Abstract
Objective
To assess the technical feasibility and local efficacy of biplane fluoroscopy plus US-guided percutaneous radiofrequency ablation (RFA) for viable hepatocellular carcinoma (HCC) around retained iodized oil after transcatheter arterial chemoembolization (TACE).
Materials and Methods
Our prospective study was approved by our institutional review board and informed consent was obtained from all participating patients. For patients with viable HCC around retained iodized oil after TACE, biplane fluoroscopy plus US-guided RFA was performed. We evaluated the rate of technical success and major complications on a post-RFA CT examination and local tumor progression with a follow-up CT.
Results
Among 40 consecutive patients, 19 were excluded due to one of the following reasons: poorly visible HCC on fluoroscopy (n = 13), high risk location (n = 2), RFA performed under monoplane fluoroscopy and US guidance (n = 2), and poorly identifiable new HCCs on US (n = 2). The remaining 21 patients with 21 viable HCCs were included. The size of total tumors ranged from 1.4 to 5.0 cm (mean: 3.2 cm) in the longest diameter. Technical success was achieved for all 21 HCCs, and major complications were observed in none of the patients. During the follow-up period (mean, 20.3 months; range, 6.5-29.9 months), local tumor progression was found in two patients (2/21, 9.5%). Distant intrahepatic metastasis developed in 76.2% (16/21) of patients.
Conclusion
When retained iodized oil around the tumor after TACE hampers the targeting of the viable tumor for RFA, biplane fluoroscopy plus US-guided RFA may be performed owing to its technical feasibility and effective treatment for viable HCCs.
Keywords: Liver; Guidance; Radiofrequency ablation; Hepatocellular carcinoma; Biplane fluoroscopy; Ultrasonography; Transcatheter arterial chemoembolization, iodized oil
INTRODUCTION
The transcatheter arterial chemoembolization (TACE) method has been widely used for the management of unresectable, advanced hepatocellular carcinoma (HCC) in patients with compensated liver cirrhosis (1). Compared to the curative treatment options including hepatic resection, liver transplantation, and local ablation therapy, the survival benefit from a TACE in patients with unresectable HCC has been controversial (2, 3). Although a TACE could decrease the HCC burden in selected cases, the proper management of local tumor progression or incompletely treated viable HCCs after a TACE is a challenging task, due to poor local efficacy of TACE (4-6). Even when a TACE is repeated, the viable HCC sometimes remains unchanged or even grows. Therefore, if viable tumors after a TACE are small in number and size, local ablation therapy, such as radiofrequency ablation (RFA) can be utilized as an alternative option for further treatment.
In general, percutaneous RFA has been performed under the guidance of ultrasonography (US) or CT (7, 8). In cases of viable HCC around retained iodized oil after TACE, since the retained iodized oil in the tumor can be a radio-opaque landmark for targeting HCC, CT/CT fluoroscopy or biplane fluoroscopy (both anteroposterior and lateral projections) can be used as a guiding modality for targeting HCCs (9, 10). However, in some hospitals, a CT machine is occupied by too many examinations for in- and outpatients, thus making it hard to use CT guidance for RFA procedures which may require a long procedure time. Therefore, biplane fluoroscopy can be considered as an alternative guiding modality for percutaneous RFA for HCCs with retained iodized oil. However, to the best of our knowledge, there have been no studies investigating the role of biplane fluoroscopy as a guidance modality in cases of local tumor progression or incompletely treated viable HCC around retained iodized oil after a TACE. The purpose of our study was therefore to assess the technical feasibility and local efficacy of biplane fluoroscopy-guided, percutaneous RFA assisted by US guidance, for the management of viable HCC after a TACE.
MATERIALS AND METHODS
Patients and Enrollment Criteria
Our prospective study was approved by the institutional review board and informed consent was obtained from all participant patients. In this study, a viable HCC around retained iodized oil was defined when local tumor progression was found around HCC with compact iodized oil accumulation by a prior TACE or when an incompletely treated viable HCC was found on follow-up CT or MR images following a TACE. From September 2008 to May 2009, patients with viable HCCs around the retained iodized oil by a prior TACE were enrolled, if they met the following inclusion criteria: 1) viable HCC around the retained iodized oil after a TACE was found at a follow-up liver CT and/or MRI, 2) in cases with a single viable HCC, the size of tumor was smaller than 5 cm in diameter, which was measured by the longest tumor diameter, including both viable HCCs and retained iodized oil based on CT or MR images, 3) in cases with multinodular HCCs (n ≤ 3), where each tumor had a longest diameter under 3 cm and at least one tumor was a viable HCC around retained iodized oil, 4) no portal vein invasion, 5) no extrahepatic metastases; 6) Child-Pugh class A or B; and 7) absence of severe coagulopathy (i.e., prothrombin activity < 40% or platelet count < 40000/mL).
Exclusion criteria were as follows: 1) the retained iodized oil around viable HCC was poorly visible on an anteroposterior and/or lateral projection image on a planning fluoroscopy for RFA; 2) in cases with multinodular HCCs, any new HCCs without retained iodized oil were poorly visible on planning US, and thus unsuitable for percutaneous US-guided RFA (even though the index tumor with retained iodized oil was visible on planning fluoroscopy); 3) percutaneous RFA was infeasible due to the high risk location of the collateral thermal injury or could result in incomplete ablation due to not having an adequate electrode path; 4) monoplane fluoroscopy was used due to the unavailability of biplane fluoroscopy at the time of the RFA procedure.
The diagnosis of a viable HCC around the retained iodized oil was based on the typical imaging features (arterial enhancement followed by delayed washout) on dynamic contrast-enhanced CT and/or MRI (11-13). Histopathologic confirmation was not obtained in any patient. The subphrenic location of the tumor was defined when the index tumor was either located within 1 cm from or abutting the diaphragm.
