Abstract
Objective
To evaluate tumor responses in patients treated with anti-angiogenic agents for non-small cell lung cancer (NSCLC) by assessing intratumoral changes using a dual-energy CT (DECT) (based on Choi's criteria) and to compare it to traditional Response Evaluation Criteria in Solid Tumors (RECIST) criteria.
Materials and Methods
Ten NSCLC patients treated with bevacizumab underwent DECT. Tumor responses to anti-angiogenic therapy were assessed and compared with the baseline CT results using both RECIST (size changes only) and Choi's criteria (reflecting net tumor enhancement). Kappa statistics was used to evaluate agreements between tumor responses assessed by RECIST and Choi's criteria.
Results
The weighted κ value for the comparison of tumor responses between the RECIST and Choi's criteria was 0.72. Of 31 target lesions (21 solid nodules, 8 lymph nodes, and two ground-glass opacity nodules [GGNs]), five lesions (16%) showed discordant responses between RECIST and Choi's criteria. Iodine-enhanced images allowed for a distinction between tumor enhancement and hemorrhagic response (detected in 14% [4 of 29, excluding GGNs] of target lesions on virtual nonenhanced images).
Conclusion
DECT may serve as a useful tool for response evaluation after anti-angiogenic treatment in NSCLC patients by providing information on the net enhancement of target lesions without obtaining non-enhanced images.
Keywords: Targeted therapy, Tumor response assessment, Response criteria, Guideline, Non-small cell lung cancer, Dual energy CT
INTRODUCTION
The tumor responses of solid tumors to cytotoxic therapy are usually assessed by traditional Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1), and is dependent on only the size change of the target lesion. However, the RECIST guideline may have some limitations (1) particularly when it's used for treating solid tumors by using anti-angiogenic agents, which have recently emerged as new treatment options for cancer (2, 3). With anti-angiogenic agent treatment, intratumoral morphologic changes may occur such as cavitation, hemorrhage and/or necrosis which usually develop as a response to the agent and may lead to tumor growth inhibition (4-6). When such morphologic changes occur, the tumor response to anti-angiogenic agents may be misinterpreted during the application of the RECIST 1.1 version in spite of the presence of an ongoing intratumoral response to anti-angiogenic agents (4, 7).
Intratumoral hemorrhage may cause the tumor to appear larger in size as compared with the tumor size on baseline CT scans. Further, the hemorrhage may mask changes in tumor attenuation which is important factor in the modified response criteria evaluation devised by Choi et al. (7). Thus, estimating the net enhancement of a tumor, irrespective of the presence of tumor hemorrhage, is crucial for the accurate evaluation of tumor response. In order to calculate net tumor enhancement, both enhanced and nonenhanced scans should be acquired. However, to obtain both the scans as a routine protocol is worrisome because of the large radiation dose the patient will receive.
With the advent of dual-energy CT (DECT), many clinical applications of the technique have been introduced (8, 9). The DECT technique enables one to differentiate an iodine substance from other materials by the material decomposition principle (10). The iodine component of lung nodules can be measured on iodine-enhanced images of DECT and this is comparable to the real value (net enhancement) of the extent of enhancement (11). A recent study demonstrated that the iodine-related attenuation of DECT in primary lung cancer correlates with the maximum standardized uptake value (SUVmax) of F-18-fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT (12). It suggests that DECT could be a useful functional imaging test for patients with NSCLC, reflecting angiogenesis of tumor. In addition, hemorrhage in the brain can be detected on virtual non-enhanced images with the removal of the iodine component within the lesion (13). Thus we expect that DECT can help to accurately assess the intrarumoral changes of non-small cell cancer (NSCLC) after anti-angiogenic angiogenic agents on virtual non-enhanced and iodine enhanced images, which are available without the acquisition of the non-enhanced images with the use of material decomposition technique at DECT. Thus, the purpose of our study was to evaluate the tumor responses in patients treated with anti-angiogenic agents for NSCLC by assessing intratumoral changes using DECT (based on Choi's criteria) and to compare it to traditional RECIST criteria.
MATERIALS AND METHODS
Institutional Review Board of Samsung Medical Center located in Seoul, Korea approved this study and informed consent was waived.
