Abstract
Thermophilic bacteria have gained increased attention as candidates for bioethanol production from lignocellulosic biomass. This study investigated ethanol production by Thermoanaerobacter strain J1 from hydrolysates made from lignocellulosic biomass in batch cultures. The effect of increased initial glucose concentration and the partial pressure of hydrogen on end product formation were examined. The strain showed a broad substrate spectrum, and high ethanol yields were observed on glucose (1.70 mol/mol) and xylose (1.25 mol/mol). Ethanol yields were, however, dramatically lowered by adding thiosulfate or by cocultivating strain J1 with a hydrogenotrophic methanogen with acetate becoming the major end product. Ethanol production from 4.5 g/L of lignocellulosic biomass hydrolysates (grass, hemp stem, wheat straw, newspaper, and cellulose) pretreated with acid or alkali and the enzymes Celluclast and Novozymes 188 was investigated. The highest ethanol yields were obtained on cellulose (7.5 mM·g−1) but the lowest on straw (0.8 mM·g−1). Chemical pretreatment increased ethanol yields substantially from lignocellulosic biomass but not from cellulose. The largest increase was on straw hydrolysates where ethanol production increased from 0.8 mM·g−1 to 3.3 mM·g−1 using alkali-pretreated biomass. The highest ethanol yields on lignocellulosic hydrolysates were observed with hemp hydrolysates pretreated with acid, 4.2 mM·g−1.
1. Background
More than 95% of the ethanol produced today is from simple biomass like mono- and disaccharides and starch [1]. The use of this type of biomass has been increasingly debated due to its impact on food and feed prices as well as for environmental reasons [2]. Therefore, complex (lignocellulosic) biomass has been put forward as a feasible alternative due to its abundance in nature and the large quantities generated as waste from agricultural activities [2, 3]. Lignocellulosic biomass is primarily composed of cellulose, hemicellulose, and lignin. Cellulose and hemicellulose are the main substrates used for ethanol production, but lignin is composed of aromatic lignols that need to be separated and removed before enzymatic hydrolysis. Today, expensive pretreatments are the main reason for unsuccessful implementation of complex lignocellulosic biomasses as a starting material for ethanol production [2].
The best-known microorganisms used for ethanol production today are the yeast Saccharomyces cerevisiae and the bacterium Zymomonas mobilis. Both organisms have very high yields of ethanol (>1.9 mol ethanol/mol hexose) but very narrow substrate spectra and thus are not suitable for ethanol production from complex substrates. Therefore, the use of thermophilic bacteria with broad substrate range and high yields may be a better option for ethanol production from complex biomasses. It has been known for some time now that many thermophilic bacteria are highly efficient ethanol producers [4]. After the oil crisis in the 1980s, there was a peak in investigations on thermophilic ethanol-producing bacteria; bacteria within the genera of Thermoanaerobacterium, Thermoanaerobacter, and Clostridium have demonstrated good ethanol yields and fast growth rates [5–8]. There are several advantages in using these thermophilic bacteria: the increased temperature deters contamination from mesophilic bacteria and fungi, possible self-distillation of ethanol avoiding the generally low ethanol tolerance problem with those bacteria, and broad substrate spectrum [9, 10]. Some of these strains produce more than 1.5 mol ethanol/mol hexose [11–16], whereas the theoretical maximum yield is 2.0 mol ethanol/mol hexose degraded. The main reasons for low yields are the formation of other end products such as acetate, butyrate, and CO2 [11–16].
The present study focuses on a recently isolated thermophilic bacterium, strain J1, which is most closely related to species within the genus Thermoanaerobacter. Bacteria within this genus seem to be among the most efficient ethanol producers known and show very high yields from simple sugar fermentations [12–14, 16] as well as from complex lignocellulosic biomass [10, 13, 17–19]. These bacteria are Gram-variable rods with broad substrate spectrum (mostly sugars) and produces ethanol, acetate, lactate, hydrogen, and carbon dioxide during anaerobic fermentation [20, 21]. The physiological characteristics of Thermoanaerobacter strain J1, isolated from Icelandic hot spring, were investigated in detail with the main aim of exploring the ethanol production capacity both from simple sugars as well as from various lignocellulosic biomass.
2. Methods
2.1. Medium
The composition and preparation of the medium used has been described earlier [12]. This medium, referred to as basal medium (BM) hereafter, contains yeast extract (2 g/L) in addition to glucose or other carbon sources. All experiments were performed at 65°C at pH 7.0 without agitation with the exception of the temperature and pH optimum experiments. The inoculum volume was 2% (v/v) in all experiments which were always performed in duplicates.