Planning US and Planning Fluoroscopy
One abdominal radiologist performed both planning of the US fluoroscopy on the same day, to assess the feasibility of percutaneous RFA for all referred patients meeting the above inclusion criteria. The radiologist had 4 years of experience in performing percutaneous RFAs of liver tumors (more than 150 cases), at the starting point of this study. All planning US were performed with a multi-frequency 4C1 convex array probe (Acuson Sequoia 512, Siemens Medical Solutions, Mountain View, CA, USA). At the time of the planning US, the radiologist was aware that the intent of the RFA was to cover not only the viable HCC, but also the retained iodized oil; thus, lesion conspicuity was determined based on the US findings of the whole tumor. The radiologist searched thoroughly for the viable HCC with retained iodized oil, and determined whether the percutaneous US-guided RFA was feasible (14). The lesion conspicuity of the index tumor on planning US was classified as follows: visible group, conspicuous enough for percutaneous US-guided RFA; invisible group, inconspicuous and thus unsuitable for percutaneous US-guided RFA.
In addition, all patients with viable HCC around the retained iodized oil underwent a planning fluoroscopy with a flat-panel radiographic/fluoroscopic table system (SONIALVISION Safire 2; Shimadzu, Kyoto, Japan) by the same radiologist. The radiologist determined whether the retained iodized oil in the index tumor was radio-opaque enough to be seen on both anteroposterior and lateral projections, and thus could be targeted under the biplane fluoroscopy. Anteroposterior and lateral projection images were obtained with the patients in the supine and left lateral decubitus position, respectively. In terms of visibility of the index tumor on fluoroscopy, the HCCs were divided into two groups. When the retained iodized oil in the tumors was visible on both anteroposterior and lateral projection images, thus rendering the index tumor suitable for targeting under the guidance of biplane fluoroscopy, they were regarded as the fluoroscopically visible group. If the index tumors were invisible or poorly visible on anteroposterior, lateral or both images, thus not feasible for targeting the tumor, they were regarded as the fluoroscopically invisible group.
Ablation Procedure
Percutaneous RFA was performed for the fluoroscopically visible group by the same radiologist in the interventional suite equipped with a biplane fluoroscopy/angiography instrument (Allura Xper FD 20/10, Philips Healthcare, Best, the Netherlands). The patients were placed in the supine position and were treated under local anesthesia or general anesthesia depending on tumor size and location. For the RFA procedure, a 200-W generator (Cool-tip; Valley lab, Boulder, CO, USA) and a 17-gauge cooled-tip electrode with a 3 cm exposed tip (Cool-tip; Valleylab, Boulder, CO, USA) or 18-gauge active-tip internally cooled electrode (Well-point RF Electrode; Taewoong Medical, Gimpo, Korea) were used. Artificial ascites were infused whenever they were required to improve the sonic window and decrease the degree of collateral thermal injury to the adjacent diaphragm or colon (15, 16). The intent of the RFA was to cover not only a viable HCC, but also the retained iodized oil where local tumor progression could subsequently occur (17). Targeting the index tumor was primarily guided by biplane fluoroscopy, using the retained iodized oil as an anatomic landmark. US guidance throughout the procedure was also needed to determine the skin entry site of the electrode, to avoid traversing dangerous structures such as the colon and the gallbladder, to estimate the ablation zone during the procedure and to avoid collateral thermal injury. Since the viable HCC itself was invisible on fluoroscopy, we placed an electrode based on CT or MR findings showing anatomic relationship between the viable HCC and the retained iodized oil. If viable HCC was visible on US, we used both US and biplane fluoroscopy as guiding modalities. However, when the viable tumor was invisible on US, biplane fluoroscopy was mainly used for the placement of an electrode during the ablation procedure, while US was used for monitoring the ablation process. Multiple overlapping ablations were facilitated by the retained iodized oil, which remained nearly unchanged after repeated cycles of ablation under biplane fluoroscopic guidance. Thus, with the multiple overlapping strategy, we could adopt a short ablation time, rather than the standard 12 minute-single ablation (i.e., 6 minutes × 2 overlapping ablations, rather than 12 minutes × single ablation), because we believe that the former could create a larger ablation zone than the latter (18). For hepatic dome lesions, the transthoracic approach (which can be complicated by the occurrence of a pneumothorax) could be avoided by the oblique approach from the lower intercostal space, under the guidance of biplane fluoroscopy.
If the index tumor was closely located to a critical structure, such as the central bile duct or large vessels, percutaneous ethanol injection (PEI) was combined with RFA simultaneously to avoid thermal injury and the heat-sink effect. Generally, absolute ethanol was injected to the parts of the tumor abutting or closely located to the critical structure and a RFA was applied to the other parts of the tumor located away from critical structure. First, a 21-gauge needle with six side holes (PEIT; Hakko, Tokyo, Japan) was introduced into the index tumor under the dual guidance of biplane fluoroscopy and US, and then the RF electrode was placed. The amount of ethanol injected into the tumor was always kept at less than the estimated volume (V): V (mL) = 4/3 π (γ + 0.5)3, where γ represents the radius of the tumor in centimeters. We injected absolute ethanol slowly into the deepest part of the tumor from the skin puncture site, and then into the superficial part of the tumor. The injection of ethanol was stopped when the echogenic zone was encompassing the parts of the tumor abutting or closely located to the critical structure and high resistance when ethanol injection was felt. Immediately after the PEI procedure, a RFA was performed. After the RFA procedure, the PEI needle was withdrawn slowly.
