from 18 to 55 years were enrolled.
Main exclusion criteria were primary or secondary progressive MS, clinically isolated syndrome (CIS), previous therapy with monoclonal antibodies, mitoxantrone, other cytotoxic or immunosuppressive drugs, and IFNB or glatiramer acetate within the last 12 months.
Study endpoints
The primary endpoint was the proportion of patients with new lesions on T2-weighted MR images at month 15 compared to baseline at month three.
Secondary endpoints were the number of new lesions on T2-weighted images, change in total lesion volume on T2-weighted images (burden of disease), total number of new gadolinium (Gd-)-enhancing lesions on T1-weighted images, changes in total brain volume, volume of grey matter and volume of white matter, clinical disease progression (EDSS, MSFC), relapse rate, time to first relapse, number of relapse-free patients, and neutralizing antibodies (NAbs).
Adverse events (AE), laboratory data, vital signs and concomitant medication were analyzed as safety variables.
Study procedures
IFNB-1b was started at a dose of 0.0625 mg e.o.d. and then increased weekly by 0.0625 to 0.25 mg e.o.d for the baseline phase of 3 months.
At month three, atorvastatin 40 mg/day was given to patients randomized to the atorvastatin/IFNB-1b group for 12 months. The other patients continued with IFNB-1b monotherapy for the whole study period.
Regular visits were performed at month one, three, four, six, nine, 12, and 15 for the assessment of EDSS, MSFC, NAbs, laboratory tests, MR, and efficacy and safety endpoints. Atorvastatin use was controlled by counting the returned tablets at visits at months six, nine, and 15. A patient was considered as compliant when he took at least 80 % of all atorvastatin tablets.
A relapse was defined as a newly appearing objective neurological abnormality in the absence of fever or known infection, lasting for at least 24 h and occurring at least 30 days after a preceding clinical event, correlating with the patient’s reported symptoms and increasing the total EDSS score or at least one of the functional systems of the EDSS score. Fatigue, mental, and/or vegetative symptoms were not classified as relapse.
Relapses were treated within 7 days with intravenous methylprednisolone 500 mg/day for 5 days followed by tapering-out with oral prednisolone.
MR scans were acquired on 1.5-Tesla scanners at screening, months three, nine, and 15. The MR protocol included T1-weighted axial spin-echo, T1-weighted sagittal 3D MPRAGE, axial dual-echo, i.e., proton-density, T2-weighted turbo-spin-echo images and axial T1-weighted spin-echo images after intravenous Gd injection.
MR scans were assessed centrally by two neuroradiologists at the Institute of Diagnostic and Interventional Neuroradiology of the University of Bern [27, 28]. A T2 lesion was defined as an area of increased signal on both the proton-density and the T2-weighted images. Disagreeing interpretations were discussed among the neuroradiologists to reach consensus. The image processing was performed with an algorithm enabling semi-automatic volumetry [29].
Laboratory analyses except NAbs were performed by Viollier AG. Atorvastatin was reduced to 20 mg/day in case of a more than threefold increase and stopped in case of more than fivefold increase of transaminases. Afterwards liver enzymes were controlled regularly and atorvastatin was continued when transaminases were below a threefold increase.
NAbs were assessed at the Ospedale San Luigi, Orbassano, Italy. The cytopathic effect assay was used as recommended by the World Health Organization [30]. Data from the neutralization assay were reported as reciprocal of the highest dilution of serum inducing 50 % neutralization. The neutralization titer was calculated according to Kawade’s formula and expressed in laboratory units (LU). A concentration of >20 LU/ml was considered positive [31]. Patients with one or more NAb-positive titers were defined NAb-positive. Two centers did not collect NAbs, explaining the lower numbers of individuals for this analysis.
Statistical analysis
SAS version 9.2 was used for all statistical analyses. To obtain a power of 84 % to detect the difference between the monotherapy group proportion, π1, of 0.610 and the combination therapy group proportion, π2, of 0.910 with a 0.05 two-sided significance level in the Fisher’s exact test, a sample size of 38 patients in each group was needed [32]. All patients who took at least one dose of study medication and had at least one follow-up observation were analyzed [Full Analysis Set (FAS)]. Missing data because of drop-outs on the primary endpoint were replaced with MR data from the last available examination, which was month nine in all drop-outs. The same approach was used for other efficacy endpoints. Missing values for other parameters were treated as missing, except for severity and relationship of AEs to study drugs, which was regarded as severe and related to study drug.
Categorical data were described by frequency and percentage, continuous data by mean, standard deviation, minimum, 1st quartile, median, 3rd quartile, and maximum. Hypothesis tests were carried out with a α-level of 0.05, two-sided. All inferential analyses were presented by p values, point estimations and two-sided 95 % CI for treatment differences. If the assumption of normality in the linear models was not fulfilled, transformations of the data or non-parametric approaches like the Wilcoxon signed-rank test were used.
Differences between treatment groups at baseline were tested using t test or Fisher’s exact test depending on the distribution of the data.