Patients
Between March and July in 2010, 10 patients (mean age, 60 years ± 10.5 [standard deviation]; range, 44-78 years) with stage IV of NSCLC and treated with bevacizumab (at a dose of 15 mg/kg on day 1) combined with cytotoxic chemotherapeutic agents (gemcitabine 1000 mg/m2 IV on days 1 and 8, and cisplatin 70 mg/m2 IV on day 1) were prospectively enrolled. They were seven men (mean age, 59 years ± 7.5; range, 50-70 years) and three women (mean age, 63 years ± 17.5; range, 44-78 years) (Table 1).
Table 1.
Patient Demographics and Target Lesions Characteristics
Note.- *Numbers in parentheses are numbers of target lesions. NSCLC = non-small cell lung cancer, NOS = not otherwise specified, LN = lymph node, DECT = dual-energy CT
Dual-Energy CT
Image Acquisition
The initial CT scans were performed within two weeks prior to the initial cycle of chemotherapy, and the follow-up CT scans were conducted after two cycles of chemotherapy. All patients underwent a CT examination using a dual-source CT scanner (Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany) with the dual-energy technique. This DECT system was composed of two X-ray tubes and two corresponding 128-row detectors mounted in a perpendicular arrangement. DECT scanning was obtained 90 seconds after the administration of contrast material (100 mL of iopamidol: Iomeron 300; Bracco, Milan, Italy) at a rate of 1.5 mL/sec by using a power injector. This was followed by 20 cc saline flushing at a rate of 1.5 mL/sec. Scan parameters were as follows: 105 mAs (effective) at 140 kV, 248 mAs (effective) at 80 kV, 32 × 0.6-mm collimation, a pitch of 0.7, a rotation time of 0.5 second, and a 512 × 512-pixel matrix. Scanning was performed from the thoracic inlet to the middle portion of the kidneys. Three types of data sets were generated from the DECT scanning: the 80 kV, 140 kV, and enhanced weighted-average images. The weighted-average images were generated by combining the 140-kV and 80-kV data sets with a weighting factor of 0.6 (60% of the information derived from the 80 kV image and 40% derived from the 140 kV image) and these were approximately 120 kV images.
Data Postprocessing and Image Reconstruction
The virtual non-enhanced images and iodine-enhanced images were made by using the liver Virtual Non-Contrast (VNC) application mode of dedicated dual-energy postprocessing software (Syngo Dual Energy; Siemens Medical Solutions, Forchheim, Germany). Since measurable target lesions were classified into three types, namely, solid pulmonary nodules, ground-glass opacity nodules (GGNs), and lymph nodes, the postprocessing software that was used was different depending on the type of target lesion. In cases of solid nodules and lymph nodes, the material parameters for the material decomposition method were as follows: -110 HU for fat at 80 kV, -87 HU for fat at 140 kV, 52 HU for soft tissue at 80 kV, and 51 HU for soft tissue at 140 kV. In cases of GGNs, since the lesion was composed of a mixture of air and soft tissue, the HU value of fat in the liver VNC application mode should be replaced with that of air, which is a HU value located at the interconnecting line between air and soft tissue (11, 13). Thus, the material parameters were -110 HU for fat at 80 kV, -115 HU for fat at 140 kV, 60 HU for soft tissue at 80 kV, and 54 HU for soft tissue at 140 kV (Fig. 1).
Fig. 1.
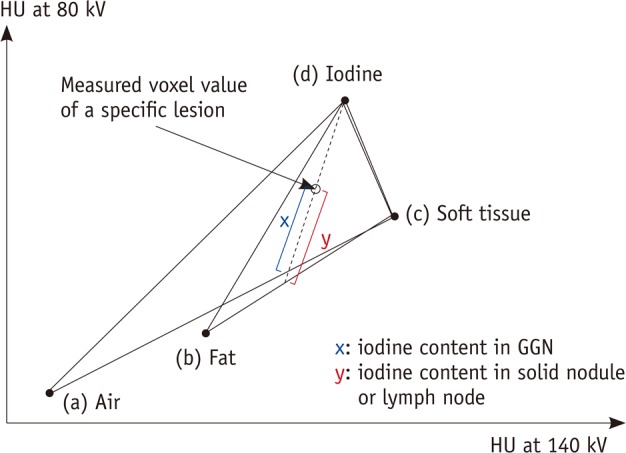
Diagram of three-material decomposition of voxel used by dual-energy software. This software splits every voxel in 80- and 140-kV image pair into three components represented by air, soft tissue, and iodine (for ground-glass nodule) or fat, soft tissue, and iodine (for solid nodule or lymph node). (a), (b), (c), and (d) are fixed points of CT attenuation values from two different energies for air, fat, soft tissue, and iodine, respectively. Intercept x or y along iodine axis represents iodine content of voxel on this two-energy plot. Virtual unenhanced images display noniodine component of voxel, and iodine-enhanced images display z intercept (i.e., iodine content). CGN = ground-glass opacity nodule
Image data were reconstructed with a section thickness of 1 mm by using a D30f (medium smooth) kernel for the iodine-enhanced image and a D45f (medium sharp) kernel for the virtual nonenhanced image.