Follow-Up after RFA
A CT scan was performed immediately after a RFA to evaluate the technical success and immediate post-RFA complication. Various multi-detector row CT scanners (Brilliance 40 [Philips Medical Systems, Cleveland, OH, USA], Lightspeed VCT [GE Medical Systems, Milwaukee, WI, USA], or Aquilion [Toshiba America Medical Systems, Tustin, CA, USA]) were used. A total of 120 mL of non-ionic contrast material (Ultravist 300, 300 mg/mL iopromide, Schering AG, Berlin, Germany) was administered intravenously, using an automatic injector at a rate of 3-4 mL/s. Triple phase CT Images from the dome of the diaphragm to the lower pole of the right kidney were obtained during a single breath-hold at 25-35, 60-70, and 180 seconds after initiating contrast material injection, representing the hepatic arterial, portal venous, and equilibrium phases, respectively. Axial images were reconstructed at a 5.0 mm slice thickness and reconstruction interval. Coronal and sagittal images were also reconstructed with both 3.0 mm slice thickness and reconstruction interval.
Technical success was defined as the presence of a non-enhancing area surrounding the tumor on CT images (19, 20). It was subdivided into two categories, primary and secondary technical success (Fig. 1). If only viable tumor around retained iodized oil was completely ablated on immediate follow up CT scan, it was defined as a primary technical success. When both viable tumor and retained iodized oil were completely ablated, it was defined as a secondary technical success. When primary technical success was not achieved, a second RFA session was performed within 24 hours. However, when secondary technical success was not achieved, we did not perform a second RFA session, since there was no evidence of a viable HCC based on CT images. Instead of an additional RFA session, a close follow-up was adopted for these patients.
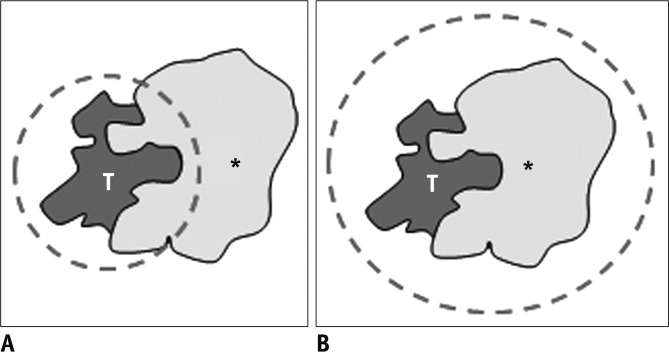
Fig. 1.
Diagram showing definition of technical success. If viable tumor (T) around retained iodized oil (asterisk) was completely ablated on immediate follow-up CT scan, it was defined as primary technical success (A). When both viable tumor (T) and retained iodized oil (asterisk) were completely ablated, it was defined as secondary technical success (B).
After a post-RFA CT scan, the patients were followed up 1 month after the RFA and every 3 months thereafter, using liver function tests, serum α-fetoprotein, and dynamic liver CT. The primary end point was the rate of technical success and major complication. Major complications were defined as any event leading to substantial morbidity and disability, increasing the level of care, and resulting in substantially lengthening a hospital stay, requiring a blood transfusion, or an interventional drainage procedure (21). All others were considered to be minor complications. The secondary end point was the rate of local tumor progression. The rate of local tumor progression and distant metastasis (both intra- and extrahepatic metastasis) at follow-up CT were assessed. Local tumor progression was defined as a new enhancing lesion within or adjacent to the previously completely ablated site on dynamic liver CT/MR imaging (22). Distant metastasis was defined as a new HCC in the liver, located distant from the index tumor or in extrahepatic regions.
