Abstract
AIM: To investigate the incidence of clinically detected port-site metastasis (PSM) in patients who underwent robotic surgery for biliary malignancies.
METHODS: Using a prospective database, the patients undergoing fully robotic surgery for biliary malignancies between January 2009 and January 2011 were included. Records of patients with confirmed malignancy were reviewed for clinicopathological data and information about PSM.
RESULTS: Sixty-four patients with biliary tract cancers underwent robotic surgery, and sixty patients met the inclusion criteria. The median age was 67 year (range: 40-85 year). During a median 15-mo follow-up period, two female patients were detected solitary PSM after robotic surgery. The incidence of PSM was 3.3%. Patient 1 underwent robotic anatomatic left hemihepatectomy and extraction of biliary tumor thrombi for an Klatskin tumor. She had a subcutaneous mass located at the right lateral abdominal wall near a trocar scar. Patient 2 underwent robotic pancreaticoduodenectomy for distal biliary cancer. She had two metachronous subcutaneous mass situated at the right lateral abdominal wall under a same trocar scar at 7 and 26 mo. The pathology of the excised PSM masses confirmed metastatic biliary adenocarcinoma.
CONCLUSION: The incidence of PSMs after robotic surgery for biliary malignancies is relatively low, and biliary cancer can be an indication of robotic surgery.
Keywords: Robotic surgery, Trocar, Port-site metastasis, Recurrence, Biliary tract cancer
INTRODUCTION
Cholangiocarcinoma (CCA) is an epithelial malignant neoplasm arising from the bile ducts with features of cholangiocyte differentiation. CCA is the second most common primary hepatobiliary malignant cancer after hepatocellular carcinoma, and epidemiologic studies suggest its incidence is increasing worldwidely[1]. Advanced unresected CCA has an extremely dismal prognosis, with a median survival of < 24 mo; meanwhile, five-year survival rates after R0 resection for hilar CCA are 11% to 41% and for distal CCA are 27% to 37%[2]. The treatment of CCA is challenging, and surgical therapy is the only therapeutic option with a chance of cure. Owing to its unique patterns of invasion and metastasis, the disease recurrence rate was very high (> 50%) after R0 resection, either locoregional recurrence or distant metastases[3,4]. Recently, cutaneous or subcutaneous metastasis from CCA and gallbladder cancer was also noted[5-7], especially port-site metastasis (PSM) after laparoscopic surgery[8-14].
Since Mouret performed the first laparoscopic cholecystectomy in 1987, this technique has rapidly become the gold standard for the management of cholelithiasis. In the past decade, laparoscopic and robotic techniques have quickly expanded to the indication of various malignancies[15-17]. The advantages of a minimally invasive surgery are well established, such as shorter convalescence and decreased analgesic requirements, along with better cosmetic results. Laparoscopic oncology continues to be confirmed with favorable outcomes[18,19], however, reports of PSMs, a phenomenon of tumor seeding or implantation at the port-site of entry of the laparoscopic trocars, remain an area of concern[8-14,20-26]. There are very limited reports on the risk of PSMs occurring from robotic surgery for malignancies[27,28].
The goal of our study was to determine the incidence of PSMs in patients undergoing robotic surgery for biliary malignancies, which can somewhat extrapolate whether robotic surgery is indicated for advanced biliary cancers or not.
MATERIALS AND METHODS
This study was conducted with the approval of the institutional review board of PLA Second Artillery General Hospital. We maintain a prospective database of all patients undergoing robotic procedures performed by the Department of Hepatobiliary Surgery, and we reviewed all cases performed between January 2009 and January 2011. All patients with a confirmed biliary malignancy who underwent robotic surgery were included. Patients with benign disease, those who had died postoperatively or lost postoperative following-up, and those whose procedure converted to open or laparotomy were excluded. Data abstracted included the patient’s age at diagnosis, site of biliary tumor, primary histological diagnosis, American Joint Committee on Cancer (AJCC) stage, surgical procedures, intraoperative findings, presence of ascites, information regarding adjuvant radiochemotherapy after initial surgery, detection of PSM, time to the development of PSM, findings at the time of the diagnosis of PSM, and the overall survival for patients who developed PSMs. AJCC stage was assigned as delineated in the National Comprehensive Cancer Network Clinial Practice Guidelines on Hepatobliary Cancers (Version 2, 2012)[29]. The medical records were reviewed for all robotic procedures, including operative notes, radiology reports, pathology reports, and all involved progress notes. All patients included in this analysis had a regular every 3-mo follow-up physical examinations.