2.2. Isolation of Strain J1
The strain was isolated in BM with glucose (20 mM) from a hot spring (69°C, pH 7.5) in Grensdalur in Southwest of Iceland. Samples were enriched on glucose, and positive samples (increase in growth and production of hydrogen) were reinoculated five times. From the final enrichment series, end point dilutions were performed by using BM containing agar (30 g·L−1). Colonies were picked from final positive dilution and reinoculated to liquid BM with glucose. Isolation of the hydrogenotrophic methane producing strain has been described elsewhere [22].
2.3. Optimum pH and Temperature Growth Experiments
To determine the strain's growth characteristics at various pHs and temperatures, the strain was cultivated on glucose (20 mM), and cell concentration was measured by increase in absorbance at 600 nm by a Perkin-Elmer Lambda 25 UV-Vis spectrophotometer. Maximum (specific) growth rate (μmax) for each experiment was derived from absorbance data. For pH optimum experiments, the initial pH was set to various levels in the range from 3.0 to 9.0 with increments of 1.0 pH unit. The experimental bottles were supplemented with acid (HCl) and alkali (NaOH) to set the pH accordingly. To determine the optimum temperature for growth, the incubation temperature varied from 35°C to 80°C. For the pH optimum experiments, the strain was cultivated at 65°C, and for the temperature optimum experiments, the pH was 7.0. Optimal pH and temperature were used in all experiments performed. Experiments were done in 117.5 mL serum bottles with 50 mL liquid medium.
2.4. Phylogenetic Analysis
Full 16S rRNA analysis of 1479-nucleotide long sequence was done according to Orlygsson and Baldursson [23] and references therein. Sequences from 16S rRNA analysis were compared to sequences in the NCBI database using the nucleotide-nucleotide BLAST (BLAST-N) tool. The most similar sequences were aligned with the sequencing results in the programs BioEdit [24] and CLUSTAL_X [25]. Finally, the trees were displayed with the program TreeView. Caloramator viterbiensis was used as an outgroup.
2.5. Effect of Initial Glucose Concentration on End Product Formation
The effect of initial glucose concentration on strain J1, by varying the concentration from 5 to 200 mM, was tested. Control samples contained only yeast extract. Glucose, hydrogen, acetate, and ethanol concentrations were measured at the beginning and at the end of incubation time (7 days). Experiments were done in 117.5 mL serum bottles with 60 mL liquid medium, and the pH was measured at the end of incubation time.
2.6. Substrate Utilization Spectrum
The ability of strain J1 to utilize different substrates was tested using the BM medium supplemented with various carbon substrates (xylose, arabinose, glucose, mannose, galactose, fructose, rhamnose, maltose, cellobiose, sucrose, lactose, trehalose, raffinose, starch, cellulose, CMC, avicel, xylan (from oat spelt), glycerol, pyruvate, serine, and threonine). All substrates were added from filter-sterilized (0.45 μm) substrates except for xylan, starch, CMC, cellulose, and avicel which were autoclaved with the medium. In all cases, the concentration of substrates was 20 mM except for xylan, starch, CMC, cellulose, and avicel when 2 g·L−1 was used. Hydrogen, acetate, and ethanol concentrations were analysed after one week of incubation. Experiments were performed in 24.5 mL serum bottles with 10 mL liquid medium.
2.7. Pretreatment of Biomass and Hydrolysates Preparation
Hydrolysates (HLs) were made from different biomasses: Whatman no. 1 filter paper, newspaper, hemp stem (Cannabis sativa), barley straw (Hordeum vulgare), and grass (Phleum pratense). Hydrolysates were prepared according to Sveinsdottir et al. [12], and the final concentration of each biomass type was 22.5 g·L−1. Biomass was pretreated chemically by using 0.50% (v/v) of acid (H2SO4) or alkali (NaOH) (control was without chemical pretreatment) before heating (121°C, 60 min). Two commercial enzyme solutions, Celluclast (Novozyme, 750 U·g−1) and Novozyme 188 (Sigma C6105, 200 U·g−1), were added to each bottle after chemical pretreatment; the bottles were cooled down to room temperature and the pH adjusted to 5.0 before enzymes were added. The hydrolysates were incubated in water bath at 45°C for 68 h. After the enzyme treatment, the pH was adjusted with NaOH or HCl to pH 7.0 which is the pH optimum of the strain. The hydrolysates were then filtered (Whatman-WeiBrand; 0.45 μm) into sterile bottles.