The primary efficacy variable was the proportion of patients with new T2 lesions at month 15 compared to baseline at month three. Based on a logistic regression model with the factors treatment and gender and the covariates number of T2 lesions, number of Gd-enhancing lesions, EDSS, relapse rate and time since MS diagnosis at baseline at month three, the two-sided hypothesis of equality between the two treatments was tested at an α-level of 0.05. The results were presented as odds ratios and the associated two-sided 95 % CI and p values. Furthermore, a Fisher’s exact test for proportions was executed to test for the unadjusted treatment effect.
Secondary efficacy variables were analyzed with covariance, logistic regression models, or Fisher’s exact test depending on the distribution. Time to first relapse was analyzed with non-parametric methods for failure time data (Wilcoxon test) and illustrated by a Kaplan–Meier plot.
Assessments of safety and tolerability variables were presented by treatment group. AEs were summarized for each treatment group by presenting the number and percentage of subjects having an event, the number and percentage of event in each system organ class and preferred term, as well as severity and relationship to the study drug.
Any medication taken during the study was classified as concomitant and coded using WHO-Drug 2007.1.
Results
The recruitment period was from May 2005 to December 2008, in which 88 patients were screened. Seventy-seven patients fulfilled the study criteria and were included and all of them were randomized at baseline at month three. None of them had previous immunomodulatory or immunosuppressive therapy. Five patients dropped out, four in the atorvastatin/IFNB-1b and one in the IFNB-1b group (Fig. 1). The EDSS score of one patient at screening was too high as we realized only in retrospect. This patient was excluded from the efficacy analysis (76 patients) but remained in the safety analysis (77 patients). The atorvastatin compliance was >80 % in the randomized phase. All relapses were treated with steroids as defined above.
Demographic and baseline characteristics are presented in Table 2. Patients of the atorvastatin/IFNB-1b group were younger than patients of the IFNB-1b group. Gender, ethnic origin, height, weight, and BMI were well matched.
Table 2.
Patient characteristics
| Characteristics | Atorvastatin/interferon beta-1b | Interferon beta-1b | p value |
|---|---|---|---|
| n = 38 | n = 38 | ||
| Demographic characteristics at screening/baseline | |||
| Age (years) | |||
| Mean ± SD | 30.5 ± 7.9 | 35.7 ± 7.95 | 0.0032 |
| Median (range) | 28 (19 to 50) | 36 (18 to 49) | |
| Gender (n, %) | |||
| Male | 17 (44.7 %) | 15 (39.5 %) | |
| Female | 21 (55.3 %) | 23 (60.5 %) | 0.82 |
| Caucasian (n, %) | 38 (100 %) | 38 (100 %) | |
| Height (cm) | |||
| Mean ± SD | 171.8 ± 8.30 | 170.6 ± 9.75 | |
| Median (range) | 170 (156 to 191) | 166.5 (157 to 192) | 0.34 |
| Weight (kg) | |||
| Mean ± SD | 71.42 ± 14.98 | 72.48 ± 18.18 | |
| Median (range) | 70 (43 to 106) | 69 (44.5 to 124) | 0.91 |
| BMI (kg/m2) | |||
| Mean ± SD | 24.15 ± 4.61 | 24.74 ± 5.3 | |
| Median (range) | 23.39 (16.4 to 34.6) | 23.07 (17.2 to 45.5) | 0.70 |
| MR findings at baseline at month 3 | |||
| No. of T2 hyperintense lesions | |||
| n | 36 | 37 | |
| Mean ± SD | 27.3 ± 24.24 | 21.2 ± 19.24 | |
| Median (range) | 21 (1 to 113) | 14 (2 to 80) | 0.19 |
| Total volume of T2 hyperintense lesions (cm3) | |||
| n | 36 | 37 | |
| Mean ± SD | 3.5 ± 3.24 | 2.7 ± 2.68 | |
| Median (range) | 2.96 (0.3 to 12.4) | 1.69 (0.1 to 9.6) | 0.22 |
| No. of GD-enhancing lesions on T1-weighted images | |||
| n | 37 | 38 | |
| Mean ± SD | 1.1 ± 2.9 | 0.3 ± 0.64 | |
| Median (range) | 0 (0 to 17) | 0 (0 to 2) | 0.08 |
| Total volume of GD-enhancing lesions on T1-weighted images (cm3) | |||
| n | 37 | 38 | |
| Mean ± SD | 0.09 ± 0.21 | 0.02 ± 0.07 | |
| Median (range) | 0 (0 to 1.1) | 0 (0 to 0.4) | 0.043 |
| Total brain volume (cm3) | |||
| n | 28 | 31 | |
| Mean ± SD | 1,476.8 ± 161.9 | 1,418.6 ± 151.97 | |
| Median (range) | 1,431.6 (1,209 to 1,898) | 1,411.4 (1,129 to 1,782) | 0.16 |
