The goal of this Review is to describe the pathways responsible for the metabolism of alcohol (ethanol) and understand the factors which regulate this oxidation. Understanding pathways of alcohol oxidation is important because it allows us to:
Learn how the body disposes of alcohol and its metabolites.
Discern some of the factors which influence this process.
Learn how alcohol influences the metabolism of nutrients and drugs.
May learn how alcohol damages various organs.
May help to identify individuals who are at increased or decreased risk for alcohol toxicity.
Some suggested causes for alcohol toxicity are linked to changes produced by the metabolism of ethanol such as redox state changes in the NAD+/NADH ratio, acetaldehyde formation, oxidative stress, and mitochondrial function are shown in LIST 1 and will be discussed below. General reviews on alcohol metabolism can be found in (1–9).
Distribution of Alcohol in the Body
The equilibrium concentration of alcohol in a tissue depends on the relative water content of that tissue. Equilibration of alcohol within a tissue depends on the water content, rate of blood flow and the tissue mass Ethanol is practically insoluble in fats and oils, although like water, it can pass through biological membranes. Ethanol distributes from the blood into all tissues and fluids in proportion to their relative content of water. The concentration of ethanol in a tissue is dependent on the relative water content of the tissue, and reaches equilibrium quickly with the concentration of ethanol in the plasma. There is no plasma protein binding of alcohol.
The same dose of alcohol per unit of body weight can produce very different blood alcohol concentrations in different individuals because of the large variations in proportions of fat and water in their bodies, and the low lipid: water partition coefficient of ethanol. Women generally have a smaller volume of distribution for alcohol than men because of their higher percentage of body fat. Women will have higher peak blood alcohol levels than men when given the same dose of alcohol as g per kg body weight but no differences occur when given the same dose per liter of body water. First pass metabolism of alcohol by the stomach, which may be greater in males, may also contribute to the higher blood alcohol levels found in women (10,11).
The breath analyzer test for estimating blood alcohol concentrations is dependent on the diffusion of ethanol from pulmonary arterial blood into the alveolar air. The ethanol vapor in breath is in equilibrium with the ethanol dissolved in the water of the blood at a blood : breath partition coefficient of about 2100:1. An excellent recent review which summarizes many of these pharmacokinetic interactions can be found in (12).
Factors Affecting Alcohol Absorption
LIST 2 describes some factors which affect the absorption of alcohol. Absorption of alcohol from the duodenum and jejunum is more rapid than from the stomach, hence the rate of gastric emptying is an important determinant of the rate of absorption of orally administered alcohol.
Alcohol crosses biological membranes by passive diffusion, down its concentration gradient. Therefore, the higher the concentration of alcohol, the greater is the resulting concentration gradient, and the more rapid is the absorption.
Rapid removal of alcohol from the site of absorption by an efficient blood flow will help maintain the concentration gradient and thereby promote absorption.
Alcohol has irritant properties and high concentrations can cause superficial erosions, hemorrhages and paralysis of the stomach smooth muscle. This will decrease alcohol absorption,
Peak blood alcohol levels are higher if ethanol is ingested as a single dose rather than several smaller doses, probably because alcohol concentration gradient will be higher in the former case.
In general, there is little difference in the rate of absorption of the same dose of alcohol administered in the form of different alcoholic beverage i.e., blood ethanol concentration is not significantly influenced by the type of alcoholic beverage consumed.
The presence of food in the stomach retards gastric emptying and thus will reduce the absorption of alcohol, the “don't drink on an empty stomach” concept. Meals high in either fat, or carbohydrate or protein are equally effective in retarding gastric emptying. The major factor governing the absorption rate of alcohol is whether the drink is taken on an empty stomach or together with or after a meal (13–15).
The blood alcohol concentration is determined by the amount of alcohol consumed, by the presence or absence of food in the stomach, factors which affect gastric emptying and the rate of alcohol oxidation.
First Pass Metabolism of Alcohol in the Stomach
Some of the alcohol which is ingested orally does not enter the systemic circulation but may be oxidized in the stomach by ADH isoforms such as σADH and class I and class III ADH. This first pass metabolism could modulate alcohol toxicity since its efficiency determines the bioavailability of alcohol. Ethanol is rapidly passed into the duodenum from the stomach in the fasted state. This will minimize first pass metabolism and thereby play a role in the higher blood alcohol concentrations observed in the fasted versus the fed state.
First pass metabolism has been reported to be low in alcoholics, especially in alcoholic women because of decreased ADH activity. This may be important in the increased sensitivity to alcohol and the higher blood alcohol concentrations in women than in men after an equivalent oral dose of ethanol. Several drugs, including H2 receptor blockers such as cimetidine or ranitidine, or aspirin inhibit stomach ADH activity. This will decrease first pass metabolism by the stomach, and hence, increase blood alcohol concentrations.
The overall significance of first pass metabolism by the stomach is controversial. The speed of gastric emptying modulates gastric and hepatic first pass metabolism of alcohol. Considering the greater levels of alcohol metabolizing enzymes in the liver compared to the stomach, it seems likely that liver plays the major role in alcohol metabolism (16–18).
Alcohol Metabolism-General Principles (1–9)
LIST 3 describes some general principles of alcohol metabolism.
The major enzyme system(s) responsible for the oxidation of ethanol, alcohol dehydrogenase, and to a lesser extent, the cytochrome P450-dependent ethanol-oxidizing system, are present to the largest extent in the liver. Liver damage lowers the rate of alcohol oxidation and hence, elimination from the body. Ethanol is a nutrient and has caloric value (about 7 kcal per gram; carbohydrates and protein produce 4 kcal per gram, while fat produces 9 kcal). However, unlike carbohydrates (glycogen in liver and muscle) and fat (triglycerides in adipose tissues and liver) which can be stored and utilized in time of need e.g. fasting, alcohol is not stored and remains in body water until eliminated. Whereas metabolism of the major nutrients is under hormonal control, e.g insulin/glucagon, leptin, catecholamine, thyroid hormones, generally, there is little hormonal regulation to pace the rate of alcohol elimination. In view of these considerations, there is a major burden on the liver to oxidize alcohol in order to remove this agent from the body.
Animals with small body weight metabolize alcohol at faster rates than larger animals e.g. the rate of alcohol elimination in mice is 5 times greater than the rate in humans. These rates of alcohol metabolism correlate with the basal metabolic rate for that species, indicating that the capacity to oxidize ethanol parallels the capacity to oxidize the typical nutrients. However, it is important to note that alcohol-derived calories are produced at the expense of the metabolism of normal nutrients since alcohol will be oxidized preferentially over other nutrients (19–23).
Kinetics of Alcohol Elimination In-vivo (12–14)
Alcohol elimination was originally believed to be a zero-order process, meaning that alcohol was removed from the body at a constant rate, independent of the concentration of alcohol. Since the Km of most ADH isozymes for ethanol is low (about 1 mM), ADH is saturated at low concentrations of alcohol, hence, the overall elimination process proceeds at maximal velocity and is independent of the alcohol concentration. However, linearity is not observed at low alcohol concentration since ADH is no longer saturated with ethanol. Alcohol elimination now follows Michaelis-Menten kinetics; the rate of change in the concentration of alcohol depends on the concentration of alcohol and the kinetic constants Km and Vmax (23,24).