2.8. Fermentation during External Electron-Scavenging Systems
In one set of experiments, strain J1 was incubated on glucose (20 mM) in the presence of sodium thiosulfate (40 mM) and in coculture with a hydrogenotrophic methanogen. The methanogen was precultivated in BM medium with a gas phase consisting of 80% of H2 and 20% of CO2 for one week. Then the experimental culture bottles were flushed with nitrogen prior to the addition of glucose (20 mM) and strain J1. The coculture was incubated at 65°C for one week.
2.9. Fermentation of Hydrolysates
Fermentation of carbohydrates present in the hydrolysates after chemical and enzymatic pretreatment was performed in 24.5 mL serum bottles. The BM medium and inoculum (8.0 mL) were supplemented with different hydrolysates (2.0 mL, total liquid volume of 10 mL) giving a final hydrolysate concentration of 4.5 g·L−1. Control samples did not contain hydrolysate; the only carbon source was yeast extract.
2.10. Analytical Methods
Hydrogen, ethanol, and volatile fatty acids were measured by gas chromatography as previously described [23]. Glucose was determined by slight modification of the method from Laurentin and Edwards [26]; supernatant broth (400 μL) was mixed with 2 mL of anthrone solution (0.2% (w/v) of anthrone in 72% (v/v) of sulphuric acid). The sample was boiled for 11 minutes and then cooled down on ice. Absorbance was then measured at 600 nm by using Perkin-Elmer Lambda 25 UV-Vis spectrophotometer.
3. Results and Discussion
3.1. Phylogeny
Figure 1 shows that strain J1 belongs to the genus Thermoanaerobacter with its closest neighbours being T. uzonensis (97.7% homology) and T. sulfurigenes (95.5%). The genus Thermoanaerobacter falls into clusters V in the phylogenetic interrelationship of Clostridium according to Collins and coworkers [27]. All species within the genus are obligate anaerobes and ferment various carbohydrates to ethanol, acetate, lactate, hydrogen, and carbon dioxide [20], while some species can degrade amino acids [28]. Most strains can reduce thiosulfate to hydrogen sulphide [20, 28]. Today, the genus consists of 18 species according to the Euzeby list of prokaryotes.
Figure 1.
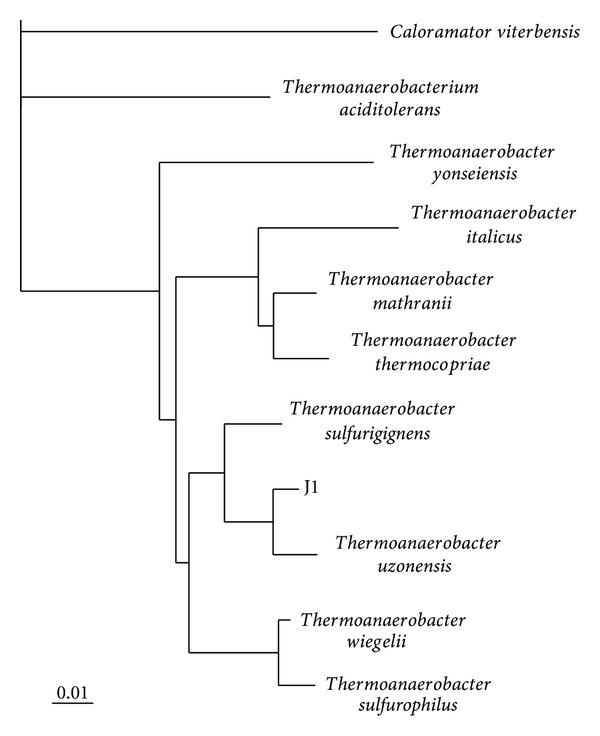
Phylogeny of strain J1 based on partial 16S rRNA sequence analysis. The phylogenetic tree was generated by using distance matrix and neighbor-joining algorithms. Caloramator viterbensis was selected as outgroup. The bar indicates 0.01 substitutions per nucleotide position.
3.2. Optimum Growth Conditions
The strain was able to grow between 55.0°C and 75.0°C with optimal temperature being 65.0°C (μmax; 0.23 h). The pH optimum was 7.0 (μmax; 0.19 h). No growth was observed below pH 4.0 and above pH 9.0.
3.3. End Product Production from Sugars and Other Substrates
One of the main reasons for increased interest in using thermophilic bacteria for second-generation ethanol production is because of their broad substrate spectrum. Therefore, it was decided to cultivate the strain on the most common sugars present in lignocellulosic biomass as well as pyruvate, glycerol, serine, and threonine (Figure 2). Clearly, the strain is a very powerful ethanol producer; it produces 1.70 mol ethanol/mol glucose and 1.25 mol ethanol/mol xylose (control values subtracted) or 85.0 and 75.0% of theoretical yields, respectively. The following stoichiometry from glucose and xylose was observed:
| (1) |
| (2) |
Figure 2.