Statistical Analysis
Statistical analyses were performed with the MedCalc software version 9.3.9.0 (MedCalc Software, Mariakerke, Belgium). The fluoroscopically visible group was compared with the fluoroscopically invisible group in terms of the size of retained iodized oil, location of tumor (subphrenic or not), and lesion conspicuity on planning US using the Mann-Whitney test or chi-square test. P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Study Population and Ablation Procedure
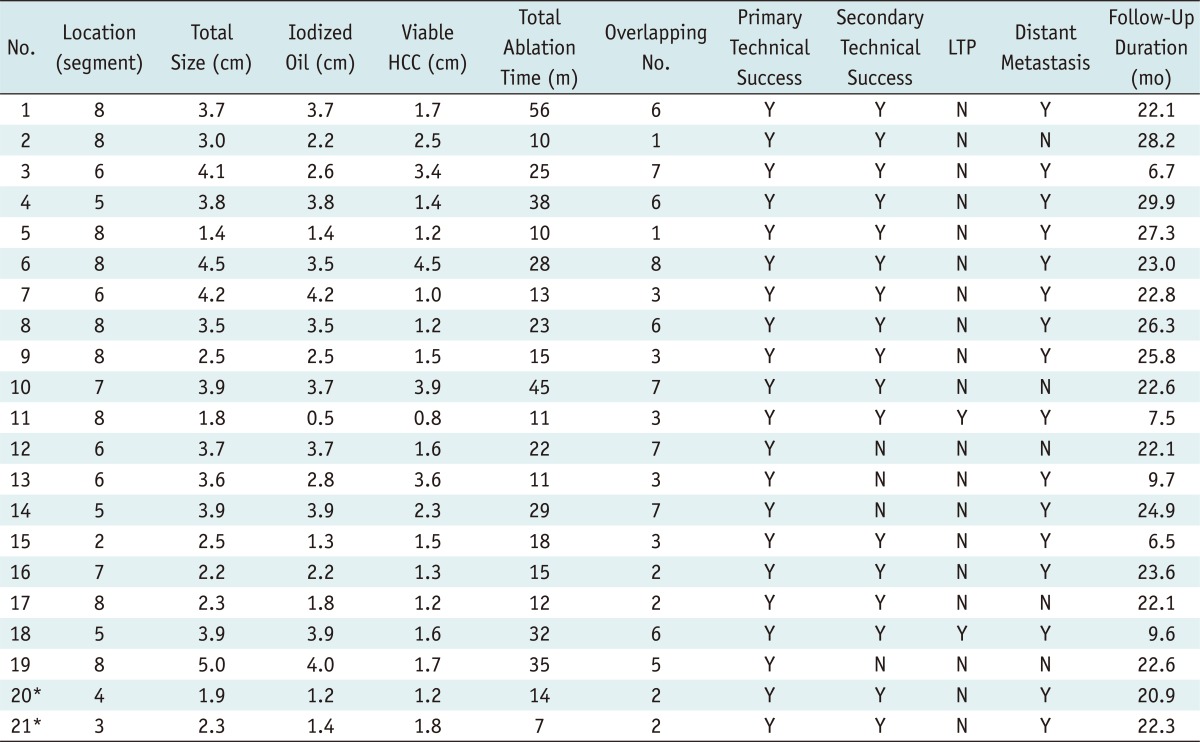
During the study period, 40 consecutive patients met the inclusion criteria and were referred for planning US and planning fluoroscopy for percutaneous RFA (Fig. 2). Among the 37 patients with a single viable HCC around the retained iodized oil, 25 patients had fluoroscopically visible tumors, whereas the other 12 patients had fluoroscopically invisible tumors. Of the 25 patients with fluoroscopically visible tumors, four patients who had tumors in high risk locations (n = 2) or were treated under the guidance of monoplane fluoroscopy and US (n = 2) were excluded. The remaining 21 tumors were ablated under the guidance of biplane fluoroscopy assisted by US guidance. On the other hand, 3 patients with multiple HCCs were excluded because the viable HCC around the retained iodized oil was invisible on fluoroscopy (n = 1) or new HCCs were invisible on US (n = 2). Finally, a total of 21 patients with 21 viable HCCs with retained iodized oil were treated under the guidance of biplane fluoroscopy assisted by US guidance, and were included in the final analysis in this study (Figs. 2, 3). Baseline characteristics of 21 patients are summarized in Table 1. Ten patients had residual untreated viable HCCs after a TACE, while the other 11 patients had local tumor progression around HCC with compact iodized oil accumulation on follow-up CT images. The number of previous TACE procedures on viable HCCs with retained iodized oil before a RFA was 3.4 ± 2.4 (range: 1-10). The time interval between the last TACE and the ablation procedure was 9.4 ± 8.7 months, ranging from 1.0 to 29.7 months. The size of the retained iodized oil and viable portion of the HCC ranged from 0.5 to 4.2 cm (mean ± standard deviation [SD]: 2.8 ± 1.1 cm) and 0.8 to 4.5 cm (mean ± SD: 1.9 ± 1.0 cm), respectively. The total size of the tumor, including both the viable HCC and retained iodized oil, ranged from 1.4 to 5.0 cm (mean ± SD: 3.2 ± 1.0 cm). Twelve of 21 (57.1%) HCCs were located in the subphrenic region and the other 9 (42.9%) were located elsewhere.
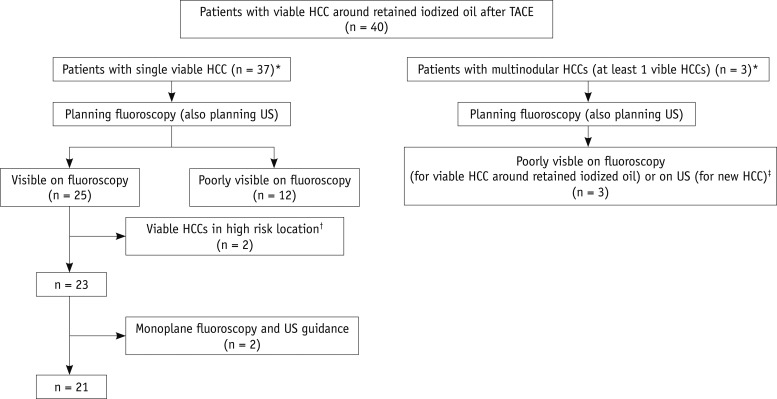
Fig. 2.