PSM was defined as subcutaneous tumor recurrence in the abdominal wall, near or within the scar tissue of the previous robotic-trocar site, which was detected either by clinical examination or by radiologic findings.
In all cases, the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) under a carbon dioxide pneumoperitoneum with the maximum intraabdominal pressure of 14 mmHg was used. On average, 5 or 6 trocars were placed during the procedure, and incision size ranged from 8 mm to 12 mm. One 12-mm trocar, usually infraumbilical, served as the camera port, and 3 robotic 8-mm trocars were placed 8-cm relative to each other. One or two additional 12-mm troars were used as assistant and choledochoscopic access. The entry into the abdominal cavity was achieved under direct visualization. The non-robotic trocar used in all cases was the Versaport trocar system (United States Surgical, North Heaven, Connecticut). The trocars were removed after the abdomen was deflated with the trocars in place. The 12-mm trocar incisions were closed in 2 layers at the fascia and the skin level, and the 8-mm trocar incisions were usually closed at the skin only with an attempt to close all layers. A lavage of the port sites with povidone-iodine solution was performed at the discretion of the surgeon.
Standard statistical analyses were used and performed using SPSS software, Version 17.0 (SPSS Inc., Chicago, IL). Descriptive statistics and proportions were used to report relevant demographic characteristics.
RESULTS
One hundred and ninety-four patients underwent robotic surgery procedures during the study period, including 64 cases with biliary cancers: intrahepatic biliary cystadenocarcinoma in 3, hilar CCA in 39, middle and distal biliary tract cancer in 11, and gallbladder cancer in 11. After exclusion of 2 patients with conversion to open procedures and 2 patients with postoperative death, we finally identified 60 patients who met inclusion criteria. Meanwhile, the 4 patients with open conversion or death were evaluated together when needed. The clinical characteristics of the patients are listed in Table 1.
Table 1.
Demographic and clinicopathological characteristics of the patients undergoing robotic surgery for biliary tract cancers
| Characteristics | No. of patients (n = 64) |
| Age (yr) | |
| Median | 67 |
| Range | 40-85 |
| Gender | |
| Male | 37 |
| Female | 27 |
| Tumor locations | |
| Intra-hepatic biliary cancer | 3 |
| Hiliar biliary cancer | 39 (1 conversion, 1 death) |
| Middle biliary cancer | 1 |
| Distal biliary cancer | 10 (1 conversion, 1 death) |
| Gallbladder cancer | 11 |
| Conversion to open procedures | 2 |
| Presence of ascites | |
| Yes | 8 |
| No | 56 |
| Use of retrieval bags | |
| Yes | 55 |
| No | 9 |
| Postoperative adjuvant radiochemotherapy | |
| Yes | 29 |
| No | 35 |
| Port-site metastasis | 2 |
All patients had no pre- and intra-operative evidence of distant intra-abdominal tumor metastasis. Eight severely jaundiced patients had an intraoperative finding of minor ascites. For retrieval of specimens, we usually applied surgical glove or ladle-like forcep to retrieve the freezing specimen or tumor thrombi intraoperatively, and use of retrieval bags for large specimens at the end of procedure. The postoperative primary histological diagnosis was biliary adenocarcinoma in 59 cases, biliary cystadenocarcinoma in 3, and miscellaneous in 2.
The tumor, node, metastasis stages of primary tumor type are outlined in Table 2. The robotic procedures performed are listed in Table 3. Among 64 patients with biliary tract cancers, 2 patients converted to open procedures, with a rate of conversion of 3.1%. The postoperative morbidity and mortality are listed in Table 4.
Table 2.