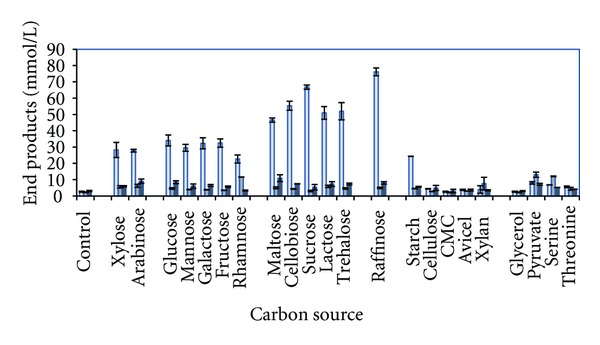
End product formation from various substrates by strain J1. Data represents average of two replicate experiments. Standard deviation are shown as error bars. From left to right; ethanol, acetate and hydrogen.
Lactate was not analysed in the present paper, but high carbon recoveries from analysed end products from glucose and xylose (92.5 and 87.4%, resp.) indicate that if it was produced, its significance is very little. The substrate spectrum of the strain shows a broad capacity in degrading pentoses (xylose, arabinose), hexoses (glucose, mannose, galactose, fructose, and rhamnose), disaccharides (maltose, cellobiose, lactose, trehalose, and sucrose) the trisaccharide raffinose, and starch, pyruvate, and serine. In all the cases, the major end product is ethanol except for serine and pyruvate in which acetate is the primary end product. The highest ethanol concentrations were produced from the trisaccharide raffinose (75.2 mM). As earlier mentioned, the strain is most closely related to T. uzonensis (strain JW/IW010) which also produces ethanol and acetate as the only volatile end products, but the ratio between ethanol and acetate is 1.35 in that strain [28]. However, T. uzonensis has a more narrow sugar degradation spectrum as compared to strain J1; it cannot degrade arabinose and rhamnose. Other well-known ethanol producers within the genus are T. ethanolicus, T. thermohydrosulfuricus, and T. finnii with yields between 1.5 and 1.9 mol ethanol/mol glucose [11, 13, 14, 29].
During growth on serine and pyruvate, the carbon flow was shifted away from ethanol to acetate and hydrogen. This can be explained by the oxidation state of these substrates as compared to sugars; the oxidation state of the carbon in glucose is zero, and during its oxidation to pyruvate, the electrons are transferred to NAD+ leading to the formation of NADH. Reoxidation of NADH to NAD+ by the strain occurs most likely through acetaldehyde dehydrogenase and alcohol dehydrogenase rendering ethanol as the main product. However, both pyruvate and serine are more oxidized substrates as compared to sugars (glucose), and there is no need to reoxidize NADH. Instead, the strain deaminates serine directly to pyruvate which is decarboxylated to acetyl phosphate (by phosphotransacetylase) and further to acetate (by acetate kinase) resulting in ATP formation. However, since hydrogen production is less as compared to acetate, it is likely that the strain is also producing formate (not analyzed) instead of hydrogen from these substrates.
3.4. Effect of Initial Glucose Loadings on Ethanol Production
High initial substrate concentrations may inhibit substrate utilization and/or decrease end product yields [5, 10, 30]. In closed systems, such as batch cultures, the limited buffer capacity of the medium may be overloaded by the accumulation of organic acids resulting in a pH drop and the inhibition of substrate fermentation utilization [30]. To investigate the influence of initial substrate concentration on end product formation, changes in pH, and substrate degradation, strain J1 was cultivated with different concentration of glucose (0 to 200 mM). The strain completely degraded glucose in all experiments, except for the highest (200 mM) initial glucose loadings, and ethanol yields were between 1.2 and 1.7 mol ethanol/mol glucose (Figure 3). Acetate formation increased from 2.7 mM in control bottles (without glucose) to 9.5 mM at 100 mM glucose concentrations which was directly linked to a decrease from pH 7.0 (control) to 5.2 (100 mM glucose). At 200 mM glucose concentrations, acetate was only slightly higher as compared to 100 mM glucose concentrations, the pH dropped from 5.2 to 4.8, and only 110 mM of glucose was degraded. Thus, the limit of glucose seems to be pH related, because of the formation of acetate, rather than substrate inhibition. The strain seems to be more tolerant for initial substrate concentrations as compared to many other thermophilic bacteria where often a concentration between 20 and 30 mM is too high for a complete degradation [7, 8]. In those cases, however, more acetate was produced as compared to ethanol and may be crucial for lowering the pH at lower substrate concentrations.