Flow chart of inclusion and exclusion criteria. "Visible on fluoroscopy" was defined when index tumor was radio-opaque enough to be visible on both anteroposterior and lateral projections, and thus could be targeted under biplane fluoroscopy with US guidance. *Among 40 referred patients, 37 had single viable HCC around retained iodized oil, while other 3 had 2 or 3 HCCs (including at least one viable HCC around the retained iodized oil), resulting in total of 42 viable HCCs around retained iodized oil and 3 new HCCs. †Two patients who had viable HCC around retained iodized oil surrounded by inferior vena cava and right hepatic vein (n = 1) or inadequate electrode path (n = 1) were excluded for percutaneous radiofrequency ablation. ‡In two patients with multinodular HCCs, although two viable HCCs around retained iodized oil were visible on fluoroscopy, other new HCCs were invisible on US. In remaining one patient, viable HCC around retained iodized oil was invisible on fluoroscopy. Therefore, total of 27 viable HCCs were visible on fluoroscopy, whereas remaining 15 viable HCCs were invisible. HCC = hepatocellular carcinoma, TACE = transcatheter arterial chemoembolization
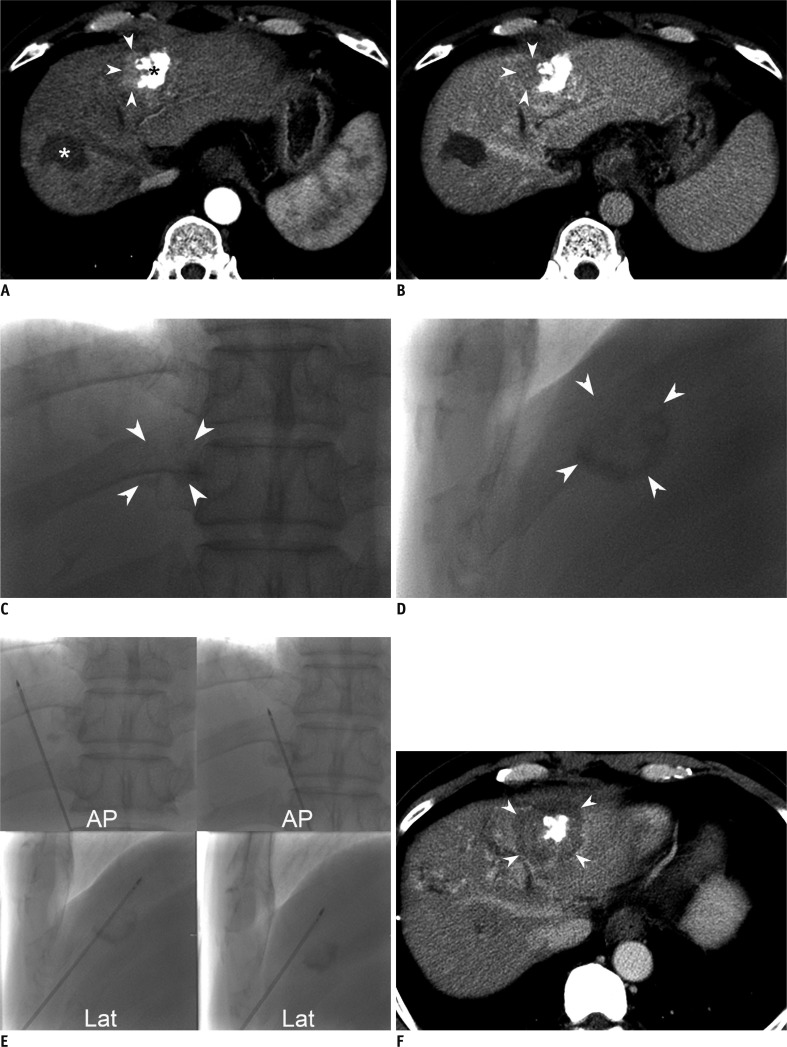
Fig. 3.
57-year-old man with viable hepatocellular carcinoma (HCC) in left hepatic dome after repeated transcatheter arterial chemoembolization (TACE).
A. Arterial phase axial CT scan obtained one month after four TACE sessions shows enhancing lesion with maximum diameter of 2.2 cm (arrowheads), located around incomplete accumulation of iodized oil (black asterisk) in hepatic segment III. Patient had previous history of radiofrequency ablation (RFA) for another HCC (white asterisk) in segment VIII of liver. B. Delayed phase CT scan shows wash-out (arrowheads) suggestive of viable HCC, in site corresponding to enhancing lesion in A. C. Fluoroscopic images demonstrate accumulation of iodized oil (arrowheads) around viable HCC on magnified anteroposterior (C) and lateral (D) projection images, which were not visualized by US. Biplane fluoroscopy using retained iodized oil as radio-opaque anatomic landmark was chosen as main guiding modality for percutaneous RFA. D. Fluoroscopic images demonstrate accumulation of iodized oil (arrowheads) around viable HCC on magnified anteroposterior (C) and lateral (D) projection images, which were not visualized by US. Biplane fluoroscopy using retained iodized oil as radio-opaque anatomic landmark was chosen as main guiding modality for percutaneous RFA. E. Representative anteroposterior (AP) and lateral (Lat) fluoroscopic images obtained during six overlapping radiofrequency ablation (RFA) procedures. We inserted electrode to lateral aspect of retained iodized oil, where viable HCC was considered to be present (left column). Multiple overlapping ablations were facilitated using steep oblique approach based on biplane fluoroscopic images in which retained iodized oil remained almost unchanged over 6 overlapping ablations. On sixth overlapping (right column), electrode was positioned in anterior side of retained iodized oil. Although not shown here, US was used for monitoring purposes during each ablation cycle. Total ablation time of six overlapping treatments was 26 minutes. F. Transverse portal phase CT image obtained immediately after single RFA session reveals partial intratumoral deposition of iodized oil, with sufficient non-enhancing ablative zone (arrowheads), with maximum diameter of 4.5 cm.
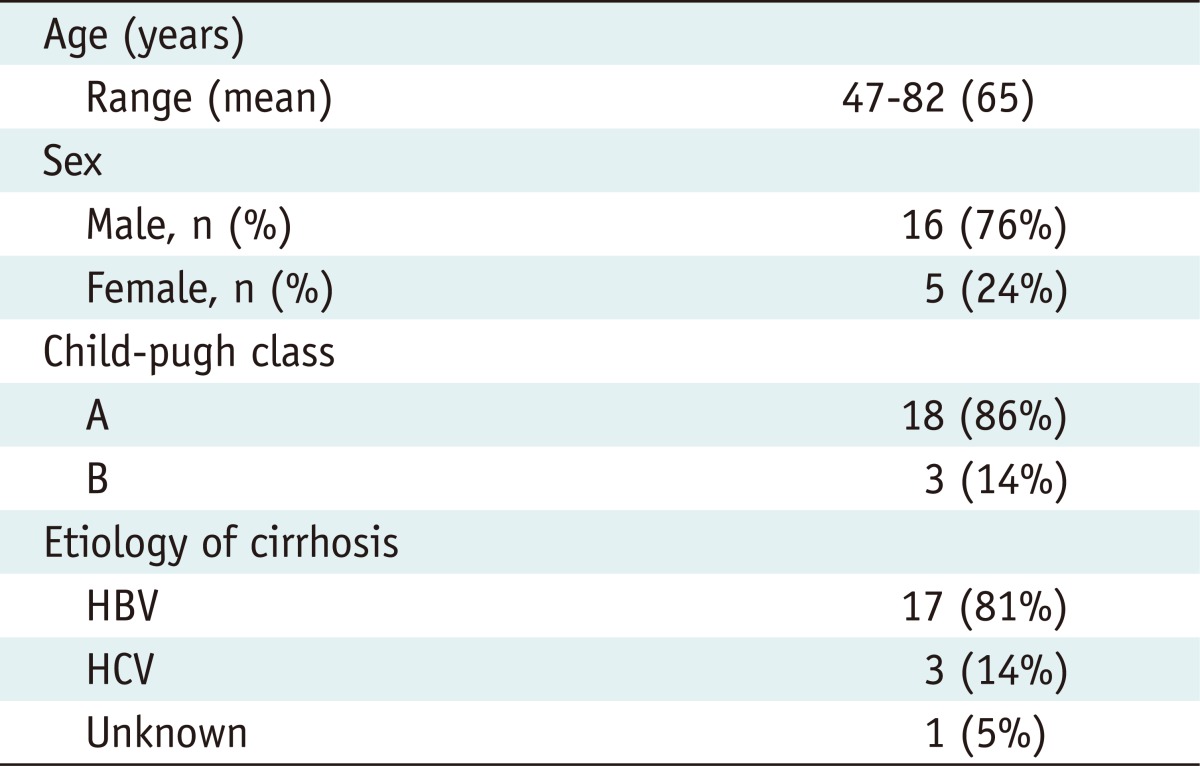
Table 1.