| Volume of grey matter (cm3) | |||
| n | 28 | 31 | |
| Mean ± SD | 734.6 ± 68.09 | 708.7 ± 73.29 | |
| Median (range) | 728.97 (620 to 867) | 694 (587 to 880) | 0.14 |
| Volume of white matter (cm3) | |||
| n | 28 | 31 | |
| Mean ± SD | 434.5 ± 46.29 | 416.3 ± 65.2 | |
| Median (range) | 428.5 (366 to 573) | 427.1 (270 to 544) | 0.21 |
| Clinical characteristics | |||
| MS duration at screening (years) | |||
| Mean ± SD | 0.88 ± 2.86 | 0.86 ± 1.46 | 0.20 |
| No. of relapses in the past 2 years before screening (n, %) | |||
| n | 38 | 38 | |
| 1 | 12 (31.6 %) | 11 (28.9 %) | |
| 2 | 19 (50.0 %) | 24 (63.2 %) | |
| 3 | 3 (7.9 %) | 3 (7.9 %) | |
| 4 | 3 (7.9 %) | 0 (0 %) | |
| 8 | 1 (2.6 %) | 0 (0 %) | 0.38 |
| EDSS at baseline at month 3 | |||
| n | 38 | 38 | |
| Mean ± SD | 1.8 ± 1.0 | 1.9 ± 1.1 | |
| Median (range) | 2 (0 to 3.5) | 2 (0 to 4) | 0.37 |
| MSFC at baseline at month 3 | |||
| n | 38 | 38 | |
| Mean ± SD | 0.26 ± 0.49 | 0.18 ± 0.46 | |
| Median (range) | 0.38 (−1.2 to 1.0) | 0.18 (−0.9 to 1.0) | 0.26 |
n number of patients, SD standard deviation, EDSS Expanded Disability Status Scale: MSFC Multiple Sclerosis Functional Composite, BMI body mass index: ns no significant difference
Mean duration since diagnosis of MS, relapse rate within the past 2 years, number and volume of lesions on T2-weighted images, number and volume of Gd-enhancing lesions on T1-weighted images, brain volume and EDSS and MSFC scores at baseline did not differ significantly. During the monotherapy phase, both groups developed equally regarding all endpoints with no statistically significant differences. At baseline at month three, there was a trend towards a higher disease activity of the atorvastatin/IFNB-1b group caused by the distribution at baseline and the decline of the arithmetic average, median, and variability.
The results for the primary and secondary efficacy variables are given in Table 3. The proportion of patients with new lesions on T2-weighted images at month 15 compared to baseline at month three was not different according to the logistic regression model (p = 0.81). The adjusted odds ratio (OR) and the 95 % CI for the treatment difference of atorvastatin/IFNB-1b versus IFNB-1b were 1.14 and 0.36–3.56. To test the unadjusted treatment differences, an exploratory analysis with Fisher’s exact test was performed. Again, no significant difference was detected (p = 0.64).
Table 3.
Efficacy endpoints (FAS, n = 76)
| Endpoint | Atorvastatin/interferon-beta-1b | Interferon-beta-1b | p value |
|---|---|---|---|
| n = 38 | n = 38 | ||
| MR endpoints | |||
| Proportion of patients with new lesions on T2-weighted images, baseline at month 3 to month 15 (n %) | |||
| n | 37 | 37 | |
| Yes | 18 (47.37) | 15 (39.47) | |
| No | 19 (50.0) | 22 (57.89) | |
| Odds ratio for atorvastatin/IFNB-1b versus IFNB-1b (95 % CI) | 1.14 (0.366 to 3.56) | 0.81 | |
| No. of new lesions on T2-weighted images, baseline at month 3 to month 15 | |||
| n | 36 | 37 | |
| Mean ± SD | 3.3 ± 6.81 | 1.7 ± 4.05 | |
| Median (range) | 0 (0 to 36) | 0 (0 to 21) | |
| Treatment difference for atorvastatin /IFNB-1b vs. IFNB-1b (95 % CI) | −0.45 (−2.12 to 1.22) | 0.59 | |
| Change in lesion volume (cm3) on T2-weighted images, baseline at month 3 to month 15 | |||
| n | 36 | 37 | |
| Mean ± SD | 0.4 ± 2.65 | 0.2 ± 1.26 | |
| Median (range) | 0 (−4 to 12) | 0 (−1 to 5) | |
| Treatment difference for atorvastatin /IFNB-1b vs. IFNB-1b (95 % CI) | −0.50 (−1.21 to 0.19) | 0.15 | |
| Total number of Gd-enhancing T1 lesions at month 9 and 15 | |||
| n | 37 | 38 | |
| Mean ± SD | 3.9 ± 12.89 | 2.2 ± 5.41 | |
| Median | 0 (0 to 65) | 0 (0 to 25) | |
| Treatment difference for atorvastatin /IFNB-1b vs. IFNB-1b (95 % CI) | −1.76 (−4.78 to 0.96) | 0.20 | |
| Change of total brain volume (cm³), baseline at month 3 to month 15 | |||
| n | 27 | 31 | |
| Mean ± SD | −13.7 ± 59.32 | −4.9 ± 33.7 | |
| Median (range) | −3.7 (−295 to 36) | −2.7 (−108 to 115) | 0.91 |
| Change of grey matter volume (cm3), baseline at month 3 to month 15 | |||
| n | 27 | 31 | |
| Mean ± SD | −4.0 ± 18.2 | −5.8 ± 41.95 | |
| Median (range) | −0.5 (−58 to 31) | −1.5 (−185 to 101) | 0.21 |