Image Analysis
Two chest radiologists with six and nine years of experience in thoracic CT interpretation, respectively, evaluated CT images. In cases of discordant interpretations, decisions on CT findings were reached by consensus.
By the comparison of the current DECT images with previous baseline CT images of all patients who underwent both non-enhanced and enhanced CT scans, we assessed the tumor response by tumor size change only according to traditional RECIST 1.1 (1) and the modified CT response criteria proposed by Choi et al. (7) we assessed the tumor response by tumor size change. The measurable lesions with the size greater than 1 cm were included as target lesions. The size of each target lesion was evaluated in mediastinal window images. For the GGNs, their size was measured on lung window images. In addition to the size measurements, tumor attenuation changes were also considered. The mean CT attenuation of a target lesion was measured in Hounsfield Units (HUs). A region of interest (ROI) with the largest size possible (covering at least two thirds of the longest diameter) was placed in the solid portion of the target lesion. In case of GGNs, ROI covered almost the entire area of target lesion. For the baseline (pretreatment) CT studies, the net enhancement was defined by subtracting CT attenuation values on non-enhanced images from those on enhanced images. For the DECT studies, net enhancement was calculated by subtracting CT attenuation values on virtual non-enhanced images from those on enhanced weighted-average images or by measuring iodine content on iodine-enhanced images. The lesion was believed to have internal hemorrhage if it had an attenuation value of 50 HU or greater on both the true non-enhanced (at baseline CT obtained before treatment) and virtual non-enhanced images (at DECT) (14).
The tumor response was divided into: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (7). According to RECIST 1.1, the sum of the longest diameter of the target lesions (SLD) is calculated, and four response categories are defined as follows: CR (complete disappearance of all lesions, confirmed at ≥ 4 weeks), PR (a ≥ 30% decrease in SLD from baseline, confirmed at ≥ 4 weeks), PD (a ≥ 20% increase in SLD from smallest SLD), and SD (neither PR nor PD). In our study, SLD was replaced by the size of one target lesion. At Choi's criteria, four response categories are defined as: CR (disappearance of all lesions), PR (a decrease in size of 10% or a decrease in tumor density [HU] ≥ 15% on CT), PD (an increase in tumor size of ≥ 10% and not meet criteria of PR by tumor density [HU] on CT), and SD (neither PR nor PD). In our study, both criteria were applied to each target lesion.
Statistical Analysis
To evaluate agreements between tumor responses assessed by RECIST 1.1 and Choi's modified response criteria, κ statistics was used. A κ value of 0.01-0.20 indicates poor agreement; 0.21-0.40 indicates fair agreement; 0.41-0.60 indicates moderate agreement; 0.61-0.80 indicates good agreement; and 0.81-1.0 indicates excellent agreement. Commercially available statistical software (MedCalc, version 7.1; MedCalc Software, Mariakerke, Belgium) was used.
RESULTS
In a total of 10 patients, the median value of the effective dose for the enhanced DECT protocol was 6.52 mSv (range, 5.37-7.65 mSv). On the other hand, the median value of the effective dose for the non-enhanced and enhanced images obtained at the previous baseline chest CT was 8.72 mSv (range, 7.67-9.79 mSv).