In addition, because the metabolism of alcohol by CYP2E1 and some ADH isozymes, such as ADH4 involves a high Km for alcohol system, a concentration-dependent rate of ethanol elimination can be observed, with higher rates of alcohol elimination at higher blood alcohol concentrations. Because of this concentration dependence, it is not possible to estimate one single rate of alcohol metabolism. Concentration-dependent metabolism of alcohol has been observed in some, but not all studies on alcohol elimination (25,26).
Although rates vary widely, the “average” metabolic capacity to remove alcohol is about 170 to 240 g per day for a person with a body weight of 70 kg. This would be equivalent to an average metabolic rate of about 7 g/hr which translates to about one drink per hr. Since alcoholics may consume 200 to 300 g of ethanol per day, equivalent to 1400 to 2100 kcal, consumption of normal nutrients is usually significantly decreased (typically, 2000–3000 kcal consumed per day in the absence of alcohol).
Factors Modifying the Alcohol Elimination Rate
There is a 3–4 fold variability in the rate of alcohol elimination by humans because of various genetic and environmental factors described below.
Sex
There is a faster rate of alcohol elimination by women when rates are corrected for lean body mass. Since women have smaller body size and therefore smaller lean body mass, ethanol elimination per unit lean body mass is higher in women. Men and women generally have similar alcohol elimination rates when results are expressed as g per hr or g per liter liver volume. Because of first pass metabolism by the stomach, it is possible that a given oral dose of alcohol may produce a higher blood ethanol concentration in females than males (11,15).
Age
Very young animals have low alcohol elimination rates because ADH (and CYP2E1) are not fully expressed. Fetal liver eliminates alcohol very poorly which may have consequences for fetal alcohol syndrome. There may be a small decline in alcohol elimination with aging, perhaps due to decreased liver mass, or body water content.
Race
Alcohol elimination is reported to be somewhat higher in subjects expressing the beta3 class I ADH isoforms compared with individuals who only express the beta 1 isoform (see ADH alleles discussed below). Some studies, but not all, suggest an increased rate of alcohol elimination by native Americans compared to Caucasians. Rates of alcohol elimination by Chinese are similar to those of Caucasians. Liver mass may explain ethnic and gender differences in alcohol elimination rates. More research on possible population differences in alcohol elimination is required (27,28).
Food
Alcohol metabolism is higher in the fed nutritional state as compared to the fasted state because ADH levels are higher, and the ability of substrate shuttle mechanisms (see below) to transport reducing equivalents into the mitochondria is elevated. Food may also increase liver blood flow. The sugar fructose increases alcohol metabolism by providing substrates which help to convert NADH to NAD+, and by enhancing mitochondrial oxygen uptake.The increase in the alcohol elimination rate by food was similar for meals of different compositions as there was no difference between carbohydrate, fat and protein on alcohol metabolic rate (29–31).
Biological Rhythms
The rate of alcohol elimination varies with the time of day, being maximal at the end of the daily dark period. This may be related to a body temperature cycle.
Exercise
unclear literature, most studies report a small increase in alcohol elimination rate, perhaps due to increased body temperature or catecholamine release.
Alcoholism
Heavy drinking increases alcohol metabolic rate ( see below). Advanced liver disease will decrease the rate of ethanol metabolism.
Drugs
Agents which inhibit ADH (pyrazoles, isobutyramide) or compete with ethanol for ADH (methanol, ethylene glycol) or which inhibit the mitochondrial respiratory chain will decrease the alcohol elimination rate. Antabuse (disulfiram) by inhibiting the elimination of acetaldehyde slows alcohol metabolism.
Scheme for Alcohol Metabolism
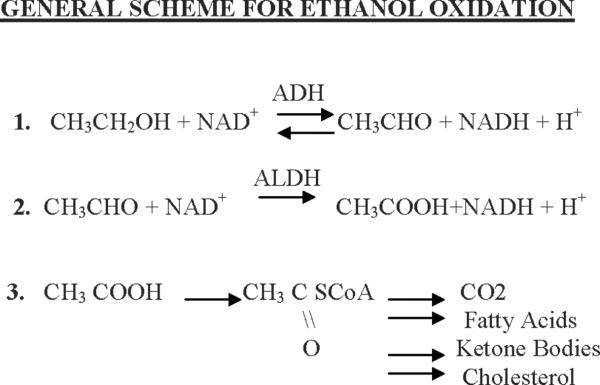
Fig 1 summarizes the basic overall metabolism of alcohol.
Fig 1.
General scheme for alcohol oxidation. Alcohol is oxidized by alcohol and aldehyde dehydrogenases eventually to acetyl CoA. Depending on the nutritional, hormonal, energetic status, the acetyl CoA is converted to the indicated products.
-
Step 1
catalyzed by the enzyme alcohol dehydrogenase, which is present largely in the liver, and consists of a family of isoforms. A vitamin-related cofactor, nicotinamide adenine dinucleotide ( NAD) (derived from the vitamin niacin) is required to accept reducing equivalents (hydrogen atoms and electrons) from the alcohol. As a result, the ethanol is oxidized to the product acetaldehyde and the vitamin cofactor, NAD+ is reduced to the product NADH + H+ (note two hydrogens are removed from alcohol). The ADH reaction is reversible.
-
Step 2
is catalyzed by the enzyme aldehyde dehydrogenase. Acetaldehyde is oxidized to acetate; NAD+ is the cofactor, and is reduced to NADH. The ALDH reaction is essentially irreversible. Much of the acetaldehyde produced from the oxidation of alcohol is oxidized in the liver to acetate; circulating levels of acetaldehyde are low under normal conditions.
-
Step 3
Much of the acetate produced by the oxidation of acetaldehyde leaves the liver and circulates to peripheral tissues where it is activated to a key Acetyl CoA. Acetyl CoA is also the key metabolite produced form all major nutrients- carbohydrate, fat and excess protein. Thus, carbon atoms from alcohol wind up as the same products produced from the oxidation of carbohydrate, fat, and protein, including CO2, fatty acids, ketone bodies, and cholesterol; which products are formed depends on the energy state and the nutritional and hormonal conditions.
ALCOHOL DEHYDROGENASE (4,32–34)
ADH is a zinc-containing enzyme, consisting of two subunits of 40 kDa each. It functions to oxidize endogenous alcohol produced by microorganisms in the gut, to oxidize exogenous ethanol and other alcohols consumed in the diet, and to oxidize substrates involved in steroid and bile acid metabolism. The enzyme has broad substrate specificity, oxidizing many primary or secondary alcohols. ADH is localized in the cytosolic fraction of the cell. ADH is found in highest amount in the liver, followed by GI tract, kidneys, nasal mucosa, testes, and uterus.
Multiple forms of ADH exist in human liver and their properties are reviewed in TABLE 1. CLASS 1 ADH contains three genes, ADH1, ADH2 and ADH3 which code for the following subunits α(ADHIA), β1, β2 and β3(ADHIB),and γ1 and γ2(ADH1C). These different subunits and polymorphic forms can combine to produce a variety of homo-or hetero-dimers e.g., αα, β1β1, αβ2. The forms are found primarily in the liver. The class I ADH forms are mainly responsible for the oxidation of alcohol. In a new classification, the family members have been classified into five distinct classes, designated ADH1 – ADH5, on the basis of the structural and kinetic characteristics. Human ADH genes that encode the subunit polypeptides α, β1, β2, β3, γ1, γ2, π, χ and (or named σ) are designated ADH1A (old ADH1), ADH1B*1 (old ADH2*1), ADH1B*2 (old ADH2*2), ADH1B*3 (old ADH2*3), ADH1C*1 (old ADH3*1), ADH1C*2 (old ADH3*2), ADH2(old ADH4), ADH3(old ADH5) and ADH4 (old ADH7), respectively. The ADH5 (old ADH6)-encoding polypeptide has not been given a Greek letter.