| Change of white matter volume (cm3), baseline at month 3 to month 15 | |||
| n | 27 | 31 | |
| Mean ± SD | 0.9 ± 12.35 | 2.5 ± 39.24 | |
| Median (range) | 1.5 (−28 to 26) | −0.7 (−141 to 126) | 0.78 |
| Clinical endpoints | |||
| Change in EDSS score, baseline at month 3 to month 15 | |||
| n | 37 | 38 | |
| Mean ± SD | 0.03 ± 0.90 | 0.17 ± 0.5 | |
| Median (range) | 0 (−2 to 2) | 0 (−2 to 2) | |
| Least squares means for effect treatment (95 % CI) | −0.11 (−0.54 to 0.32) | 0.61 | |
| Change in MSFC score, baseline at month 3 to month 15 | |||
| n | 37 | 38 | |
| Mean ± SD | 0.1 ± 0.27 | 0.1 ± 0.32 | |
| Median (range) | 0.1 (0–1) | 0.1 (−1 to 1) | |
| Least squares means for effect treatment (95 % CI) | −0.08 (−0.22 to 0.06) | 0.24 | |
| Relapse, baseline at month 3 to month 15 | |||
| n | 38 | 38 | |
| Relapse-free (n, %) | |||
| No | 18 (43.4 %) | 13 (34.2 %) | |
| Yes | 20 (52.6 %) | 25 (65.8 %) | |
| Odds ratio of atorvastatin/IFNB-1b versus IFNB-1b (95 % CI) | 0.65 (0.22 to 1.90) | 0.43 | |
| No. of relapses | |||
| Total number | 28 | 23 | |
| Mean ± SD | 0.7 ± 0.98 | 0.6 ± 1.05 | |
| Median (range) | 0 (0 to 4) | 0 (0 to 4) | 0.63 |
| Time to first relapse (25 % quartiles estimates) | |||
| Mean ± SD | 220.3 ± 18.08 | 284 ± 18.73 | 0.16 |
| Neutralizing antibodies (NAb) | |||
| NAb-positive (n, %) | |||
| n | 29 | 31 | |
| No | 13 (44.8 %) | 20 (64.5 %) | |
| Yes | 16 (55.2 %) | 11 (35.5 %) | 0.12 |
| Change from NAb-positive to NAb-negative | |||
| n | 29 | 31 | |
| No | 14/16 (87.5 %) | 6/11 (54.5 %) | |
| Yes | 2/16 (12.5 %) | 5/11 (45.5 %) | 0.22 |
Treatment differences were calculated using ANCOVA
n number of patients with data, SD standard deviation, EDSS Expanded Disability Status Scale, MSFC Multiple Sclerosis Functional Composite
The predefined secondary endpoints number of new lesions and total lesion volume on T2-weighted images, total number of Gd-enhancing lesions on T1-weighted images, total brain volume, volume of grey matter, volume of white matter, EDSS, MSFC (including subscores), relapse rate, and number of relapse-free patients did not show any significant differences between the treatment groups at month 15 (all p values >0.1). In individual patients, data on study endpoints were missing because of a variety of reasons, e.g., movement artifacts during single MR sequences or incomplete data collection at visits. Two centers did not provide adequate MRI data for the analysis of total brain volume and grey and white matter volumes. This explains the lower numbers of individuals in some endpoints.
The logistic regression model regarding the primary endpoint with new T2 lesions as dependent variable and treatment, number of T2 lesions, number of Gd-enhancing T1 lesions, volume of Gd-enhancing T1 lesions, relapse rate, EDSS, time since MS diagnosis, age and gender at baseline as influencing variables showed that age (p = 0.04), number of Gd-enhancing T1 lesions (p = 0.02) and number of T2 lesions (p = 0.01) at baseline had a significant influence on the number of new T2 lesions whereas treatment did not (p = 0.72). Furthermore, age had a significant influence on the dependent variables of relapse rate, total brain volume, and volume of white matter whereas treatment did not.
NAb were evaluated in 60 of 77 patients (29 in the atorvastatin/IFNB-1b group; 31 in the IFNB-1b group). Sixteen patients turned NAb-positive in the atorvastatin/IFNB-1b group and 11 patients in the IFNB-1b group (p = 0.12). Neither the time of occurrence of NAb nor the titers differed between the groups. Five of 11 patients in the IFNB-1b group and two of 16 patients in the atorvastatin/IFNB-1b group turned from NAb-positive to NAb-negative during the study (p = 0.22).
The time to first relapse failed to prove significance in the Wilcoxon test as well (p = 0.16). The median (50 % quartile) time to first relapse could be calculated for the atorvastatin/IFNB-1b group, but because of an insufficient number of relapses not for the IFNB-1b group. The 25 % quartiles (atorvastatin/IFNB-1b group 100 days; IFNB-1b group 220 days) showed a non-significant shorter time to the next relapse in the atorvastatin/IFNB-1b group.