Figure 3.
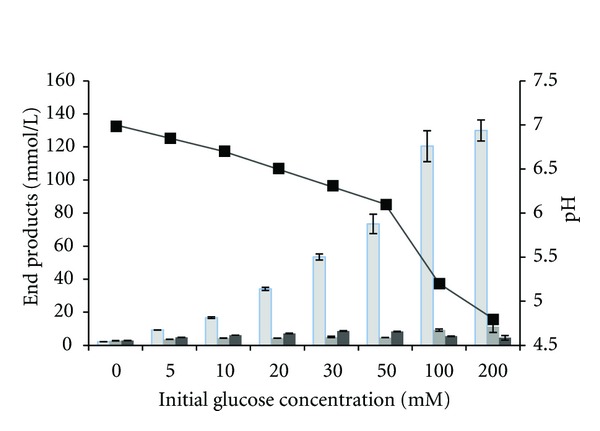
End product formation from different initial glucose concentrations. Also shown are percent of glucose degraded. Values represent means of two replicates and standard deviation are shown as error bars. Columns from left to right; ethanol, acetate, hydrogen. pH measered after fermentation (▪).
3.5. Effect of Hydrogen-Scavenging Systems on End Product Formation
It is well known that Thermoanaerobacter species are highly flexible concerning end product formation depending on the culture conditions. Fardeau et al. [31] showed a dramatic shift in end product formation by Thermoanaerobacter finnii when grown on glucose in the presence and absence of thiosulfate. In that case, both ethanol and lactate decreased during thiosulfate reduction to hydrogen sulphide, whereas the acetate concentration increased. The influence of using biological hydrogen-scavenging systems has also been investigated throughout Thermoanaerobacter brockii during amino acid degradation [27]. Both thiosulfate and the presence of a hydrogen-scavenging methanogen were crucial for the oxidative deamination of the branched chain amino acids by this strain. However, degradation of a substrate that is thermodynamically easier to degrade, for example, the amino acid serine, was completely degraded in the presence and absence of thiosulfate and Methanobacterium sp. although a shift occurred between ethanol and acetate formation [27]. To investigate the influence of low partial pressure (pH2) on end product formation, strain J1 was cultivated in the presence of thiosulfate and in coculture with a hydrogenotrophic methanogen. As observed earlier, strain J1 produced ethanol as the main end product during glucose fermentation only (Table 1). The addition of thiosulfate to glucose fermentations resulted in a shift towards acetate from ethanol where the ratio between ethanol and acetate changed from 6.90 to 1.29. Cocultivating strain J1 with a hydrogenotrophic methanogen led even to more dramatic shift towards acetate (and methane), and the ratio of ethanol and acetate was 0.14. This difference in end product formation by using thiosulfate or a hydrogenotrophic methanogen is surprisingly big considering that the concentration of hydrogen is very low at the end of experimental time (0.3 to 0.5 mmol·L−1) in both cases. This difference could be caused by more rapid uptake of hydrogen in the coculture experiment, but end products were only analysed at the end of the experimental time.
Table 1.
Utilization of glucose by strain J1 in the presence of thiosulfate or a hydrogenotrophic methanogen. Data represents average of two replicate experiments ± standard deviation.
| Concentration (mmol·L−1) | ||||
|---|---|---|---|---|
| Ethanol | Acetate | Hydrogen | Methane | |
| Control | 3.0 ± 0.1 | 2.9 ± 0.1 | 2.0 ± 0.1 | 0.0 ± 0.0 |
| Control + S2O3 | 1.1 ± 0.1 | 5.2 ± 0.2 | 0.3 ± 0.0 | 0.0 ± 0.0 |
| Control + methanogen | 0.9 ± 0.5 | 4.9 ± 0.4 | 0.0 ± 0.0 | 2.4 ± 0.0 |
| Glucose | 29.0 ± 1.5 | 4.2 ± 0.3 | 7.2 ± 0.5 | 0.0 ± 0.0 |
| Glucose + S2O3 | 20.0 ± 0.3 | 15.5 ± 2.1 | 0.3 ± 0.1 | 0.0 ± 0.0 |
| Glucose + methanogen | 4.1 ± 0.2 | 29.5 ± 1.2 | 0.5 ± 0.0 | 7.4 ± 1.2 |
3.6. Fermentation of Hydrolysates from Lignocellulosic Biomass