TABLE 1.
Kinetic constants for human liver ADH isoforms
| Constant | α α | β1β1 | β2β2 | β3β3 | γ1γ1 | γ2γ2 | π π |
|---|---|---|---|---|---|---|---|
| KmNAD+ μM | 13 | 7.4 | 180 | 530 | 7.9 | 8.7 | 14 |
| Km ethanol, mM | 4.2 | 0.049 | 0.94 | 24 | 1 | 0.63 | 34 |
| Ki 4-methylpyrazole, μM | 1.1 | 0.13 | - | 2.1 | 0.1 | - | 2000 |
| Vmax min−1 | 27 | 9.2 | 400 | 300 | 87 | 35 | 20 |
| pH-optimum | 10.5 | 10.5 | 8.5 | 7.0 | 10.5 | 10 | 10.5 |
CLASS II ADH
The ADH4 gene codes for the π subunit, which produces ππ homodimers in the liver and to a lesser extent in kidney and lung. The high Km for alcohol may make this enzyme more important in metabolism of high concentrations of alcohol.
CLASS III ADH
The ADH5 gene codes for the χ subunit which produces χχ homodimers. This isoform has a very high Km for alcohol (>2 M).
CLASS V ADH
The mRNA product produced by the ADH6 gene is present in liver and stomach, but the protein has not been characterized.
CLASS IV ADH
The ADH7 gene encodes the sigma subunit which is very efficient in oxidizing retinol to retinal. This form is present in the stomach.
The class I ADH isoforms play the most important role in alcohol oxidation (33–37). ADH is present in low levels in fetal liver and the fetus eliminates ethanol very slowly because of this late maturation of ADH genes. The ability to form many isoforms, with varying kinetic properties, probably contributes to the large variability in the capacity for metabolizing alcohol that human populations exhibit. The strong sensitivity of the Class I ADH to pyrazole inhibition explains the powerful inhibition of alcohol metabolism by these agents.
Control of ADH activity is complex and involves:
-
a)
dissociation of the product NADH is rate limiting step
-
b)
subject to product inhibition by NADH and acetaldehyde
-
c)
subject to substrate inhibition by high concentrations of ethanol
Alcohol oxidation is generally limited by the maximum capacity of ADH. The amount of ADH in the liver is greater in the fed than the fasted state which plays a major role in the increased rate of alcohol oxidation in the fed state (38,39). Inhibitors of ADH inhibit ethanol oxidation in direct proportion to their potency as inhibitors of ADH. Hormonal effects on ADH are complex; some stimulation is found after treatment with growth hormone, epinephrine or estrogens. Thyroid hormones and androgens inhibit ADH activity.
The polymorphic forms of ADH (Class I ADH1B, ADH1C) vary to some extent in different racial groups as shown in TABLE 2. To date, there are no clear associations between the various ADH isozymes and the development of alcoholic liver disease, or the susceptibility to alcohol actions, or the propensity to consume ethanol. Studies which have investigated the association between alcoholism and alcohol-induced liver damage with the ADH2, ADH3, CYP2E1 and ALDH2 polymorphisms are not conclusive. A large meta-analysis (36), showed that carriers of the ADH2*1 and ADH3*2 alleles, the less active ethanol metabolizing alcohol dehydrogenases, and the highly active ALDH2*1allele had an increased risk of alcoholism. This likely reflects low accumulation of acetaldehyde in these individuals. In liver disease, ALDH2*1 is a protective factor as it removes toxic acetaldehyde. Neither the ADH2 nor the ADH3 polymorphism were implicated in the development of liver disease. Allelic variants of CYP2E1 were not involved in determining the risk of alcoholism or in alcoholic liver disease. Further research in this area is required, as is research on what other substrates the various ADH isoforms oxidize.
TABLE 2.
Frequency of ADH Alleles in Racial Populations
| ADH1B*1 | ADH1B*2 | ADH1B*3 | ADH1C*1 | ADH1C*2 | |
|---|---|---|---|---|---|
| White-American | >95% | <5% | <5% | 50% | 50% |
| White-European | 85% | 15% | <5% | 60% | 40% |
| Japanese | 15% | 85% | <5% | 95% | 5% |
| Black-American | 85% | <5% | 15% | 85% | 15% |
Hepatic Redox State (40–42)
Because the ADH and ALDH2 reactions reduce NAD+ to NADH, the cellular NAD+/NADH redox ratio is lowered as a consequence of ethanol metabolism. This has profound effects on other liver metabolic pathways which require NAD+ or are inhibited by NADH.
Since the ADH reactions occur in the cytosol, the cytosolic NAD+/NADH redox ratio will be lowered. This ratio is reflected by the pyruvate/lactate ratio because of the reaction.
Since the ALDH reaction occurs largely in the mitochondria, the mitochondrial NAD+/NADH redox ratio will be lowered. This reaction is reflected by the beta hydroxybutyrate/acetoacetate ratio because of the reaction.
Important reactions inhibited because of this decreased NAD+/ NADH redox ratio are
Glycolysis
Citric Acid Cycle (ketogenesis favored)
Pyruvate Dehydrogenase
Fatty Acid Oxidation
Gluconeogenesis
Reoxidation of NADH Generated by the ADH Reaction
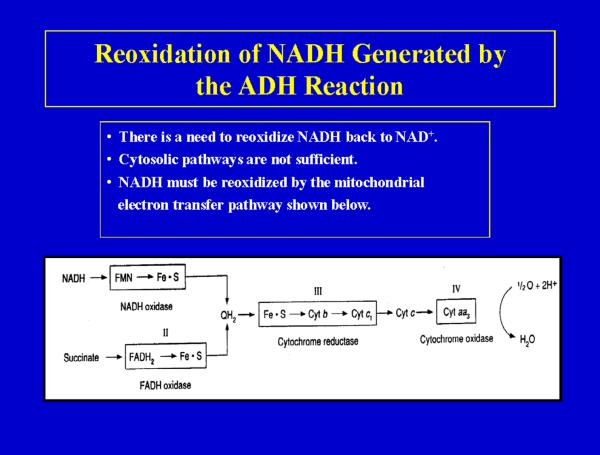
To maintain effective rates of alcohol oxidation by ADH, it is important to regenerate NAD+ from the NADH produced by the ADH reaction. Under certain conditions, the rate of oxidation of alcohol can be limited by the reoxidation of NADH. The major system for reoxidizing NADH is the mitochondrial electron transfer system. By coupling NADH reoxidation to this system, energy will be produced from alcohol metabolism (7 kcal per g ethanol). Fig 2 shows the typical mitochondrial respiratory chain found in all tissues except the red blood cell. Note the 4 complexes which make up the chain. As electrons or reducing equivalents pass through complexes I, III and IV, an energized electrochemical and pH gradient is developed which is used to synthesize ATP via complex V, the ATP synthase ( 43,44).
Fig 2.
The mitochondrial respiratory chain. Reducing equivalents (electrons) enter the respiratory chain either from NADH or from succinate and are passed through a series of electron carriers to cytochrome oxidase which reacts with molecular oxygen to produce water. The NADH produced from the oxidation of alcohol by alcohol dehydrogenase is oxidized by the respiratory chain. Energy, in the form of ATP, is produced during this oxidation, hence, alcohol is of caloric value.