The Cox regression model with the time to first relapse as dependent variable and treatment, gender, number of T2 lesions, number of Gd-enhancing lesions, EDSS, relapse rate, time since diagnosis, age and volume of T1 lesions as influencing variables showed that age (p = 0.04) had a significant influence on the time to first relapse whereas treatment did not (p = 0.33).
Details on AEs by system organ class are given in Table 4. During the monotherapy and randomized phases, any AEs including serious and severe AEs occurred equally in both groups. During the randomized phase, AEs were more frequently related to the study drug in the atorvastatin/IFNB-1b group.
Table 4.
Adverse events by system organ class MedDRA (FAS, n = 77)
| Events n (%) | Atorvastatin/ Interferon-beta-1b | Interferon-beta-1b | p value |
|---|---|---|---|
| (n = 39) | (n = 38) | ||
| Total number of adverse events | 101 | 89 | |
| Adverse events (AE) by number of subjects | |||
| Overall adverse event | 36 (92.3 %) | 27 (71.1 %) | ns |
| Monotherapy phase | |||
| Any AE | 25 (64.1 %) | 21 (55.3 %) | ns |
| Any serious AE | 0 (0 %) | 0 (0 %) | ns |
| Any severe AE | 0 (0 %) | 2 (5.3 %) | ns |
| Any AE related to study drug | 17 (43.6 %) | 17 (44.7 %) | ns |
| Any AE leading to discontinuation of study drug | 0 (0 %) | 2 (5.3 %) | ns |
| Randomized phase | |||
| Any AE | 31 (79.5 %) | 24 (63.2 %) | ns |
| Any serious AE | 0 (0 %) | 1 (2.6 %) | ns |
| Any severe AE | 1 (2.6 %) | 2 (5.3 %) | ns |
| Any AE related to study drug | 22 (56.4 %) | 12 (31.6 %) | 0.02 |
| Any AE leading to discontinuation of study drug | 0 (0 %) | 1 (2.6 %) | ns |
| Most frequently (>5 %) reported AE during the randomized phase by number of subjects | |||
| Eye disorders | |||
| Glaucoma | 0 (0 %) | 2 (5.3 %) | ns |
| Gastrointestinal disorders | |||
| Diarrhea | 2 (5.1 %) | 2 (5.3 %) | ns |
| Nausea | 3 (7.7 %) | 1 (2.6 %) | ns |
| General disorders/administration site conditions | |||
| Fatigue | 2 (5.1 %) | 4 (10.5 %) | ns |
| Influenza-like illness | 4 (10.3 %) | 5 (13.2 %) | ns |
| Pyrexia | 0 (0 %) | 2 (5.3 %) | ns |
| Infections and infestations | |||
| Influenza | 3 (7.7 %) | 3 (7.9 %) | ns |
| Nasopharyngitis | 5 (12.8 %) | 2 (5.3 %) | ns |
| Injury, poisoning, and procedural complications | |||
| Joint injury | 2 (5.1 %) | 0 (0 %) | ns |
| Abnormal laboratory values | |||
| Elevated liver enzymes | 9 (23.1 %) | 2 (5.3 %) | 0.02 |
| Musculoskeletal and connective tissue disorders | |||
| Muscle spasms | 2 (5.1 %) | 4 (10.5 %) | ns |
| Myalgia | 3 (7.7 %) | 0 (0 %) | ns |
| Nervous system disorders | |||
| Headache | 3 (7.7 %) | 4 (10.5 %) | ns |
| Muscular weakness | 0 (0 %) | 2 (5.3 %) | ns |
| Paraesthesia | 1 (2.6 %) | 2 (5.3 %) | ns |
| Psychiatric disorders | |||
| Depression | 2 (5.1 %) | 2 (5.3 %) | ns |
| Renal and urinary disorders | |||
| Bladder disorder | 0 (0 %) | 2 (5.3 %) | ns |
| Respiratory, thoracic and mediastinal disorders | |||
| Epistaxis | 0 (0 %) | 2 (5.3 %) | ns |
| Pharyngolaryngeal pain | 2 (5.1 %) | 1 (2.6 %) | ns |
| Skin and subcutaneous tissue disorders | |||
| Acne | 2 (5.1 %) | 1 (2.6 %) | ns |
| Dry skin | 1 (2.6 %) | 2 (5.3 %) | ns |
| Eczema | 2 (5.1 %) | 0 (0 %) | ns |
AE adverse event, n number, ns no significant difference
In the randomized phase, elevated liver enzymes occurred more often in the atorvastatin/IFNB-1b group (p = 0.02). All other AEs were equally distributed. Because of elevated liver enzymes, atorvastatin was transiently reduced in six patients (mean 3.1 month) and stopped for good in three patients 3.6 month on average before study termination. In the IFNB-1b group, IFNB was stopped temporarily in one patient.