Characteristics of Target Lesions
The histological subtypes of NSCLC in the 10 patients were as follows: adenocarcinoma (n = 7), NSCLC not otherwise specified (n = 2), and squamous cell carcinoma (n = 1). The number of target lesions per patient ranged from one to seven. Thirty-one target lesions (solid nodules [n = 21], lymph nodes [n = 8] and GGNs [n = 2]) in ten patients were identified. Solid nodules, lymph nodes, and GGNs ranged in their diameters from 10 to 89 mm, from 15 to 26 mm, and from 10 to 12 mm, respectively. The hemorrhagic responses within the target lesions, with the exception of two GGNs, were detected in 14% (4 of 29) of solid nodular and lymph node target lesions.
Comparison of Tumor Response between RECIST 1.1 and Choi's Criteria Based on DECT
The weighted κ value for the comparison of tumor responses between the RECIST 1.1 and Choi's criteria was 0.72 (0.65-0.79), which represented a good agreement.
Out of 31 target lesions, five (16%) target lesions showed discordant tumor responses between the traditional RECIST 1.1 and Choi's criteria (Table 2). While three of five target lesions were PD or SD when applying RECIST 1.1. They were PR based on Choi's critera with the development of new intratumoral hemorrhage (Fig. 2).
Table 2.
Comparison of Tumor Response between RECIST and Choi's Criteria
Note.- *Discordant tumor responses between RECIST 1.1 and Choi's criteria. PR = partial response, SD = stable disease, PD = progressive disease, GGN = ground-glass opacity nodule
Fig. 2.
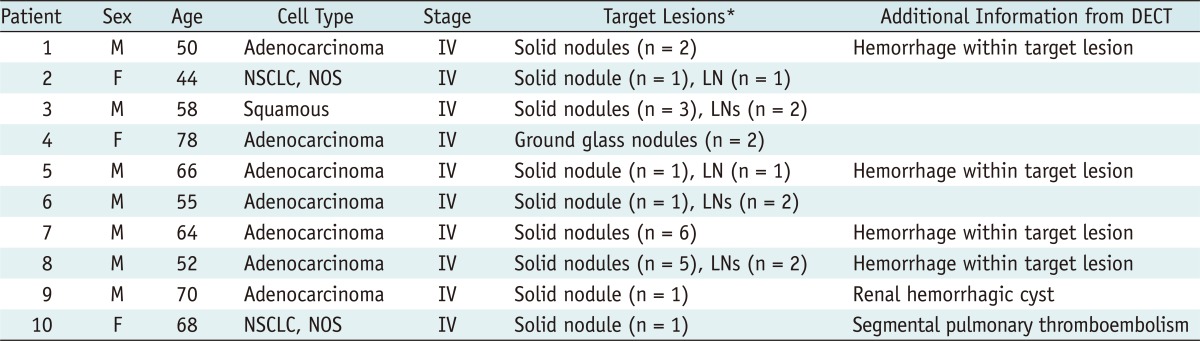
Hemorrhagic tumor response detected on dual-energy CT in 66-year-old man with lung adenocarcinoma.
A. Previous baseline enhanced CT image shows 35 mm-sized primary tumor with net enhancement of 48 HU (subtraction of CT attenuation value on nonenhanced image from that of enhanced image; 68 minus 20 HU) in left lower lobe. B. Enhanced weighted-average image obtained from dual-energy CT after chemotherapy shows about 28% increase in size of primary tumor and also increased CT attenuation value (up to 82 HU, circular regional of interest [ROI]). Thus, tumor response was assessed as progressive disease on basis of traditional RECIST 1.1. C. In contrast, virtual nonenhanced image shows primary cancer CT attenuation value of 53 HU (circular ROI), suggesting hemorrhagic component (arrows). D, E. Color coded (D) and grayscale (E) iodine-enhanced images show tumor (arrows) attenuation value of 29 HU, declined value by 40% of net enhancement calculated from previous baseline chest CT. Therefore, tumor response was assessed as partial response by Choi's criteria based on dual-energy CT.
Discordant responses were also noticed in GGNs. They were two GGNs of 10-12 mm in diameter. With RECIST 1.1, these lesions were regarded as equivocal lesions and they were categorized as SD as a response. However, the extent of enhancement for these GGNs was assessed on iodine-enhanced images, and this measured 58 HU and 89 HU, respectively. On the next follow-up chest CT scans, the GGNs changed into cavitary nodules with a definite increase in size (compatible with cavitary metastases). Thus, they were considered in PD category (Fig. 3).