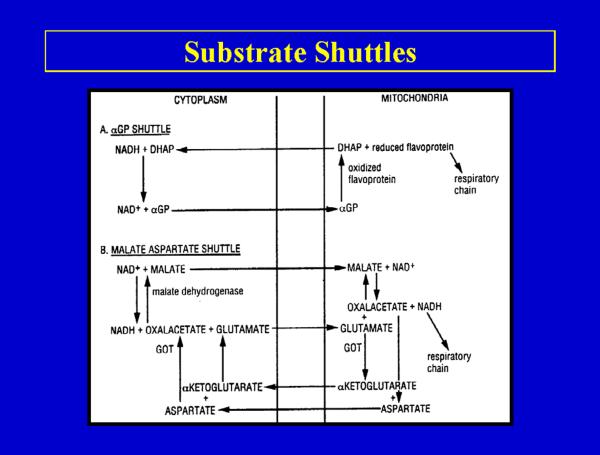
Substrate Shuttles
Because intact mitochondria are not permeable to NADH, it is necessary to transfer the reducing equivalents of NADH present in the cytosol into the mitochondria by substrate shuttle mechanisms. The two major substrate shuttles are the -glycerophosphate shuttle and the malate-aspartate shuttle (Fig 3). The malate-aspartate shuttle plays the major role in transferring reducing equivalents into the mitochondria (45–48). The rate of alcohol oxidation can be limited by the transfer of reducing equivalents into mitochondria or by the actual capacity of the respiratory chain to oxidize these reducing equivalents. Shuttle capacity may become limiting under fasting metabolic states as the levels of shuttle components decrease. This may contribute to the lower rates of alcohol oxidation (in addition to lower ADH content) in the fasting metabolic state. Agents or conditions which enhance reoxidation of NADH by the respiratory chain can increase the rate of alcohol metabolism e.g. uncoupling agents can accelerate ethanol oxidation in the fed metabolic state (38,39).
Fig 3.
Substrate shuttle mechanisms for the reoxidation of NADH by the mitochondrial respiratory chain. The alcohol dehydrogenase reaction oxidizes alcohol in the liver cytosol and therefore produces NADH in the cytosol. This NADH cannot directly enter the mitochondria for oxidation (Fig 2) and therefore has to be transported into the mitochondria by either the α-glycerophosphate (a) or the malate-aspartate (b) shuttle.
Catalase-Dependent Oxidation of Alcohol
Catalase, a heme containing enzyme, is found in the peroxisomal fraction of the cell. This is an important antioxidant enzyme since it normally catalyzes the removal of H2O2 (reaction b above) but it can also oxidize alcohol as shown in reaction (a) above. This pathway is limited by the rather low rates of H2O2 generation produced under physiological cellular conditions (less than 4 umol/g liver/hr, only 2% that of alcohol oxidation) and appears to have an insignificant role in alcohol oxidation by the liver.
A number of the central nervous system effects of ethanol are mediated by acetaldehyde. Because circulating acetaldehyde levels are very low, the metabolism of alcohol to acetaldehyde by the brain has been a major research area in alcohol research. Catalase is present throughout the brain, in the peroxisomes. Inhibitors of catalase were reported to depress oxidation of alcohol to acetaldehyde by the brain. Acetaldehyde derived from catalase-dependent oxidation of alcohol in the brain has been suggested to play a role in the development of tolerance to alcohol, to voluntary ethanol consumption and to the positive reinforcing actions of ethanol, perhaps via interaction with catecholamines to produce various condensation products (49–51).
Microsomal (Cytochrome P450) Oxidation of Ethanol
Cytochrome P450s are a family of heme enzymes which are involved in the oxidation of steroids, fatty acids, and numerous xenobiotics ingested from the environment. Highest levels of cytochrome P450 are in the liver, where they are present mainly in the endoplasmic reticulum (microsomal fraction). Some P450's are also found in mitochondria. P450 functions in conjunction with other microsomal enzymes such as NADPH-cytochrome P450 reductase and cytochrome b5 (52–54).There are many isoforms of P450; over 100 gene families have been identified. The P450s arranged in families based on sequence homologies. CYP2E1 is a P450 which has the highest activity for oxidizing alcohol to acetaldehyde. Besides ethanol, CYP2E1 can oxidize many other compounds including acetone, benzene, and other alcohols. A clear physiological function for CYP2E1 has not been identified. Some of the significant properties of CYP2E1 are listed in LIST 4 (55–58).
The Km of CYP2E1 for alcohol is 10 mM ,10-fold higher than the Km of ADH for ethanol but still within the range of alcohol concentrations seen in social drinking. At low alcohol concentrations, CYP2E1 may account for about 10% of the total alcohol oxidizing capacity of the liver. However in view of its higher Km, the relevance of CYP2E1 in ethanol oxidation increases as blood alcohol concentrations increase. Alcohol oxidation increases at higher ethanol concentrations, and much of this increase is due to CYP2E1 metabolism of alcohol Many P450s are induced by their substrates; this helps to remove the xenobiotic from the body. CYP2E1 levels are increased by chronic ethanol administration by a mechanism largely involving protection of the enzyme against proteolysis by the macromolecular proteasome complex. CYP2E1 is also induced in diabetics, in the fasted nutritional state and by certain drugs. Because of its inducibility, CYP2E1 may play an important role in alcohol metabolism after chronic ethanol consumption, i.e. in alcoholics. As many as 13 different CYP2E1 polymorphisms have been identified. Some of these may be important as risk factors for carcinogenicity of tobacco or certain toxins; however, there is no evidence linking any of these polymorphisms to the frequency of alcohol liver damage.
Alcohol-Drug Interactions
Since ethanol and certain drugs compete for metabolism by CYP2E1, active drinkers will often display an enhanced sensitivity to certain drugs as alcohol will inhibit the metabolism of the drug and thereby prolong its half-life. Conversely, since CYP2E1 is induced after chronic alcohol consumption, metabolism of drugs which are also substrates for CYP2E1 will be increased. This will decrease the half-life of the drug, and thus decrease the effectiveness of the drug when ethanol is not present. CYP2E1 is very active in oxidizing many chemicals to reactive intermediates, e.g. carbon tetrachloride, benzene, nitrosamines, acetaminophen, halothane. Toxicity of these agents is enhanced in alcoholics (55,57–59).
The CYP2E1 catalytic turnover cycle results in the production of large amounts of reactive oxygen intermediates such as the superoxide radical and hydrogen peroxide. This may be important in mechanisms of alcoholic liver injury involving oxidative stress (60). Regulation of CYP2E1 is complex involving transcription, translational and protein turnover mechanisms.
Metabolic Adaptation (Tolerance)
Besides CNS adaptation, alcoholics (in the absence of liver disease) often display an increased rate of blood ethanol clearance. This is metabolic tolerance or adaptation. Suggested mechanisms for this metabolic tolerance are shown in LIST 5 (55,61–63).
Class I ADH is not inducible. Further work with the many human isoforms is needed.
Substrate shuttle capacity and transport of reducing equivalents into the mitochondria is not altered by chronic alcohol consumption.
A major theory to explain metabolic adaptation – the “Hypermetabolic state hypothesis “ – postulates that changes in thyroid hormone levels increases (Na+ + K+)-activated ATPase, with the subsequent increase of ADP levels. This increases the state 3 mitochondrial oxygen consumption, therefore, increasing NADH reoxidation. Increased oxygen consumption may cause hypoxia, especially to hepatocytes of zone 3 of the liver acinus, the region where alcohol toxicity originates (centrilobular hypoxia hypothesis).