The American Joint Committee on Cancer stage of the 64 patients with biliary tract cancers
| Stage | Hilar biliary cancer (n = 39) | Mid- and distal biliary cancer (n = 11) | Gallbladder cancer (n = 11) | Intrahepatic biliary cystadenocarcinoma (n = 3) |
| T1 | 1 | 1 | 0 | 0 |
| T2 | 8 | 9 | 0 | 2 |
| T3 | 14 | 1 | 4 | 0 |
| T4 | 16 | 0 | 7 | 1 |
Table 3.
The diagnosis and robotic surgical procedures of the 64 patients with biliary tract cancer
| Tumor location | Surgical procedure | Number | Average time of procedures (min) |
| Intrahepatic biliary cystadenocarcinoma (n = 3) | Hepatic segmentectomy | 3 | 220 (170-260) |
| Hilar biliary cancer (n = 39) | Anatomical left hemihepatectomy | 3 (1 open conversion) | 530 (410-650) |
| Excision of tumor and GD-bridged biliary reconstruction | 3 | 415 (390-460) | |
| Excision of tumor and Roux-en-Y hepaticojejunostomy | 15 | 400 (350-510) | |
| Excision of tumor and biliary reconstruction | 1 | 350 | |
| Excision of tumor and external biliary drainage | 7 | 210 (190-290) | |
| Excision of tumor and T-tube biliary drainage | 10 (1 death) | 230 (210-280) | |
| Middle biliary cancer (n = 1) | Excision of tumor and Roux-en-Y hepaticojejunostomy | 1 | 330 |
| Distal biliary cancer (n = 10) | Whipple procedures | 10 (1 conversion, 1 death) | 720 (570-870) |
| Gallbladder cancer (n = 11) | Excision of GD tumor and Roux-en-Y hepaticojejunostomy | 2 | 450 (410-490) |
| Cholecystectomy | 3 | 200 (170-300) | |
| Cholecystectomy and internal biliary drainage | 1 | 220 | |
| Cholecystectomy and T-tube biliary drainage | 5 | 230 (210-280) |
GD: Gallbladder.
Table 4.
The postoperative morbidites and mortalities of the patients undergoing robotic surgery for biliary tract cancer (%)
| Complications | No. of patients (n = 64) |
| Open conversion | 2 (3.1) |
| Morbidity | 9 (14.1) |
| Minor biliary leakage | 3 |
| Intra-abdominal hemorrhage | 1 |
| Pancreatic leakage | 2 |
| Pulmonary infection | 2 |
| Acute renal failure | 1 |
| Mortality | 2 (3.1) |
| Pulmonary infection | 1 |
| Acute renal failure | 1 |
The median follow-up period for all of the patients was 15 mo (range: 11-34 mo). PSMs were detected in 2 (3.3%) of the 60 patients, both of them were absent of intraoperative ascites. The interval between robotic surgery and detection of PSM was 27 mo in patient 1 and 7 mo in patient 2.
Patient 1 was a 51-year-old woman, who admitted with complaints of abdominal pain, jaundice and generalized pruritus in February 2009. A diagnosis of Klatskin tumor (Bismuth classification IIIb) was suspected. Robotic anatomatic left hemihepatectomy and extraction of biliary tumor thrombi were performed. During the procedures, the glove-fingers were used for retrieval of tumor thrombi specimens (Figure 1). The pathology revealed well-differentiated papillary adenocarcinoma and biliary tumor thrombi arising from the left biliary duct. In order to prevent local tumor recurrence, a prophylactic stereotactic body radiation therapy (SBRT) with total dose of 42 Gy (12 times) was given to the hepatic margin at 6th postoperative month.
Figure 1.

During robotic procedure, the glove-fingers were used for retrieval of tumor thrombi specimens. 1, 2 and 3: The robotic arms, arm 1, 2 and 3.
In May, 2011, she was readmitted to our hospital for noting a subcutaneous mass at right lateral abdominal wall. Upon physical examination, a painless well-circumscribed, hard, subcutaneous mass near a trocar scar was palpated at the right lateral abdominal wall. Computed tomography (CT) scans confirmed a 2 cm × 1.5 cm subcutaneous mass (Figure 2A); no other obvious radiologic abnormalities were discovered. A subcutaneous tumor seeding was considered, and the mass was locally excised from the abdominal wall muscle (Figure 3A). Postoperative pathology confirmed metastatic biliary adenocarcinoma.