Fig. 3.
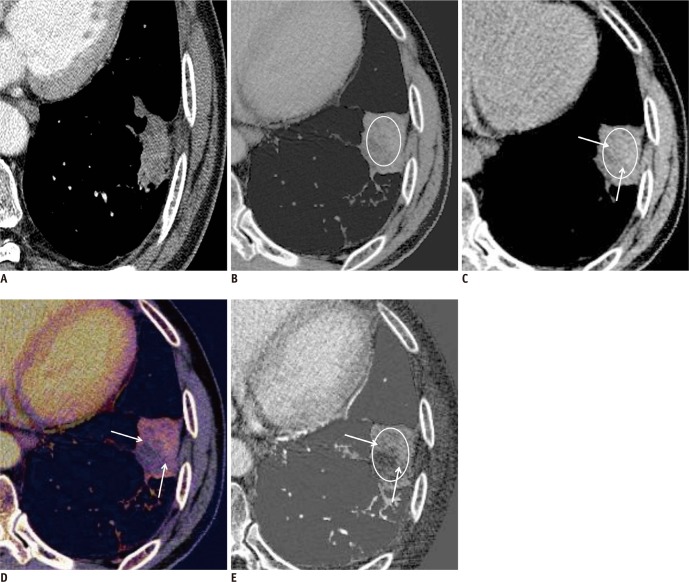
New pulmonary metastases manifested as ground-glass opacity nodules (part solid) in 78-year-old woman with lung adenocarcinoma in right upper and lower lobes.
A, B. Enhanced weighted-average image obtained from dual-energy CT shows newly developed 10-mm (A) and 12 mm-sized (B) ground-glass opacity nodules in right lung (arrows). Ground-glass opacity nodules are equivocal (not definitely defined) lesions on RECIST 1.1 version. Thus, tumor response was assessed as stable disease. C, D. These nodules shows substantial enhancement (58 HU in C and 89 HU in D) on color coded iodine-enhanced images obtained from dual-energy CT (arrows). Therefore, tumor response was assessed as progressive disease. E, F. Conventional images on further follow-up CT scans (two months after dual energy CT) show marked increase in nodule size with internal new cavitation (arrows), suggestive of cavitary metastases. Tumor response was again confirmed as progressive disease.
DISCUSSION
According to RECIST 1.1, the tumor size change was the only factor determining the tumor response to cytotoxic chemotherapy (1). However, a few recent studies have demonstrated that with RECIST 1.1 we could not perform an exact assessment of the tumor response for the targeted agent. Because the targeted agents induce intratumoral hemorrhage, necrosis or cavitation rather than tumor shrinkage, the morphological change within a tumor has been emphasized as a kind of tumor response (4, 7, 15). Choi et al. (7) proposed new CT response criteria to assess the response of gastrointestinal stromal tumors (GISTs) after imatinib treatment, and these criteria include evaluating the changes in tumor size or attenuation values. Lee et al. (4, 16) also devised a new CT response criteria in NSCLC patients who are treated with epidermal growth factor receptor tyrosine kinase inhibitor. These criteria reflect the change of the tumor attenuation value and cavity changes as well as the size changes. They confirmed that when these criteria are applied in tumor response evaluation, they are significantly associated with the overall patient survival. According to these new criteria, the tumor density is defined as the CT attenuation value as measured on enhanced CT images.
To evaluate a tumor's response to treatment when measuring CT attenuation changes, it would be ideal to measure the net enhancement of the tumor and thus evaluate the exact tumor response even in cases with intratumoral hemorrhage or necrosis. Intratumoral hemorrhage might lead to overestimation of tumor size and thus misinterpretation of SD or PR as PD using the traditional tumor response criteria. Moreover, the hemorrhage may be mistakenly regarded as an enhancing solid component when only the enhanced CT images are obtained.
Gupta et al. (13) recently reported that DECT is helpful in detecting intracerebral hemorrhage (ICH) in patients who have recently undergone intraarterial or intravenous iodinated contrast material injection. In Gupta et al. (13), the iodine component could be effectively differentiated from ICH with DECT with high sensitivity and specificity.