CYP2E1 levels are enhanced after alcohol treatment Since CYP2E1 is the most active P450 for oxidizing alcohol, this may play an important role in metabolic tolerance.
Ethanol, perhaps via increasing endotoxin levels, may activate non-parenchymal cells such as Kupffer cells to release mediators (cytokines and prostaglandins) which stimulate oxygen consumption, thereby NADH reoxidation, by parenchymal cells.
The so-called swift increase in alcohol metabolism (SIAM) refers to an increased rate of ethanol metabolism within a few hours after alcohol administration in vivo or in vitro. Mechanisms responsible for SIAM are quite complex and appear to involve three major pathways, the mitochondria, the peroxisome and endotoxin activation of Kupffer cells (64).
Zonal Metabolism of Alcohol in the Hepatic Acinus (65–67)
Liver injury after chronic alcohol treatment originates in the perivenous zone of the hepatic lobule. Possible factors to explain this include:
-
1.
Oxygenation is low in this zone since there is an oxygen gradient across the liver lobule and less oxygen reaches the hepatocytes in the perivenous zone. This is exacerbated after chronic alcohol administration which increases hepatic oxygen uptake, so even less oxygen reaches perivenous hepatocytes
-
2. & 3-
ADH and ALDH2, and rates of alcohol and acetaldehyde metabolism are evenly distributed across the liver lobule. However, because of the lower oxygen tension, there is a more pronounced reduction of the hepatic redox state produced by ethanol in the perivenous zone
-
4.
CYP2E1 is largely in the perivenous zone which explains why toxicity of drugs metabolized by CYP2E1 to reactive metabolites, e.g. CCl4, or acetaminophen occurs in the perivenous zone.
-
5.
Level of antioxidants, such as glutathione are lower in the perivenous zone.
Other Pathways of Alcohol Metabolism
1. Conjugation reactions
Ethanol can react with glucuronic acid to form ethylglucuronide. Such soluble conjugates are readily excreted. Cofactor availability and the poor affinity for alcohol by most conjugation enzymes limit these pathways. Ethyl glucuronide (68) is a non-volatile, water-soluble direct metabolite of ethanol. It can be detected in body fluids, tissue, sweat and hair for an extended time after alcohol has been eliminated from the body. These led to the suggestion that ethyl glucuronide may be a marker for alcohol consumption or for the detection of relapse of alcoholics. Ethyl glucuronide is not detectable in abstinent patients, non-drinkers or teetotalers and is thus specific for alcohol consumption.
3. Fatty Acyl Synthases
Fatty acid ethyl ester synthases catalyze the reaction between ethanol and a fatty acid to produce a fatty acyl ester. These synthases are present in most tissues, especially the liver and pancreas, organs most susceptible to alcohol toxicity (69). These esters are synthesized in the endoplasmic reticulum, and transported to the plasma membrane and then removed from the cell by binding to lipoproteins and albumin and transported in the circulation. Fatty acid ethyl esters can be toxic, inhibiting DNA and protein synthesis. When oxidative metabolism of ethanol is blocked, there is an increase in ethanol metabolism to the fatty acid ethyl ester. These esters can be detected in the blood after alcohol is no longer detectable and therefore detection of fatty acid ethyl esters may serve as a marker of alcohol intake.
Acetaldehyde Metabolism
The balance between the various ADH and ALDH isoforms regulates the concentration of acetaldehyde, which is important as a key risk factor for the development of alcoholism (70–74). Most of the acetaldehyde produced from the oxidation of alcohol is further oxidized in the liver by a family of ALDH isoforms. Major ALDH isoforms exist in the mitochondrial, microsomal, and cytosolic compartments. Mitochondria contain a low Km ALDH in the matrix space (class II ALDH) and a high Km ALDH in the outer membrane, microsomes contain a high Km ALDH, while the cytosol contains an intermediate (class I ALDH) and a high Km (class III ALDH) ALDH. Acetaldehyde can also be oxidized by aldehyde oxidase, xanthine oxidase, and by CYP2E1, but these are insignificant pathways. The low Km mitochondrial ALDH oxidizes most of the acetaldehyde produced from the oxidation of alcohol, although in human liver, the class I cytosolic ALDH may also contribute (75). The class I and II ALDHs are tetrameric enzymes, with subunit molecular weights of 54 kDa.
In general, the capacity of ALDH to remove acetaldehyde exceeds the capacity of acetaldehyde generation by the various pathways of alcohol oxidation. Therefore, circulating levels of acetaldehyde are usually very low. Chronic alcohol consumption decreases acetaldehyde oxidation, either due to decreased ALDH2 activity or to impaired mitochondrial function. Acetaldehyde generation is increased by chronic alcohol consumption because of metabolic adaptation. As a result, circulating levels of acetaldehyde are usually elevated in alcoholics because of increased production, decreased removal or both.
The basis of action for certain alcohol-aversive drugs such as disulfiram (Antabuse) or cyanamide is to inhibit ALDH, and therefore alcohol oxidation. The resulting accumulation of acetaldehyde causes a variety of unpleasant effects such as nausea, sweating, vomiting, and increased heart rate, if ethanol is consumed with these drugs. Certain individuals, usually of Asian extraction, have an inactive mitochondrial ALDH2 becauseof a single amino acid substitution; glutamate 487 is converted to a lysine residue; this causes a large decrease in affinity for the NAD+ cofactor. Thus inactive enzyme can be found in 15 to 40% of the population of East Asia and when these individuals consume ethanol, blood levels of acetaldehyde are 5-to 20- fold higher than those found in individuals with the active ALDH allele. Individuals with the inactive ALDH show marked vasodilator, nausea and dysphasia when consuming alcohol, and are virtual abstainers if homozygous for the ALDH2*2 allele. Acetaldehyde is poorly eliminated by these individuals and as a consequence, little alcohol is consumed. ALDH2 deficient individuals are at lower risk for alcoholism. They may have possible increased risk for liver damage if alcohol continues to be consumed.
Acetaldehyde is a reactive compound and can interact with thiol and amino groups of amino acids in proteins. Formation of acetaldehyde adducts with proteins may cause inhibition of that protein's function and/or cause an immune response (73,74). ALDH is important not only for removing acetaldehyde, but also for the removal of other aldehydes, including biogenic aldehydes and lipid peroxidation-derived aldehydes. Effective removal of acetaldehyde is important not only to prevent cellular toxicity, but also to maintain efficient removal of alcohol, e.g., acetaldehyde is a product inhibitor of ADH. The class I ALDH can oxidize retinal to retinoic acid; the possibility that high levels of acetaldehyde compete with retinal for oxidation by class I ALDH may be of developmental significance (75).
Future Considerations
While much has been learned about the pathways of ethanol metabolism and how these pathways are regulated, there are many critical questions remaining. For example:
What limits and regulates alcohol metabolism in-vivo?
What is the mechanism(s) responsible for metabolic tolerance?
Is it alcohol per se, or alcohol-derived metabolites which play a key role in organ damage? What might be the consequences of attempting to accelerate ethanol metabolism?
What is the role, if any, of the various ADH isoforms in oxidation of endogenous substrates, alcohol metabolism and alcohol toxicity? The hypothesis that alcohol or acetaldehyde inhibit the oxidation of physiologically important endogenous substrates of ADH or ALDH2 and that this may contribute to the adverse action of ethanol requires further study.
Can the various ADH and ALDH isozymes or polymorphic forms of CYP2E1 be of predictive value or serve as markers to identify individuals who are susceptible to developing alcoholism? Can non-invasive probes be developed to measure the various isoforms present?