Figure 2.
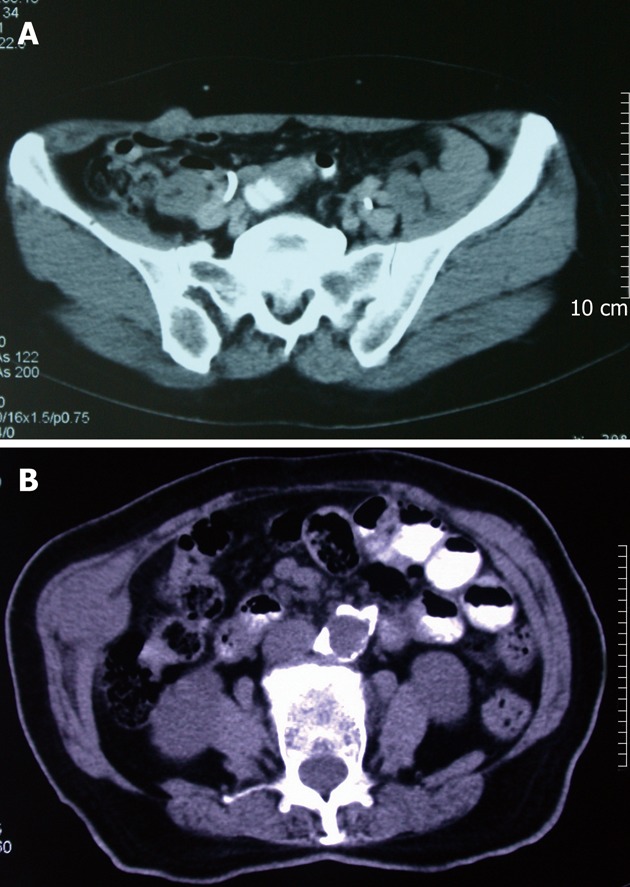
Computerized tomography scans of subcutaneous mass. A: A 2 cm × 1.5 cm subcutaneous mass at right lateral abdominal wall, which near a trocar scar on physical examination; B: A 4 cm × 3 cm × 3 cm subcutaneous mass at right lateral abdominal wall.
Figure 3.
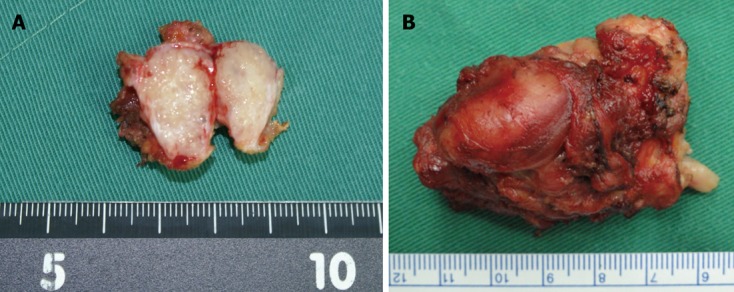
The subcutaneous mass was locally excised (A) and repeated excised locally (B) from abdominal wall muscle.
In November 2011, about 33-mo after the robotic surgery, she was discovered multiple intraabdominal masses on CT scans, and was managed by SBRT.
Patient 2 was a 75-year-old woman with painless jaun dice who was diagnosed as distal biliary tumor, and underwent robotic pylorus-preserving pancreaticoduodenectomy in July 2009. Postoperative pathology revealed poor-differentiated adenocarcinoma arising from the distal biliary duct. The preoperative serum CA199 level was 160 U/mL (normal range: 0-37 U/mL), then it dropped to normal range. An incisional hernia was occurred at infra-umbilical port site 2-mo after operation.