In the atorvastatin/IFNB-1b group, AEs were classified as mild in 16 (41 %), moderate in 14 (35.9 %), and severe in one (2.6 %) subject. The severe AE was an influenza-like illness. There was one serious AE (SAE), a lumbar herniated disk. In the IFNB-1b group, AEs were classified as mild in ten (26.3 %), moderate in 12 (31.6 %), and severe in two (5.3 %) subjects. The severe AEs were dermal herpes zoster and lumbar disk prolapse. Blood lipid levels were similar at baseline at month three. Total and low-density lipoprotein cholesterol decreased significantly (p < 0.0001) in the atorvastatin/IFNB-1b group compared to the IFNB-1b group.
Discussion
Atorvastatin 40 mg added to IFNB-1b did not have any beneficial effect on RRMS compared to IFNB-1b monotherapy over a period of 12 months. There were no significant differences in the primary or secondary endpoints between the two treatment groups.
Patients in the atorvastatin/IFNB-1b group were significantly younger, showed a trend towards higher disease activity at baseline, and had significantly larger volumes of Gd-enhancing lesions on T1-weighted images. A multiple regression analysis showed that this imbalance at baseline, and not the different treatment, was responsible for the trends towards a higher disease activity of the atorvastatin/IFNB-1b group at study end. Therefore, a negative effect of atorvastatin cannot be concluded.
The combination of atorvastatin and IFNB-1b was well tolerated and did not cause unexpected or severe side-effects. However, elevated liver enzymes without clinical symptoms occurred more often in the atorvastatin/IFNB-1b group and led to a temporary reduction or stop of atorvastatin in several patients. It cannot be distinguished whether atorvastatin alone or the combination accounted for the elevated liver enzymes. Liver enzymes normalized and atorvastatin could be continued at full dosage in most patients. However, in three patients, atorvastatin had to be stopped. Other AEs were similar in both groups.
SWABIMS also addressed the question, whether atorvastatin had an impact on NAbs against INFB-1b. There was a trend towards a higher prevalence and longer persistence of NAbs in the atorvastatin/IFNB-1b group that might indicate a negative effect of atorvastatin on NAb formation. However, for the moment, this does not have clinical implications.
The results of SWABIMS suggest that atorvastatin 40 mg added to IFNB-1b has no beneficial effect on RRMS. The results of SWABIMS are similar to the results of the SIMCOMBIN trial, the largest randomized trial that added simvastatin to IFNB-1a in RRMS as well as to a post hoc analysis of the SENTINEL trial. None of the two studies showed any beneficial effect of statins [13, 15]. Therefore, neither atorvastatin nor simvastatin are to be recommended as an add-on therapy to IFNB.
A minimal beneficial or harmful effect of other combinations of statins and IFNB cannot be definitely excluded yet. Other trials have supported positive or negative effects of statins, but this would have to be proved in larger studies (Table 1). A marked effect, however, seems unlikely because of the results of the largest trial (SIMCOMBIN), our SWABIMS study, and the comparable immunomodulatory properties of the different statins in experimental studies [6, 33].
The rationale to combine immunomodulatory drugs with different mechanisms of action is to obtain additive anti-inflammatory effects. This is the case for statins and IFNB-1b in vitro. Both inhibit the proliferation of stimulated peripheral blood mononuclear cells, reduce the expression of activation-induced adhesion molecules on T cells, modify the T helper 1/T helper 2 cytokine balance, reduce matrix metalloproteinases (MMP) -9, and downregulate chemokine receptors on both B and T cells [33]. However, combination therapies may lead to antagonistic effects as well. Besides anti-inflammatory effects, statins also show proinflammatory properties such as interferon-γ production, inhibit STAT1 phosphorylation, which is an important signaling pathway for IFNB, and antagonize the inhibitory effect of IFNB on the proteolytic activity on MMP-2 and MMP-9 [33–35]. The antagonistic mechanisms could potentially explain the negative results of studies combining IFNB and statins.
Multiple sclerosis patients with vascular risk factors and vascular disease have a more rapid disability progression than MS patients without [36, 37]. Therefore, vascular risk factors and diseases should be treated as rigorously as in non-MS patients. Provided that liver enzymes are monitored, SWABIMS suggests that atorvastatin 40 mg can be used for vascular prevention in MS patients who need a lipid-lowering therapy.
There are limitations of the SWABIMS study. It was a multi-center, randomized, parallel-group, rater-blinded trial, but not placebo-controlled. At the time of study planning and initiation, an identical placebo was not available. Therefore, we chose a prospective randomized rater-blinded end-point study design. Nevertheless, the evaluating clinicians and neuroradiologists assessing MR endpoints were blinded. Other limitations are the sample size and that we chose a surrogate marker instead of a clinical endpoint as primary endpoint. However, sample size calculations with the limited data of statins in MS available in 2005 indicated that the patient numbers of SWABIMS could give meaningful results with a primary MR endpoint. Another limitation might be the dose of atorvastatin. In vascular disease, higher doses of atorvastatin are more effective than lower doses. However, the optimal immunomodulatory dosage is unknown and it is not certain that higher doses yield higher efficacy. Therefore, and for safety reasons, we chose a daily dose of 40 mg of atorvastatin.