The strain is producing maximally 33.9 mM (1.56 g/L) of ethanol from 4.5 g/L of hydrolysates made from cellulose (Figure 4). The yields on cellulose pretreated only with enzymes and heat are 7.5 mM·g−1 dry weight (dw) which is considered lower as compared to glucose degradation alone (1.70 mol ethanol/mol glucose; 9.4 mM·g−1 glucose). No glucose was analysed in the cellulose hydrolysate after fermentation. Thus, the lower ethanol yields on cellulose as compared to glucose indicate that the cellulose was not completely degraded during enzymatic hydrolysis. Chemical pretreatment of cellulose by the addition of acid or alkali did not increase the end product formation yields on cellulose. The highest ethanol yields on the more complex biomass types (without chemical pretreatment) were observed on hemp (11.6 mM; 2.6 mM·g−1 dw) but lowest on straw (3.5 mM; 0.8 mM·g−1 dw). Chemical pretreatment by adding either acid or alkali increased yields substantially on most of the lignocellulosic biomasses tested. The increase was most profound on hydrolysates from straw pretreated with alkali where ethanol production was increased from 3.5 to 14.8 mM (controls subtracted). The highest ethanol yields were however observed on hemp, 4.3 mM·g−1 dw (19.0 mM). The highest ethanol yields by Thermoanaerobacter species have been reported by continuous cultures of Thermoanaerobacter strain BG1L1 on wheat straw [17] and corn stover [18], or 8.5–9.2 mM·g−1 sugar consumed. Thermoanaerobacter ethanolicus has been reported to produce 4.5 and 4.8 mM ethanol·g−1 hexose equivalent degraded from wood hydrolysate and beet molasses, respectively [13, 32]. Thermoanaerobacter mathranii, isolated from the same geographical area in Iceland [33] as strain J1 produced 5.3 mM·g−1 sugar from wheat straw hydrolysate [34]. Recently, a new Thermoanaerobacter strain, AK5 closely related to T. thermohydrosulfuricus and T. ethanolicus, was isolated from a hot spring in Iceland and has similar yields on cellulose (7.7 mM·g−1), hemp (3.1 mM·g−1), and grass (4.1 mM·g−1) hydrolysates [22].
Figure 4.
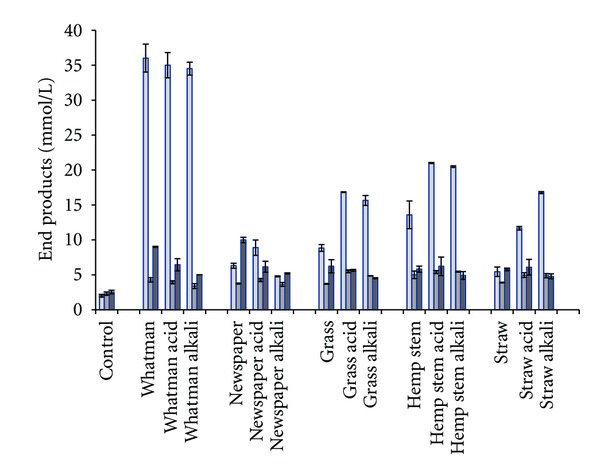
Production of end products from hydrolysates (4.5 g·L−1) from different biomasses. Values represent mean of two replicates (±standard deviation). From left to right: ethanol, acetate, and hydrogen.
4. Conclusion
Ethanol production was studied by Thermoanaerobacter J1 isolated from hot spring in Iceland. The main aim of the study was to investigate the importance of various factors on ethanol production from both sugars and complex lignocellulosic biomass. The strain produces 1.70 mol ethanol/mol glucose and 1.25 mol ethanol/mol xylose and shows a broad substrate spectrum, degrading various sugars and starch but not cellulosic substrates. High ethanol yields were observed at initial glucose concentrations up to 100 mM. During growth under hydrogen removal, a shift from ethanol to acetate formation occurs. The strain produces up to 7.5 mM ethanol·g−1 cellulose and 4.2 mM·g−1 hemp hydrolysate.
Authors' Contribution
J. E. Jessen carried out all experimental procedures. J. Orlygsson planned the experimental procedure and drafted the paper. Both authors read and approved the final paper.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgments
This work was sponsored by RANNÍS, Technology Development Fund, projects 081303408 (BioEthanol) and RAN091016-2376 (BioFuel), and the Research Fund of the University of Akureyri. Special thanks are due to Margret Audur Sigurbjornsdottir for aligning the 16S rRNA sequences and building the phylogenetic tree and to Sean M. Scully for proofreading the paper.