Are there population and gender differences in rates of alcohol elimination, and if so, are such differences explained by the varying isoforms present in that population?
What controls the expression of the various isoforms at the transcriptional level, and are there posttranscriptional modifications? What dictates the turnover of these enzymes which may be important in regulating the amount of active enzyme present in the cells, e.g. CYP2E1?
Why are calories from alcohol not as efficient in providing energy as are calories from typical nutrients? What is the mechanism by which food increases alcohol metabolism?
What role, if any, does acetate play in the metabolic actions of alcohol?
Can we build appropriate models and rate equations to kinetically describe the process of alcohol elimination under various conditions? [author query: the Guest Editor has requested this section be replaced with a “conclusions” paragraph.]
KEY POINTS
The equilibrium concentration of alcohol in a tissue depends on the relative water content of that tissue.
The rate of alcohol absorption depends on the rate of gastric emptying, the concentration of alcohol and is more rapid in the fasted state.
The blood alcohol concentration is determined by the amount of alcohol consumed,the presence or absence of food and the rate of alcohol metabolism.
First pass metabolism of alcohol occurs in the stomach and is decreased in alcoholics.
Liver alcohol dehydrogenase is the major enzyme system for metabolizing alcohol; this requires the cofactor NAD and the products produced are acetaldehyde and NADH.
The acetaldehyde is further oxidized to acetate, the same final metabolite produced from all other nutrients-carbohydrates, fats and proteins; the acetate can be converted to CO2, fatty acids, ketone bodies, cholesterol and steroids.
Oxidation of alcohol by cytochrome P450 pathways, especially CYP2E1 which is induced by alcohol, are secondary pathways to remove alcohol especially at high concentrations.
Alcohol metabolism is regulated by the nutritional state, the concentration of alcohol,specific isoforms of alcohol dehyrogenase, need to remove acetaldehyde and regenerate NAD and induction of CYP2E1.
Substrate shuttles and the mitochondrial respiratory chain are required to regenerate NAD from NADH, and this can limit the overall rate of alcohol metabolism.
Metabolism of alcohol is increased in alcoholics without liver disease: this metabolic tolerance to alcohol may involve induction of CYP2E1, elevated regeneration of NAD or endotoxemia.
SYNOPSIS.
This review describes the pathways and factors which modulate blood alcohol (alcohol and ethanol are used interchangeably) levels and alcohol metabolism and describe how the body disposes of alcohol. The various factors which play a role in the distribution of alcohol in the body, influence the absorption of alcohol and contribute to first pass metabolism of alcohol will be described. Most alcohol is oxidized in the liver and general principles and overall mechanisms for alcohol oxidation will be summarized. The kinetics of alcohol elimination in-vivo and the various genetic and environmental factors which can modify the rate of alcohol metabolism will be discussed. The enzymatic pathways responsible for ethanol metabolism, in particular, the human alcohol dehydrogenase alleles will be described. Rate-limiting steps in the overall metabolism of ethanol, including the activity of alcohol dehydrogenase isoforms, and the necessity to reoxidize NADH by substrate shuttle pathways and the mitochondrial respiratory chain will be discussed. The impact of alcohol metabolism on other liver metabolic pathways, and on cytochrome P450-dependent metabolism of xenobiotics and drugs will be briefly described. Factors playing a role in the metabolic adaptation i.e., increased rate of ethanol metabolism by chronic alcoholics will be discussed. The metabolism and role of acetaldehyde in the toxic actions of alcohol and ethanol drinking behavior will be discussed. Despite much knowledge of alcohol pharmacokinetics and metabolism, numerous questions remain for further evaluation and research, including what regulates alcohol metabolism in-vivo, the role of alcohol metabolites in organ damage, functions and physiological substrates of the various ADH isoforms, population and gender differences in alcohol metabolism, need for developing markers to identify individuals susceptible to alcohol and other considerations are discussed.
LIST 1.
SOME SUGGESTED CAUSES FOR ALCOHOL TOXICITY
Redox state changes in the NAD/NADH ratio
Acetaldehyde formation
Mitochondrial damamge
Cytokine formation (TNFα)
Kupffer cell activation
Membrane actions of ethanol
Hypoxia
Immune actions
Oxidative stress
LIST 2.
Factors Affecting Alcohol Absorption
Concentration of alcohol
Blood flow at site of absorption
Irritant properties of alcohol
Rate of ingestion
Type of beverage
Food
LIST 3.
GENERAL PRINCIPLES OF ALCOHOL OXIDATION
< 10 % alcohol excreted in breath, sweat and urine.
~ 90 % alcohol removed by oxidation.
Most of this alcohol oxidation occurs in the liver.
Alcohol cannot be stored in the liver.
No major feedback mechanisms to pace the rate of alcohol metabolism to the physiological conditions of the liver cell.
LIST 4.
CYTOCHROME P4502E1 (CYP2E1)
A minor pathway for alcohol metabolism
Produces acetaldehyde, 1-hydroxyethyl radical
Responsible for alcohol-drug interactions
Activates toxins such as acetaminophen,CCl4, halothane,benzene,halogenated hydrocarbons to reactive toxic intermediates
Activates procarcinogens such as nitrosamines, azo compounds to active carcinogens
Activates molecular oxygen to reactive oxygen species such as superoxide radical anion, H202, hydroxyl radical
LIST 5.
SUGGESTED MECHANISMS FOR METABOLIC TOLERANCE TO ALCOHOL
Induction of alcohol dehydrogenases
Increased shuttle capacity
Increased reoxidation of NADH by mitochondria
Induction of CYP2E1
Hypermetabolic state
Increased release of cytokines or prostaglandins which elevate oxygen
consumption by hepatocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: None to report.