Baseline Characteristics of 21 Patients with 21 Viable Hepatocellular Carcinomas
HBV = hepatitis B virus, HCV = hepatitis C virus
Artificial ascites were introduced in 16 patients to minimize thermal injury to the diaphragm or colon. The number of overlapping ablations was 4.3 ± 2.3 per HCC nodule (range: 1-8). The total ablation time was 22.3 ± 13.1 minutes (range: 7-56 minutes) (Table 2).
Table 2.
Characteristics of 21 Hepatocellular Carcinomas (HCCs) Treated with Percutaneous Radiofrequency Ablation (RFA) with or without Percutaneous Ethanol Injection (PEI)
Note.- *Two nodules, either closely contacting large portal vein or stomach, were treated by combined RFA and PEI. LTP = local tumor progression
Comparison between Fluoroscopically Visible vs. Invisible Groups
On the planning fluoroscopy for a RFA, among the 42 viable HCCs with retained iodized oil in the 40 patients, 27 (64.3%) tumors were fluoroscopically visible, whereas 15 (35.7%) were fluoroscopically invisible (Table 3). Among the 27 fluoroscopically visible tumors, 6 were not ablated under biplane fluoroscopy-guidance because of the following reasons: 1) RFA was not performed since the viable HCCs were located in high risk regions (n = 2); 2) RFA was performed under monoplane and US guidance (n = 2); and 3) in cases with multi-nodular HCCs, although the viable tumors with retained iodized oil were visible on fluoroscopy, while other new HCCs were invisible on US (n = 2) (Fig. 2).
Table 3.
Comparison between Fluoroscopically Visible and Invisible Group
Note.- *When retained iodized oil in tumors was visible on both anteroposterior and lateral projection images, and thus was suitable for targeting tumor under guidance of biplane fluoroscopy, they were classified in fluoroscopically visible group. If index tumors were invisible or poorly visible on anteroposterior, lateral or both images, and thus not feasible for targeting tumor, they were regarded as part of fluoroscopically invisible group, †Subphrenic location of tumor was defined when index tumor was either located within 1 cm from diaphragm or abutting diaphragm, ‡Visible group on US was defined when index tumor was conspicuous enough to allow percutaneous US-guided RFA, whereas invisible group was defined when index tumor was inconspicuous, thus unsuitable for percutaneous US-guided RFA. US = ultrasonography, RFA = radiofrequency ablation
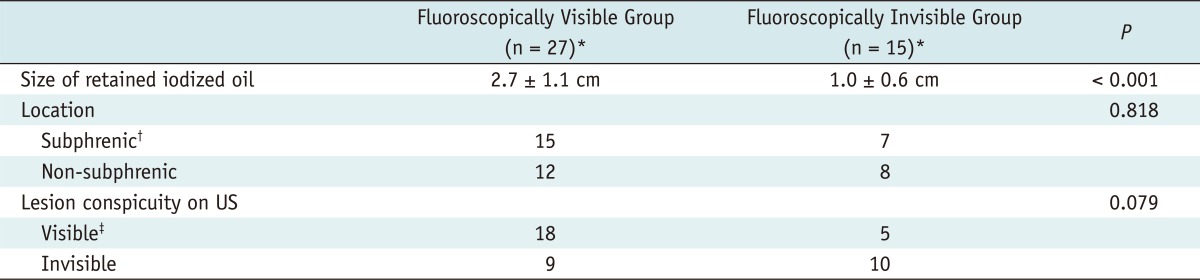
For all 42 viable tumors around the retained iodized oil, which were evaluated by planning fluoroscopy and US, the size (2.7 ± 1.1 cm) of the retained iodized oil in the fluoroscopically visible tumors was significantly larger than that (1.0 ± 0.6 cm) of fluoroscopically invisible tumors (p < 0.001). However, there was no significant difference in the location of tumor (subphrenic or not) between the two groups (p = 0.818). For the lesion conspicuity on US, there was also no significant difference between the two groups (p = 0.079) (Table 3).
Among the 21 viable HCCs around the retained iodized oil which were treated under the guidance of biplane fluoroscopy and US, 12 (57.1%) tumors were located in the subphrenic region and the other 9 (42.9%) in the non-subphrenic region. Although 15 (71.4%) tumors were conspicuous enough for US-guided RFA, the other 6 (28.6%) tumors were invisible on US.