References
- 1.Renewable Fuels Association. Choose ethanol. 2011, http://chooseethanol.com/what-is-ethanol/entry/ethanol-at-a-glance/
- 2.Sánchez ÓJ, Cardona CA. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresource Technology. 2008;99(13):5270–5295. doi: 10.1016/j.biortech.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Zaldivar J, Nielsen J, Olsson L. Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Applied Microbiology and Biotechnology. 2001;56(1-2):17–34. doi: 10.1007/s002530100624. [DOI] [PubMed] [Google Scholar]
- 4.Wiegel J. Formation of ethanol by bacteria. A pledge for the use of extreme thermophilic anaerobic bacteria in industrial ethanol fermentation processes. Experientia. 1980;36(12):1434–1446. [Google Scholar]
- 5.Lacis LS, Lawford HG. Ethanol production from xylose by Thermoanaerobacter ethanolicus in batch and continuous culture. Archives of Microbiology. 1988;150(1):48–55. [Google Scholar]
- 6.Lamed R, Zeikus JG. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. Journal of Bacteriology. 1980;144(2):569–578. doi: 10.1128/jb.144.2.569-578.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almarsdottir AR, Sigurbjornsdottir MA, Orlygsson J. Effect of various factors on ethanol yields from ligncellulosic biomass by Thermoanaerobacterium AK17. Biotechnology and Bioengineering. 2012;109(3):686–694. doi: 10.1002/bit.24346. [DOI] [PubMed] [Google Scholar]
- 8.Sigurbjornsdottir MA, Orlygsson J. Combined hydrogen and ethanol production from sugars and lignocellulosic biomass by Thermoanaerobacterium AK54. Applied Energy. 2012;97:785–791. [Google Scholar]
- 9.Taylor MP, Eley KL, Martin S, Tuffin MI, Burton SG, Cowan DA. Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends in Biotechnology. 2009;27(7):398–405. doi: 10.1016/j.tibtech.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Sommer P, Georgieva T, Ahring BK. Potential for using thermophilic anaerobic bacteria for bioethanol production from hemicellulose. Biochemical Society Transactions. 2004;32(2):283–289. doi: 10.1042/bst0320283. [DOI] [PubMed] [Google Scholar]
- 11.Wiegel J, Ljungdahl LG. Thermoanaerobacter ethanolicus gen. nov., spec. nov., a new, extreme thermophilic, anaerobic bacterium. Archives of Microbiology. 1981;128(4):343–348. [Google Scholar]
- 12.Sveinsdottir M, Baldursson SRB, Orlygsson J. Ethanol production from monosugars and lignocellulosic biomass by thermophilic bacteria isolated from Icelandic hot springs. Icelandic Agricultural Sciences. 2009;22:45–58. [Google Scholar]
- 13.Avci A, Dönmez S. Effect of zinc on ethanol production by two Thermoanaerobacter strains. Process Biochemistry. 2006;41(4):984–989. [Google Scholar]
- 14.Carreira LH, Wiegel J, Ljungdahl LG. Production of ethanol from biopolymers by anaerobic, thermophilic, and extreme thermophilic bacteria: I. Regulation of carbohydrate utilization in mutants of Thermoanaerobacter ethanolicus. Biotechnology Bioengineering Symposium. 1983;13(13):183–191. [Google Scholar]
- 15.Koskinen PEP, Beck SR, Örlygsson J, Puhakka JA. Ethanol and hydrogen production by two thermophilic, anaerobic bacteria isolated from Icelandic geothermal areas. Biotechnology and Bioengineering. 2008;101(4):679–690. doi: 10.1002/bit.21942. [DOI] [PubMed] [Google Scholar]
- 16.Lovitt RW, Shen GJ, Zeikus JG. Ethanol production by thermophilic bacteria: biochemical basis for ethanol and hydrogen tolerance in Clostridium Thermohydrosulfuricum. Journal of Bacteriology. 1988;170(6):2809–2815. doi: 10.1128/jb.170.6.2809-2815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgieva TI, Mikkelsen MJ, Ahring BK. Ethanol production from wet-exploded wheat straw hydrolysate by thermophilic anaerobic bacterium Thermoanaerobacter BG1L1 in a continuous immobilized reactor. Applied Biochemistry and Biotechnology. 2008;145(1–3):99–110. doi: 10.1007/s12010-007-8014-1. [DOI] [PubMed] [Google Scholar]
- 18.Georgieva TI, Ahring BK. Evaluation of continuous ethanol fermentation of dilute-acid corn stover hydrolysate using thermophilic anaerobic bacterium Thermoanaerobacter BG1L1. Applied Microbiology and Biotechnology. 2007;77(1):61–68. doi: 10.1007/s00253-007-1149-8. [DOI] [PubMed] [Google Scholar]