In conclusion, atorvastatin 40 mg/day in addition to IFNB-1b did not have any beneficial effect on RRMS compared to IFNB-1b monotherapy over a period of 12 months. Therefore, adding atorvastatin 40 mg/day to IFNB-1b seems to be no treatment option for patients with RRMS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from Bayer Schering Pharma (Switzerland) and Pfizer (Switzerland). The sponsor was the Department of Neurology of the University Hospital Bern, Switzerland.
Conflicts of interest
Dr. Kamm has received honoraria for lectures from Biogen-Dompé, Novartis, Bayer, Teva and Pfizer. Dr. Humpert has received honoraria for lectures from Biogen-Dompé, Merck-Serono and Novartis. Dr. Donati has received honoraria for consulting from Merck-Serono, Pfizer and Teva. Dr. Findling, Dr. von Bredow, Ms. Burren, Dr. Schwegler, Dr. Schött, Drs. Müller, Dr. Slotboom, Dr. Naegelin, and Prof. Tettenborn have no competing interests. Prof. Goebels received honoraria for lectures from, served as principal investigator, member of steering committees, or member of advisory boards in clinical trials sponsored by, or has had consulting agreements over the past 5 years with Bayer-Schering, Biogen-Idec, Merck-Serono, Novartis, Sanofi-Aventis, and other companies involved in the development of MS therapeutics, received research support by the Swiss National Research Foundation, the National Center for Competence in Research Neural Plasticity and Repair, Biogen-Idec, Merck-Serono SA Geneva, the Swiss Multiple Sclerosis Society, the 3R Research Foundation Switzerland, and the Koetser Foundation for Brain Research. Prof. Kappos received research support from the Swiss National Research Foundation, the Swiss MS Society, and from the Gianni Rubatto Foundation (Zurich); has served on scientific advisory boards and his Department at the University Hospital Basel has received research support from Acorda Therapeutics Inc., Actelion Pharmaceuticals Ltd, Abbott, AstraZeneca, Bayhill Therapeutics, Bayer Schering Pharma, Biogen Idec, Boehringer Ingelheim, Centocor Ortho Biotech Inc., Eisai Inc., Genzyme Corporation, GlaxoSmithKline, The Immune Response Corporation, MediciNova, Inc., Neurocrine Biosciences, Novartis, Sanofi-Aventis, Merck Serono, Roche, Teva Pharmaceutical Industries Ltd., UCB, and Wyeth. Prof. Mattle has received honoraria for lectures and consulting from Bayer-Schering, Biogen-Dompé, Merck-Serono, TEVA, Sanofi-Aventis, Novartis, and Pfizer.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Appendix
SWABIMS study group
Neurology
Aarau: G Schwegler, M Leichtle, B Kieser; Basel: L Kappos, L Achtnichts, Y Naegelin; Bern: H P Mattle, C P Kamm, B Rieder, S Humpert, C Lienert, O Findling, S Jung, S Kipfer, A Mugglin, J Sellner, S Wolff, I Greeve, V Blatter, I Beiser; Biel: F Donati, M Alfaro, M Braunschweig; Münsterlingen: F Müller, L Schelosky, K-W Stock, M Nuding; Luzern: M Müller, P Stellmes, T Treumann, P Wicki; St. Gallen: B Tettenborn, N Putzki, S Müller, T Hundsberger, D Schött; Zürich: J Waskönig, C Gübelin, S Kollias, N Goebels.
MR core laboratory
University Institute of Diagnostic and Interventional Neuroradiology, Inselspital, Bern University Hospital, and University of Bern, Switzerland: M El-Koussy, Y Burren, J Slotboom, M Zbinden, C Kiefer, F v Bredow, R Wiest, G Schroth.
Clinical research organization
PharmaPart Gmbh (Bahnhofstrasse 20, Postfach 173, 8800 Thalwil, Switzerland) was contracted for data management, data analysis, randomization, visit tracking, drug distribution, case report forms, and coding of adverse events.
Safety monitoring board
B Weinshenker, M Matiello, Rochester MN, USA.