Therapeutic Outcome
On immediate post-RFA CT images, the long and short diameters of the ablation zone were 4.9 ± 1.1 cm and 3.5 ± 1.0 cm, respectively. Primary technical success was achieved in all 21 HCCs. Secondary technical success was achieved in only 17 of 21 (81.0%) HCCs. However, a second RFA session was not performed in any of the 4 patients, because there was no evidence of viable HCCs on CT images. Both the sizes of the retained iodized oil (median, 2.5 cm; range, 0.5-4.2 cm vs. median, 3.8 cm; range, 2.8-4.0 cm; p = 0.066) and total tumor (median, 3.0 cm; range, 1.4-4.5 cm vs. median, 3.8 cm; range, 3.6-5.0 cm; p = 0.140), were slightly smaller in the secondary technical success group than the secondary technical failure group. However, the differences were not statistically significant. Major complications were not observed in any patient.
During follow-up period (20.3 ± 7.4 months; range, 6.5-29.9 months; and median, 22.6 months), the rate of local tumor progression was 9.5% (2/21). Because these two patients with local tumor progression had synchronous distant intrahepatic metastases, repeated TACE was performed for further management. Distant intrahepatic metastasis developed frequently in 76.2% (16/21) of patients.
DISCUSSION
This study demonstrates that biplane fluoroscopy is a feasible and effective guiding modality for percutaneous RFA of a viable HCC around the retained iodized oil by a prior TACE if the retained iodized oil around the tumor is visible on both anteroposterior and lateral projection images. Primary technical success was achieved after a single ablation session in all cases treated by using biplane fluoroscopy guidance assisted by US guidance. In addition, this treatment method showed an acceptable local tumor progression rate of 9.5% (2/21). Despite the small sample size of this study, we believe that this ablation technique can be considered an alternative treatment option for viable HCC around the retained iodized oil after a TACE, because a TACE has poor local efficacy even when it is repeated (4).
Traditionally, CT/CT fluoroscopy and US have been widely used as guiding modalities for percutaneous RFA of HCC. In cases of viable HCCs around the retained iodized oil, since the retained iodized oil provides good contrast on CT images, CT can be used as a guiding modality. Recently, CT fluoroscopy offering real-time imaging guidance for RFA procedure has been used with promising results (8, 23). However, the drawback of CT/CT fluoroscopy guidance includes a relatively high radiation dose to both the patient and operator. In addition, although the transthoracic approach for a dome HCC has been generally accepted to be safe and it can sometimes be avoided by appropriate gantry angling of CT, a pneumothorax can occur in 38-70% of RFA procedures of dome HCCs (24-27).
Compared to CT guidance, our guiding technique using biplane fluoroscopy and US has several advantages (10). First, biplane fluoroscopy allows proper needle placement through the tumor with high confidence, because it provides a real-time orthogonal projection view for the simultaneous delineation of the electrode and the retained iodized oil, with high-resolution and metallic artifact-free images. Second, with biplane fluoroscopic guidance, the radiation dose to the patient and operator is much smaller than that of CT guidance (28). Third, HCCs with retained iodized oil could be targeted even for the hepatic dome lesion by the steep oblique approach, which precludes the transpulmonary approach. In this study, pneumothorax was avoided in all 12 tumors located in subphrenic region by the oblique approach not traversing the thoracic cavity. Using the oblique approach for dome HCC, the hand of the operator was also out of the fluoroscopic field, thus direct exposure of radiation to the operator could be minimized during the RFA procedure.
In this study, we could ablate the viable HCC around retained iodized oil through multiple overlapping treatments, and repositioning of the needle was performed with high confidence whenever overlapping was required. The mean number of overlapping ablations was 4.3 per HCC nodule (range: 1-8). This was possible because the retained iodized oil, which could serve as an anatomic landmark to facilitate targeting, remained nearly unchanged on fluoroscopy during overlapping cycles. However, biplane fluoroscopy does not allow direct visualization of viable HCCs around the retained iodized oil. Therefore, the use of this technique depends on the presence of an accumulation of iodized oil that can be visualized on both anteroposterior and lateral projection images of the biplane fluoroscopy. In this study, 15 (35.7%) of 42 viable HCCs with retained iodized oils were invisible on fluoroscopy, thus precluding this guiding technique. This was due to the small size of the retained iodized oil in the fluoroscopically invisible group, compared to the visible group (mean ± SD, 1.0 ± 0.6 cm vs. 2.7 ± 1.1 cm). This implies that not all retained iodized oil around viable HCCs is radio-opaque enough to allow percutaneous RFA under biplane fluoroscopic guidance. For viable HCCs around retained iodized oil to be ablated by using our method, the retained iodized oil should be large and radio-opaque enough to be visible on fluoroscopy. This is a major drawback of biplane fluoroscopy guidance, compared to CT guidance, in which almost all HCCs with retained iodized oil could be visualized on cross sectional CT images. Meanwhile, if the size of a viable HCC is considerably larger than that of the retained iodized oil, an adequate ablative margin may be difficult to achieve under biplane fluoroscopic guidance. In this study, the mean sizes of the retained iodized oil and viable HCCs ablated by biplane fluoroscopy guidance were 2.8 cm and 1.9 cm, respectively.
For US guidance, microbubbles induced by previous ablation cycles, affect the next placement of electrode if US alone guidance is used. Nevertheless, throughout the procedure, US guidance is also essential in our guiding method to determine the skin entry site of the electrode in order to avoid traversing dangerous structures such as the colon and gallbladder, to estimate the ablation zone during ablation cycles, and to avoid collateral thermal injury to the adjacent structures. However, for targeting and overlapping the index tumor, biplane fluoroscopy was the major guiding modality, whereas US was an accessory guiding modality. In our series, although 6 (28.6%) of 21 tumors were invisible on US, we could ablate those tumors by using our guidance technique. Even though 15 (71.4%) of 21 tumors were visible on US, we also used biplane fluoroscopy as a guiding modality. This was because we believe that targeting and overlapping is more easily facilitated with dual guidance than with US guidance alone. According to a recent study (29), the lesion conspicuity of recurrent HCCs after TACE was suitable for US-guided RFA in only 60.5% of cases. Therefore, US guidance alone may be inadequate for the accurate targeting of viable HCCs with retained iodized oil. Based on this study, the concurrent use of biplane fluoroscopy and US is believed to allow precise access to viable HCCs containing iodized oil.