REFERENCES
- 1.Khanna JM, Israel Y. Ethanol. Metabolism. Int. Review of Physiol. 1980;21:275–315. [PubMed] [Google Scholar]
- 2.Crabb DW, Bosron WF, Li TK. Ethanol Metabolism. Pharmac. Ther. 1987;34:59–73. doi: 10.1016/0163-7258(87)90092-1. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy NP, Tipton KF. Ethanol Metabolism and Alcoholic Liver Disease. Essays in Biochemistry. 1990;25:137–195. [PubMed] [Google Scholar]
- 4.Riveros-Rosas H, Julian-Sanchez A, Pina E. Enzymology of Ethanol and Acetaldehyde Metabolism in Mammals. Arch. Med. Res. 1997;28:453–471. [PubMed] [Google Scholar]
- 5.Kalant H. Pharmacokinetics of ethanol: Absorption, Distribution and Elimination. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; 1996. pp. 15–58. [Google Scholar]
- 6.Cederbaum A. Metabolism of Ethanol , Acetaldehyde and Condensation Products. In: Begletier H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; 1996. pp. 59–109. [Google Scholar]
- 7.Lands WE. A Review of Alcohol Clearance in Humans. Alcohol. 1998;15:147–160. doi: 10.1016/s0741-8329(97)00110-9. [DOI] [PubMed] [Google Scholar]
- 8.Zakhari S. Overview: How is alcohol metabolized by the body. Alcohol Res and Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 9.Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 10.Frezza M, Di Padova C, Pozzato G, et al. High blood alcohol levels in women. New Engl. J. Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 11.Cole-Harding S, Wilson JR. Ethanol metabolism in men and women. J. Studies Alc. 1987;48:380–387. doi: 10.15288/jsa.1987.48.380. [DOI] [PubMed] [Google Scholar]
- 12.Norberg A, Jones WA, Hahn RG, et al. Role of variability in explaining ethanol pharmacokinetics. Clin. Pharmacokinet. 2003;42:1–31. doi: 10.2165/00003088-200342010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson PK, Sedman AJ, Sakmar E, et al. Pharmacokinetics of ethanol after oral administration in the fasting state. J. Pharmacokinet. and Biopharm. 1977;5:207–224. doi: 10.1007/BF01065396. [DOI] [PubMed] [Google Scholar]
- 14.Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcoholism: Clin Exp Res. 2001;25:502–507. [PubMed] [Google Scholar]
- 15.Kwo PY, Ramchandanl VA, O'Connor S, et al. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroent. 1998;115:1552–1557. doi: 10.1016/s0016-5085(98)70035-6. [DOI] [PubMed] [Google Scholar]
- 16.DiPadova C, Worner TM, Julkunen RJK, et al. Effects of fasting and chronic alcohol consumption on the first pass metabolism of ethanol. Gastroent. 1987;92:1169–1173. doi: 10.1016/s0016-5085(87)91073-0. [DOI] [PubMed] [Google Scholar]
- 17.Levitt MD, Furne J, DeMaster E. First pass metabolism of ethanol is negligible in rat gastric mucosa. Alcoholism: Clin Exp Res. 1997;21:293–297. [PubMed] [Google Scholar]
- 18.Lee SL, Chau GY, Yao CT, et al. Functional assessment of human alcohol dehydrogenase family in ethanol metabolism: Significance of first-pass metabolism. Alcoholism: Clin. Exp. Res. 2006;30:1132–1142. doi: 10.1111/j.1530-0277.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 19.Morgan MY, Levine JA. Alcohol and nutrition. Proc. Nutr. Soc. 1988;47:85–98. doi: 10.1079/pns19880017. [DOI] [PubMed] [Google Scholar]
- 20.Lieber CS. Perspectives: do alcohol calories count? Am. J. Clin. Nutr. 1991;54:976–982. doi: 10.1093/ajcn/54.6.976. [DOI] [PubMed] [Google Scholar]
- 21.Lands WEM. Alcohol and energy intake. Am. Soc. Clin. Nutr. 1995;62:1101S–1106S. doi: 10.1093/ajcn/62.5.1101S. [DOI] [PubMed] [Google Scholar]
- 22.Addolorato G, Capristo E, Greco AL, et al. Energy expenditure, substrate oxidation and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcoholism: Clin Exp Res. 1997;21:962–967. [PubMed] [Google Scholar]
- 23.Salaspuro MP, Lieber CS. Non-uniformity of blood ethanol elimination: its exaggeration after chronic consumption. Annals Clin. Res. 1978;10:294–297. [PubMed] [Google Scholar]
- 24.Matsumoto H, Fukui Y. Pharmacokinetics of ethanol: a review of the methodology. Addiction Biol. 2002;7:5–14. doi: 10.1080/135562101200100553. [DOI] [PubMed] [Google Scholar]
- 25.Holford NG. Clinical pharmacokinetics of ethanol. Clin. Pharmacokinet. 1987;13:273–292. doi: 10.2165/00003088-198713050-00001. [DOI] [PubMed] [Google Scholar]
- 26.Ramchandani VA, Bostron WF, Li TK. Research advances in ethanol metabolism. Pathol. Biol. 2001;49:676–682. doi: 10.1016/s0369-8114(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 27.Reed TE, Kalant H, Gibbins RJ, et al. Alcohol and acetaldehyde metabolism in Caucasians, Chinese and Amerinds. Canadian Med. Assoc. J. 1976;6:851–855. [PMC free article] [PubMed] [Google Scholar]
- 28.Bennion LJ, Li TK. Alcohol metabolism in American Indians and Whites. New Engl. J. Med. 1976;294:9–13. doi: 10.1056/NEJM197601012940103. [DOI] [PubMed] [Google Scholar]
- 29.Passanati GT, Wolff CA, Vesell E. Reproductibility of individual rates of ethanol metabolism in fasting subjects. Clin Pharmacol Ther. 1990;47:389–396. doi: 10.1038/clpt.1990.44. [DOI] [PubMed] [Google Scholar]
- 30.Wissel PS. Dietary influences on ethanol metabolism. Drug-Nutrient Interact. 1987;5:161–168. [PubMed] [Google Scholar]
- 31.Ramchandani VA, Kwo PY, Li TK. Effect of food and food composition on alcohol elimination rates in healthy men and women. J. Clin. Pharmacol. 2001;41:1345–1350. doi: 10.1177/00912700122012814. [DOI] [PubMed] [Google Scholar]
- 32.Edenberg H. The genetics of alcohol metabolism. Alcohol Res. and Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 33.Crabb DW. Ethanol oxidizing enzymes: Roles in alcohol metabolism and alcoholic lliver disease. Prog. Liver Dis. 1995;13:151–172. [PubMed] [Google Scholar]
- 34.Bosron W, Ehrig T, Li TK. Genetic factors in alcohol metabolism and alcoholism. Semin. Liver Dis. 1993;13:126–135. doi: 10.1055/s-2007-1007344. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson CJP, Fukunaga T, Sarkola T, et al. Functional relevance of human ADH polymorphism. Alcoholism: Clin Exp. Res. 2001;25:157S–163S. doi: 10.1097/00000374-200105051-00027. [DOI] [PubMed] [Google Scholar]
- 36.Zintzaras E, Stefanidis I, Santos M, et al. Do alcohol-metabolizing enzyme gene polymorphisms increase the risk of alcoholism and alcoholic liver disease. Hepatology. 2006;43:352–361. doi: 10.1002/hep.21023. [DOI] [PubMed] [Google Scholar]
- 37.Kimura M, Miyakawa T, Matsushita S, et al. Gender differences in the effects of ADHIB and ALDH2 polymorphisms in alcoholism. Alcoholism: Clin. Exp. Res. 2011;35:1923–1927. doi: 10.1111/j.1530-0277.2011.01543.x. [DOI] [PubMed] [Google Scholar]
- 38.Meijer AJ, Van Wuerkom GM, Williamson JR, et al. Rate-limiting factors in the oxidation of ethanol by isolated rat liver cells. Biochem. J. 1975;150:205–209. doi: 10.1042/bj1500205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cederbaum AI, Dicker E, Rubin E. Transfer and reoxidation of reducing equivalents as the rate-limiting steps in the oxidation of ethanol by liver cells isolated from fed and fasted rats. Arch. Biochem. Biophys. 1977;183:638–646. doi: 10.1016/0003-9861(77)90398-8. [DOI] [PubMed] [Google Scholar]
- 40.Gordon ER. The effect of chronic consumption of ethanol on the redox state of the rat liver. Canadian J. Biochem. 1972;50:949–957. doi: 10.1139/o72-131. [DOI] [PubMed] [Google Scholar]