- 19.Rani KS, Swamy MV, Seenayya G. Production of ethanol from various pure and natural cellulosic biomass by Clostridium Thermocellum strains SS21 and SS22. Process Biochemistry. 1998;33(4):435–440. [Google Scholar]
- 20.Lee YE, Jain MK, Lee C, et al. Taxonomic distinction of saccharolytic thermophilic anaerobes: description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov., reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes , and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb. nov., Thermoanaerobacter thermosulfurigenes comb. nov., and Thermoanaerobacter thermohydrosulfuricus comb. nov., respectively, and transfer of Clostridium Thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. International Journal of Systematic Bacteriology. 1993;43(1):41–51. [Google Scholar]
- 21.Fardeau ML, Salinas MB, L’Haridon S, et al. Isolation from oil resorvoirs of novel thermophilic anaerobes phylogenetically related to Thermoanaerobacter subterraneus: reassignment of T. subterraneus, Thermoanaerobacter yonseiensis, Thermoanaerobacter tengcongensis and Carboxydibrachium pacificum to Caldanaerobacter subterraneus gen. nov., sp. nov., comb. nov. as four novel subspecies. International Journal of Systematic and Evolutionary Microbiology. 2004;54(2):467–474. doi: 10.1099/ijs.0.02711-0. [DOI] [PubMed] [Google Scholar]
- 22.Brynjarsdottir H, Wawiernia B, Orlygsson J. Ethanol production from sugars and complex biomass by Thermoanaerobacter AK5: the effect of electron scavenging systems on end product formation. Energy & Fuels. 2012;26(7):4568–4574. [Google Scholar]
- 23.Orlygsson J, Baldursson SRB. Phylogenetic and physiological studies of four hydrogen-producing thermoanaerobes from Icelandic geothermal areas. Icelandic Agricultural Sciences. 2007;93:93–106. [Google Scholar]
- 24.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- 25.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurentin A, Edwards CA. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Analytical Biochemistry. 2003;315(1):143–145. doi: 10.1016/s0003-2697(02)00704-2. [DOI] [PubMed] [Google Scholar]
- 27.Collins MD, Lawson PA, Willems A, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. International Journal of Systematic Bacteriology. 1994;44(4):812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 28.Fardeau ML, Patel BKC, Magot M, Ollivier B. Utilization of serine, leucine, isoleucine, and valine by Thermoanaerobacter brockii in the presence of thiosulfate or Methanobacterium sp. as electron accepters. Anaerobe. 1997;3(6):405–410. doi: 10.1006/anae.1997.0126. [DOI] [PubMed] [Google Scholar]
- 29.Wagner ID, Zhao W, Zhang CL, Romanek CS, Rohde M, Wiegel J. Thermoanaerobacter uzonensis sp. nov., an anaerobic thermophilic bacterium isolated from a hot spring within the Uzon Caldera, Kamchatka, Far East Russia. International Journal of Systematic and Evolutionary Microbiology. 2008;58(11):2565–2573. doi: 10.1099/ijs.0.65343-0. [DOI] [PubMed] [Google Scholar]
- 30.Van Ginkel S, Sung S, Lay JJ. Biohydrogen production as a function of pH and substrate concentration. Environmental Science and Technology. 2001;35(24):4726–4730. doi: 10.1021/es001979r. [DOI] [PubMed] [Google Scholar]
- 31.Fardeau ML, Faudon C, Cayol JL, Magot M, Patel BKC, Ollivier B. Effect of thiosulphate as electron acceptor on glucose and xylose oxidation by Thermoanaerobacter finnii and a Thermoanaerobacter sp. isolated from oil field water. Research in Microbiology. 1996;147(3):159–165. doi: 10.1016/0923-2508(96)80215-4. [DOI] [PubMed] [Google Scholar]
- 32.Wiegel J, Carreira LH, Mothershed CP, Puls J. Production of ethanol from biopolymers by anaerobic, thermophilic, and extreme thermophilic bacteria. II. Thermoanaerobacter ethanolicus JW200 and its mutants in batch cultures and resting cell experiments. Biotechnology Bioengineering Symposium. 1983;13(13):193–205. [Google Scholar]
- 33.Larsen L, Nielsen P, Ahring BK. Thermoanaerobacter mathranii sp. nov., an ethanol-producing, extremely thermophilic anaerobic bacterium from a hot spring in Iceland. Archives of Microbiology. 1997;168(2):114–119. doi: 10.1007/s002030050476. [DOI] [PubMed] [Google Scholar]
- 34.Ahring BK, Licht D, Schmidt AS, Sommer P, Thomsen AB. Production of ethanol from wet oxidised wheat straw by Thermoanaerobacter mathranii. Bioresource Technology. 1999;68(1):3–9. [Google Scholar]