In this study, although local tumor control was relatively acceptable, distant intrahepatic metastasis developed in more than two thirds of the patients (76.2%, 16/21); this can be explained by the fact that the patients included in this study were initially managed by a TACE, which means that the tumor burden of the patients was not as good as in patients who could be treated by RFA or surgical resection. Even if we completely ablated the viable HCC around retained iodized oil, new HCCs or local tumor progression around other HCCs treated by TACE occurred frequently. Therefore, our treatment method may be applicable to selected patients. For instance, patients with single nodular HCCs larger than 5 cm in diameter could be initially treated with TACE, and then followed by RFA using our method after several months, when the tumor may be shrunken to be smaller than 5 cm. Moreover, our treatment strategy would be also helpful for combined TACE and RFA of intermediate-sized (3-5 cm) HCCs.
Some limitations of this study should be mentioned. First, the number of enrolled patients was relatively small. Second, the fluoroscopy machines used for planning fluoroscopy and RFA guidance were different from each other. In our institution, the biplane fluoroscopy machine was frequently unavailable at the time of planning fluoroscopy because it was occupied for other interventional procedures. Although not analyzed, the visibility of the retained iodized oil on fluoroscopy might have been different between the two machines. Third, all planning US, planning fluoroscopy and RFA procedures were performed by a single radiologist, in a single institution. Therefore, the results of this study might have been influenced by both the experience of the radiologist and the patient population. Finally, the mean follow up period of 20.3 months may be not sufficient for the evaluation of local efficacy. However, this is a preliminary study to evaluate the technical feasibility of biplane fluoroscopy-guided RFA for viable HCCs after TACE. Therefore, a larger cohort of patients and prolonged follow-up period are required to assess its long-term therapeutic efficacy.
In conclusion, biplane fluoroscopy-guided RFA assisted by US guidance is technically feasible and effective for the treatment of viable HCC with retained iodized oil after TACE, if the retained iodized oil around the tumor is visible on both anteroposterior and lateral projection images.
References
- 1.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 2.Saccheri S, Lovaria A, Sangiovanni A, Nicolini A, De Fazio C, Ronchi G, et al. Segmental transcatheter arterial chemoembolization treatment in patients with cirrhosis and inoperable hepatocellular carcinomas. J Vasc Interv Radiol. 2002;13:995–999. doi: 10.1016/s1051-0443(07)61863-6. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 6.Uchida H, Matsuo N, Sakaguchi H, Nagano N, Nishimine K, Ohishi H. Segmental embolotherapy for hepatic cancer: keys to success. Cardiovasc Intervent Radiol. 1993;16:67–71. doi: 10.1007/BF02602980. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012;13:34–43. doi: 10.3348/kjr.2012.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247:260–266. doi: 10.1148/radiol.2471070818. [DOI] [PubMed] [Google Scholar]
- 9.Lee MW, Kim YJ, Park SW, Jeon HJ, Yi JG, Choe WH, et al. Percutaneous radiofrequency ablation of liver dome hepatocellular carcinoma invisible on ultrasonography: a new targeting strategy. Br J Radiol. 2008;81:e130–e134. doi: 10.1259/bjr/16397365. [DOI] [PubMed] [Google Scholar]
- 10.Lee MW, Kim YJ, Park SW, Yu NC, Choe WH, Kwon SY, et al. Biplane fluoroscopy-guided radiofrequency ablation combined with chemoembolisation for hepatocellular carcinoma: initial experience. Br J Radiol. 2011;84:691–697. doi: 10.1259/bjr/27559204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 14.Rhim H, Choi D, Kim YS, Lim HK, Choe BK. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol. 2010;75:253–258. doi: 10.1016/j.ejrad.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim SW, Rhim H, Park M, Kim H, Kim YS, Choi D, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:366–376. doi: 10.3348/kjr.2009.10.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung YH. A strategy for early detection of recurrent hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. Intervirology. 2005;48:46–51. doi: 10.1159/000082094. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol. 1995;2:399–404. doi: 10.1016/s1076-6332(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 20.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S377–S390. doi: 10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, et al. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225–1232. doi: 10.1016/s1051-0443(07)61969-1. [DOI] [PubMed] [Google Scholar]
- 24.Miura H, Yamagami T, Terayama K, Yoshimatsu R, Matsumoto T, Nishimura T. Pneumothorax induced by radiofrequency ablation for hepatocellular carcinoma beneath the diaphragm under real-time computed tomography-fluoroscopic guidance. Acta Radiol. 2010;51:613–618. doi: 10.3109/02841851003786001. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T, Shibata T, Maetani Y, Kubo T, Itoh K, Togashi K, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004;15:1323–1327. doi: 10.1097/01.RVI.0000132297.97113.C4. [DOI] [PubMed] [Google Scholar]
- 26.Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol. 2006;12:608–611. doi: 10.3748/wjg.v12.i4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato T, Yamagami T, Hirota T, Matsumoto T, Yoshimatsu R, Nishimura T. Transpulmonary radiofrequency ablation for hepatocellular carcinoma under real-time computed tomography-fluoroscopic guidance. Hepatogastroenterology. 2008;55:1450–1453. [PubMed] [Google Scholar]
- 28.Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy--guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001;220:161–167. doi: 10.1148/radiology.220.1.r01jl29161. [DOI] [PubMed] [Google Scholar]
- 29.Min JH, Lee MW, Rhim H, Choi D, Kim YS, Kim YJ, et al. Recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: planning sonography for radio frequency ablation. J Ultrasound Med. 2011;30:617–624. doi: 10.7863/jum.2011.30.5.617. [DOI] [PubMed] [Google Scholar]