Footnotes
For the SWABIMS Study Group. Members of this group listed in “Appendix”
References
- 1.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis. An overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanislaus R, Pahan K, Singh AK, Singh I. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci Lett. 1999;269:71–74. doi: 10.1016/S0304-3940(99)00414-0. [DOI] [PubMed] [Google Scholar]
- 4.Stanislaus R, Singh AK, Singh I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental allergic encephalomyelitis. J Neurosci Res. 2001;66:155–162. doi: 10.1002/jnr.1207. [DOI] [PubMed] [Google Scholar]
- 5.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 6.Markovic-Plese S, Singh AK, Singh I. Therapeutic potential of statins in multiple sclerosis: immune modulation, neuroprotection and neurorepair. Future Neurol. 2008;3:153–167. doi: 10.2217/14796708.3.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klopfleisch S, Merkler D, Schmitz M, et al. Negative impact of statins on oligodendrocytes and myelin formation in vitro and in vivo. J Neurosci. 2008;28:13609–13614. doi: 10.1523/JNEUROSCI.2765-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miron VE, Zehntner SP, Kuhlmann T, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174:1880–1890. doi: 10.2353/ajpath.2009.080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 10.Paul F, Waiczies S, Wuerfel J, et al. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PLoS ONE. 2008;3:e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togha M, Karvigh SA, Nabavi M, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Mult Scler. 2010;16:848–854. doi: 10.1177/1352458510369147. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology. 2008;71:1390–1395. doi: 10.1212/01.wnl.0000319698.40024.1c. [DOI] [PubMed] [Google Scholar]
- 13.Rudick RA, Pace A, Rani MR, et al. Effect of statins on clinical and molecular responses to intramuscular interferon beta-1a. Neurology. 2009;72:1989–1993. doi: 10.1212/WNL.0b013e3181a92b96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzillo R, Orefice G, Quarantelli M, et al. Atorvastatin combined to interferon to verify the efficacy (ACTIVE) in relapsing-remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Mult Scler. 2010;16:450–454. doi: 10.1177/1352458509358909. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen PS, Lycke J, Erälinna JP, et al. Simvastatin as add-on therapy to interferon beta-1a for relapsing-remitting multiple sclerosis (SIMCOMBIN study): a placebo-controlled randomised phase 4 trial. Lancet Neurol. 2011;10:691–701. doi: 10.1016/S1474-4422(11)70144-2. [DOI] [PubMed] [Google Scholar]
- 16.IFNB Multiple Sclerosis Study Group Interferon beta-lb is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/WNL.43.4.655. [DOI] [PubMed] [Google Scholar]
- 17.Kamm CP, Mattle HP, SWABIMS Study Group SWiss atorvastatin and interferon beta-1b trial in multiple sclerosis (SWABIMS)—rationale, design and methodology. Trials. 2009;14:110–115. doi: 10.1186/1745-6215-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beller EM, Gebski V, Keech AC. Randomisation in clinical trials. Med J Aust. 2002;177:565–567. doi: 10.5694/j.1326-5377.2002.tb04955.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 20.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The multiple sclerosis functional composite measure (MSFC). An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 21.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 22.Arca M. Atorvastatin: a safety and tolerability profile. Drugs. 2007;67(1):63–69. doi: 10.2165/00003495-200767001-00007. [DOI] [PubMed] [Google Scholar]
- 23.Bernini F, Poli A, Paoletti R. Safety of HMG-CoA reductase inhibitors: focus on atorvastatin. Cardiovasc Drugs Ther. 2001;15:211–218. doi: 10.1023/A:1011908004965. [DOI] [PubMed] [Google Scholar]
- 24.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 25.ICH harmonised tripartite guideline—guideline for good clinical practice: E6(R1). Geneva: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, June 10, 1996. Accessed January 8, 2010, at http://www.ich.org/LOB/media/MEDIA482.pdf
- 26.Declaration of Helsinki: ethical principles for medical research involving human subjects. Ferney-Voltaire, France: World Medical Association, 2006. Accessed Jan 8, 2010, at http://www.wma.net/en/30publications/10policies/b3/index.html
- 27.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Prohovnik I. Cross-validation of brain segmentation by SPM5 and SIENAX. Psychiatry Res. 2008;164:172–177. doi: 10.1016/j.pscychresns.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slotboom J, Schaer R, Ozdoba C, et al. A novel method for analyzing DSCE-images with an application to tumor grading. Invest Radiol. 2008;43:843–853. doi: 10.1097/RLI.0b013e3181893605. [DOI] [PubMed] [Google Scholar]
- 30.WHO Expert Committee on Biological Standardization Thirty-fifth report. World Health Organ Tech Rep Ser. 1985;725:1–140. [PubMed] [Google Scholar]
- 31.Bertolotto A, Malucchi S, Sala A, et al. Differential effects of three interferon betas on neutralising antibodies in patients with multiple sclerosis: a follow up study in an independent laboratory. J Neurol Neurosurg Psychiatry. 2002;73:148–153. doi: 10.1136/jnnp.73.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paty DW, Li DK, The UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:662–667. doi: 10.1212/WNL.43.4.662. [DOI] [PubMed] [Google Scholar]
- 33.Neuhaus O, Strasser-Fuchs S, Fazekas F, et al. Statins as immunomodulators: comparison with interferon-beta 1b in MS. Neurology. 2002;59:990–997. doi: 10.1212/WNL.59.7.990. [DOI] [PubMed] [Google Scholar]
- 34.Dhawan N, Reder AT. Statins block interferon signaling in human immune cells: potential loss of the therapeutic effect of IFN-B in multiple sclerosis. Neurology. 2007;68(suppl 1):A364. [Google Scholar]
- 35.Kieseier BC, Archelos JJ, Hartung HP. Different effects of simvastatin and interferon beta on the proteolytic activity of matrix metalloproteinases. Arch Neurol. 2004;61:929–932. doi: 10.1001/archneur.61.6.929. [DOI] [PubMed] [Google Scholar]
- 36.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74:1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrie RA, Horwitz R, Cutter G, et al. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72:117–124. doi: 10.1212/01.wnl.0000333252.78173.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.