These have also been reports that virtual non-enhanced images on DECT can replace the true non-enhanced images for liver or kidney lesion evaluation (17, 18). In addition, Chae et al. (11) corroborated that the CT numbers in the virtual non-enhanced image are similar to the non-enhanced-weighted image, and DECT can help to correctly measure the enhancement degree of pulmonary nodules. In addition, Schmid-Bindert et al. (12) reported that a moderate correlation was found between maximum iodine-related attenuation of DECT and SUVmax of PET/CT in all primary lung cancers. Thus, we expected that DECT could have been the method to enable tumor response monitoring by allowing the detection of intratumoral hemorrhage and evaluating the tumor net enhancement (measuring the iodine component) without additional nonenhanced scanning.
Intratumoral hemorrhage, which was readily assessed on virtual non-enhanced images, was detected in 14% (4 of 29) of the solid target lesions. To the best of our knowledge, the current study is the first that has assessed a hemorrhagic tumor response by using DECT in a patient with NSCLC and who was treated with anti-angiogenic agents. Interestingly most target lesions that showed a hemorrhagic response (75%, 3 of 4 lesions) to anti-angiogenic treatment, the tumor response evaluations based on RECIST 1.1 and Choi's criteria were discordant. Hemorrhagic target lesions seemed to increase or not be altered according to their CT attenuation values on enhanced weighted-average images as compared with those seen on baseline enhanced CT images. However, the lesions had substantially declined attenuation values of greater than 15% on iodine-enhanced images as compared with net enhancement calculated from baseline CT images. Thus, when applying Choi's criteria for tumor response evaluation, the response should be categorized as a PR.
A GGN is defined as a lesion of hazy increased lung attenuation with the preservation of the underlying bronchial and vascular margins (19). The lesion usually does not appear on mediastinal window images. Thus, the extent of its enhancement is not easily measured on the mediastinal window images of routine chest CT. In our study, we could quantify the enhancement extent of the GGNs with the help of the DECT technique, which allowed the pure iodine component of GGN to be differentiated from an air component of the lesion. Two newly developed GGNs in NSCLC patients treated with anti-angiogenic therapy had internal high-attenuation values on iodine-enhanced images, which represented the enhancing solid component. The lesions showed a marked increase in size on further follow up CT images, thus confirming that the GGNs represented metastatic lesions. Such results may imply that the DECT technique allows for quantification of the extent of the nodule enhancement even in GGNs and this facilitates monitoring the GGN internal morphologic changes over time and a more accurate evaluation of the tumor response.
Recent studies have reported that thoracic DECT is performed without an additional radiation dose as compared with conventional chest CT and without a hampered image quality (11, 20). Our study also showed that the median value of the effective dose with DECT was smaller than that of a standard chest CT. The cumulative radiation dose in cancer patients may not be a great concern, but having DECT images that are comparable with the images of standard chest CT without increasing the radiation dose to patients is encouraging news. In the near future, we may use DECT as a routine study protocol in oncologic patients for lesion characterization and evaluation of the tumor response.
Our study had several limitations. First, the generalized applicability of our study's DECT protocol for oncology patients might be limited by the small sample size. Further studies with large numbers of patients are needed to ensure the feasibility of DECT for evaluating the tumor response after anti-angiogenic treatment. Second, we did not measure the volume of the enhancing solid portion. In addition to the change in the net enhancement value, the volume of enhancing solid component within the tumor could also be an important parameter for evaluating the tumor response. Assessment of both of these two parameteres might draw a more exact evaluation of the tumor response. To corroborate this, future studies will be needed. Third, we could not pathologically prove a hemorrhagic response within the tumor. However, the non-enhancing portion showed a high density, greater than 50 HU in a non-enhanced scan which may exclusively be a hemorrhage component. Lastly, the scatter radiation-related artifacts increase if a dual source system of CT scanner is used. Therefore, it might affect the exact measurement of the HU values on DECT images.
In conclusion, DECT may serve as a useful tool for response evaluation after anti-angiogenic treatment in NSCLC patients by providing information on the extent of tumor nodules and lymph nodes enhancement, which can be accomplished without obtaining non-enhanced images. The virtual non-enhanced and iodine-enhanced DECT images may facilitate identifying intratumoral morphologic changes such as hemorrhage associated with anti-angiogenic therapy and differentiating such changes from true tumor growth (i.e., the enhancing portion of the tumor).