- 41.Stubbs M, Veech RL, Krebs HA. Control of the redox of the nicotinamide adenine-dinucleotide couple in rat liver cytoplasm. Biochem. J. 1972;126:59–65. doi: 10.1042/bj1260059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veech RL, Guynn R, Veloso D. The time course of the effects of ethanol in the redox and phosphorylation states of rat liver. Biochem. J. 1972;127:387–397. doi: 10.1042/bj1270387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szabo G, Hoek JB, Darley-Usmar V, et al. Alcohol and mitochondrial metabolism: at the crossroads of life and death. Alcoholism: Clin Exp Res. 2005;29:1749–1752. doi: 10.1097/01.alc.0000179318.48376.cd. [DOI] [PubMed] [Google Scholar]
- 44.Teplova VV, Belosludtsev KN, Belosludtseva NV, et al. Role of mitochondria in hepatotoxicity of ethanol. Cell Biophys. 2010;55:951–958. [PubMed] [Google Scholar]
- 45.Cederbaum AI, Lieber CS, Beattie DS, et al. Characterization of shuttle mechanisms in the transport of reducing equivalents into mitochondria. Arch. Biochem. Biophys. 1973;158:763–781. doi: 10.1016/0003-9861(73)90571-7. [DOI] [PubMed] [Google Scholar]
- 46.Dawson AG. Rapid oxidation of NADH via the reconstituted malate-aspartate shuttle in systems containing mitochondrial and soluble fractions of rat liver: implications for ethanol metabolism. Biochem. Pharmacol. 1982;31:2733–2738. doi: 10.1016/0006-2952(82)90126-5. [DOI] [PubMed] [Google Scholar]
- 47.Cederbaum AI, Lieber CS, Toth A, et al. Effect of ethanol and fat on the transport of reducing equivalents into rat liver mitochondria. J. Biol. Chem. 1973;248:4977–4986. [PubMed] [Google Scholar]
- 48.Sugano T, Handler JA, Yoshihara H, et al. Acute and chronic ethanol treatment in vivo increases malate-aspartate shuttle capacity in perfused rat liver. J. Biol. Chem. 1990;265:21549–21553. [PubMed] [Google Scholar]
- 49.Zimatkin SM, Liopo AV, Deitrich RA. Distribution and kinetics of ethanol metabolism in rat brain. Alcoholism: Clin. Exp. Res. 1998;22:1623–1627. [PubMed] [Google Scholar]
- 50.Thurman RG, Handler JA. New perspectives in catalase-dependent ethanol metabolism. Drug Metab. Rev. 1989;20:679–688. doi: 10.3109/03602538909103570. [DOI] [PubMed] [Google Scholar]
- 51.Deng XS, Deitrich RA. Putative role of brain acetaldehyde in ethanol addiction. Current Drug Abuse Reviews. 2008;1:3–8. doi: 10.2174/1874473710801010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guengerich FR. Mammalian cytochrome P450. CRC Press Boca Raton; 1987. [Google Scholar]
- 53.Nelson DR, Koymans L, Kamataki T, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogen. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Lewis DFV, Pratt JM. The P450 catalytic cycle and oxygenation mechanism. Drug Metab. Rev. 1998;30:739–786. doi: 10.3109/03602539808996329. [DOI] [PubMed] [Google Scholar]
- 55.Lieber CS. Cytochrome P4502E1: its physiological and pathological role. Physiol. Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 56.Caro AA, Cederbaum AI. Oxidative stress, toxicology and pharmacology of CYP2E1. Annu Rev. Pharmacol. Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 57.Bolt M, Koos PH, Their H. The cytochrome P450 isoenzyme CYP2E1 in the biological processing of industrial chemicals. Int. Arch. Occup. Environ. Health. 2003;76:174–185. doi: 10.1007/s00420-002-0407-4. [DOI] [PubMed] [Google Scholar]
- 58.Koop DP. Oxidative and reductive metabolism by cytochrome P4502E1. FASEB J. 1992;6:724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez FJ. Roles of cytochromes P450 in chemical toxicity and oxidative stress:studies with CYP2E1. Mutat. Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 60.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Rad. Biol. Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernstein J, Videla L, Israel Y. Role of the sodium pump in the regulation of liver metabolism in experimental alcoholism. Ann NY Acad. Sci. 1974;242:560–572. doi: 10.1111/j.1749-6632.1974.tb19117.x. [DOI] [PubMed] [Google Scholar]
- 62.Cederbaum AI, Dicker E, Lieber CS, et al. Ethanol oxidation by isolated hepatocytes from ethanol-treated and control rats; factor contributing to the metabolic adaptation after chronic ethanol consumption. Biochem. Pharmacol. 1978;27:7–15. doi: 10.1016/0006-2952(78)90250-2. [DOI] [PubMed] [Google Scholar]
- 63.Videla L, Israel Y. Factors that modify the metabolism of ethanol in rat liver and adaptive changes produced by its chronic administration. Biochem. J. 1970;118:275–281. doi: 10.1042/bj1180275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bradford BU, Rusyn I. Swift increase in alcohol metabolism (SIAM): understanding the phenomenon of hypermetabolism in liver. Alcohol. 2005;35:13–17. doi: 10.1016/j.alcohol.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Kashiwagi T, Ji S, Lemasters JJ, et al. Rates of alcohol dehydrogenase-dependent ethanol metabolism in periportal and pericentral regions of the perfused rat liver. Mol. Pharmacol. 1982;21:438–443. [PubMed] [Google Scholar]
- 66.Vaananen H, Lindros KO. Comparison of ethanol metabolism in isolated periportal or perivenous hepatocytes: effects of chronic ethanol treatment. Alcoholism: Clin. Exp. Res. 1985;9:315–321. doi: 10.1111/j.1530-0277.1985.tb05551.x. [DOI] [PubMed] [Google Scholar]
- 67.Chen L, Sidner RA, Lumeng L. Distribution of alcohol dehydrogenase and the low km form of aldehyde dehydrogenase in isolated perivenous and periportal hepatocytes in rats. Alcoholism: Clin. Exp. Res. 1992;16:23–29. doi: 10.1111/j.1530-0277.1992.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 68.Seidl S, Wurst FM, Alt A. Ethyl glucuronide- a biological marker for recent alcohol consumption. Addict. Biol. 2001;6:205–212. doi: 10.1080/13556210120056535. [DOI] [PubMed] [Google Scholar]
- 69.Laposata M. Fatty acid ethyl esters: non oxidative metabolites of ethanol. Addict. Biol. 1998;3:5–14. doi: 10.1080/13556219872308. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal DP, Goedde HW. Human aldehyde dehydrogenases: their role in alcoholism. Alcohol. 1989;6:517–523. doi: 10.1016/0741-8329(89)90061-x. [DOI] [PubMed] [Google Scholar]
- 71.Goedde HW, Agarwal DP. Pharmacogenetics of aldehyde dehydrogenase. Pharmac. Ther. 1990;45:345–371. doi: 10.1016/0163-7258(90)90071-9. [DOI] [PubMed] [Google Scholar]
- 72.Lindros KO, Eriksson CJP. The role of acetaldehyde in the action of ethanol. Finnish Foundation Stud. Alc. 1975;23 [Google Scholar]
- 73.Niemela O. Acetaldehyde adducts of proteins: diagnostic and pathogenic implications in diseases caused by excessive alcohol consumption. Scand. J. Clin lab Invest. 1993;53:45–54. doi: 10.3109/00365519309090673. [DOI] [PubMed] [Google Scholar]
- 74.Sorrell MF, Tuma DJ. Hypothesis: alcoholic liver injury and the covalent binding of acetaldehyde. Alcoholism: Clin. Exp. Res. 1989;9:306–309. doi: 10.1111/j.1530-0277.1985.tb05549.x. [DOI] [PubMed] [Google Scholar]
- 75.Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: the 2002 update. Chemico Biolog. Interact. 2002;143–144:5–22. doi: 10.1016/s0009-2797(02)00163-1. [DOI] [PubMed] [Google Scholar]