Acknowledgments
Many thanks to RTs Min Chan Kim, Yung Joon Yoon, Sul Kyoung Shin, and Wan Yuk Kim for helping us with the CT acquisition, the data postprocessing, and the image reconstruction.
References
- 1.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Pirker R, Filipits M. Targeted therapies in lung cancer. Curr Pharm Des. 2009;15:188–206. doi: 10.2174/138161209787002915. [DOI] [PubMed] [Google Scholar]
- 3.Bertino EM, Otterson GA. Benefits and limitations of antiangiogenic agents in patients with non-small cell lung cancer. Lung Cancer. 2010;70:233–246. doi: 10.1016/j.lungcan.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Lee HY, Lee KS, Ahn MJ, Hwang HS, Lee JW, Park K, et al. New CT response criteria in non-small cell lung cancer: proposal and application in EGFR tyrosine kinase inhibitor therapy. Lung Cancer. 2011;73:63–69. doi: 10.1016/j.lungcan.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Sandler AB, Schiller JH, Gray R, Dimery I, Brahmer J, Samant M, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with Carboplatin and Paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 8.Coursey CA, Nelson RC, Boll DT, Paulson EK, Ho LM, Neville AM, et al. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010;30:1037–1055. doi: 10.1148/rg.304095175. [DOI] [PubMed] [Google Scholar]
- 9.Kang MJ, Park CM, Lee CH, Goo JM, Lee HJ. Dual-energy CT: clinical applications in various pulmonary diseases. Radiographics. 2010;30:685–698. doi: 10.1148/rg.303095101. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17:1510–1517. doi: 10.1007/s00330-006-0517-6. [DOI] [PubMed] [Google Scholar]
- 11.Chae EJ, Song JW, Seo JB, Krauss B, Jang YM, Song KS. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology. 2008;249:671–681. doi: 10.1148/radiol.2492071956. [DOI] [PubMed] [Google Scholar]
- 12.Schmid-Bindert G, Henzler T, Chu TQ, Meyer M, Nance JW, Jr, Schoepf UJ, et al. Functional imaging of lung cancer using dual energy CT: how does iodine related attenuation correlate with standardized uptake value of 18FDG-PET-CT? Eur Radiol. 2012;22:93–103. doi: 10.1007/s00330-011-2230-3. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R, Phan CM, Leidecker C, Brady TJ, Hirsch JA, Nogueira RG, et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. 2010;257:205–211. doi: 10.1148/radiol.10091806. [DOI] [PubMed] [Google Scholar]
- 14.Ling D, Korobkin M, Silverman PM, Dunnick NR. CT demonstration of bilateral adrenal hemorrhage. AJR Am J Roentgenol. 1983;141:307–308. doi: 10.2214/ajr.141.2.307. [DOI] [PubMed] [Google Scholar]
- 15.Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009;27:404–410. doi: 10.1200/JCO.2008.16.2545. [DOI] [PubMed] [Google Scholar]
- 16.Lee HY, Lee KS, Hwang HS, Lee JW, Ahn MJ, Park K, et al. Molecularly targeted therapy using bevacizumab for non-small cell lung cancer: a pilot study for the new CT response criteria. Korean J Radiol. 2010;11:618–626. doi: 10.3348/kjr.2010.11.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graser A, Johnson TR, Hecht EM, Becker CR, Leidecker C, Staehler M, et al. Dual-energy CT in patients suspected of having renal masses: can virtual nonenhanced images replace true nonenhanced images? Radiology. 2009;252:433–440. doi: 10.1148/radiol.2522080557. [DOI] [PubMed] [Google Scholar]
- 18.Barrett T, Bowden DJ, Shaida N, Godfrey EM, Taylor A, Lomas DJ, et al. Virtual unenhanced second generation dual-source CT of the liver: is it time to discard the conventional unenhanced phase? Eur J Radiol. 2012;81:1438–1445. doi: 10.1016/j.ejrad.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 19.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 20.Schenzle JC, Sommer WH, Neumaier K, Michalski G, Lechel U, Nikolaou K, et al. Dual energy CT of the chest: how about the dose? Invest Radiol. 2010;45:347–353. doi: 10.1097/RLI.0b013e3181df901d. [DOI] [PubMed] [Google Scholar]