In February 2010, she had complained of a palpable painless subcutaneous mass of 3 cm × 2 cm size at right lateral abdominal wall under a robotic trocar scar, and which was removed by local excision. The pathology revealed seeding nodule of CCA. In September 2011, another 4 cm × 3 cm × 3 cm subcutaneous mass at the same site (Figure 2B) with obvious pain was noted, no other distal metastases were determined on CT scans. Meanwhile, the incisional hernia enlarged rapidly to a size of 13 cm × 13 cm; the serum CA199 level increased to 91.26 U/mL. Repeated local excision of subcutaneous mass (Figure 3B) and simultaneous incisional hernia repair with mesh implantation were performed. She recovered uneventfully. Postoperative pathology confirmed metastatic adenocarcinoma, and the serum CA199 level had returned to normal at that time.
DISCUSSION
Oncology-related laparoscopic PSMs were first reported in 1978, Döbrönte et al[20] described a PSM occurring 2 wk after laparoscopy in a patient with malignant ascites. Since then, a number of PSMs cases after diagnostic or therapeutic laparoscopy have been reported, involving nearly all abdominopelvic malignancies[8-14,20-26]. However, given the sparse published data, and the few events of PSM, the precise incidence of PSM has still not been well defined. Ramirez et al[23] estimated that the overall incidence of PSMs after laparoscopic surgery was 1%-2%; and in general laparoscopic surgery the incidence of tumor seeding ranges from 0.8% to 21%[5,30]. Noticeably, the incidence of PSMs after laparoscopic cholecystectomy for incidental gallbladder cancer was as high as 14%-29% within the first 2 years after the initial operation[11-14].
Our study showed that the incidence of PSM in patients undergoing robotic surgery for the management of biliary malignancies is 3.3% (2/60). The 3.3% PSMs incidence in this study seems much lower than the reported incidence after laparoscopic surgery for incidental gallbladder cancer, and a little higher than that in general laparoscopic surgery[23]. The relatively low incidence of PSMs reveals that advanced biliary malignancies can be selected as an indication of robotic or laparoscopic techniques. To the best of our knowledge, this is the first study evaluating the incidence of PSMs after robotic procedures for biliary malignancies. Although there are rare reported cases of PSM after robotic procedures in malignancies[27,28], the risk of PSM between robotic and laparoscopic surgery would be not significantly different, because of procedures and requirements such as CO2 pneumoperitoneum pressure (12-14 mmHg), the trocars placement are all much similar[28].
There are several postulated mechanisms for deve loping PSMs[23,30-34]. The most commonly discussed hypotheses include direct wound contamination and implantation, the biological aggressiveness of the primary tumor, haematogenous spread, effects of pneumoperitoneum that including the type of insufflating gas, chimney effect, aerosolization, surgical technique and the local immune response. Likewise the occurrence of PSMs is secondary to the above-mentioned multiple factors. Accordingly, several precautions were adopted with attempt to minimize the risk of PSMs[23,32-38].
The incidence of PSMs is much higher after laparoscopic surgery for incidental gallbladder carcinoma, which is mostly caused by tumor cells contamination due to gallbladder perforation during procedure and retrieval[8-14,39]. In this study, most of our patients had advanced biliary tract cancers. Their bile potentially contains tumor cells exfoliated from the primary tumor; bile ducts mobilization and dissection will probably detach more tumor cells into the bile; the robotic or laparoscopic instruments are inevitably contaminated by the spilled bile and exfoliated tumor cells. These possible scenarios resulted in consequent peritoneal carcinomatosis and PSM, especially in those with tumor thrombus, or undergoing R1 and R2 resection[5-7,40]. For patient 1, the procedures of tumor thrombi exposure and retrieval probably increase the risk of direct wound contamination of tumor cells, during specimen retrieval and instrument transfers.
This study raises a question regarding the indication of robotic or laparoscopic surgery for advanced biliary tract cancers. The initial result of this study justifies robotic surgery for biliary cancer on low incidence of PSMs. However, while either based on the surgical approaches, being robotic, laparoscopic or open, it is less important than the adherence to the principles of oncological surgery. All the procedures of violation of the primary tumor boundaries or damage of tumor-bearing lymph nodes, may promote tumor cell dissemination[41]. The conditions such as a presence of tumor thrombus and extensive intraabdominal metastases, may be a contraindication of robotic surgery for biliary tract cancer, because direct tumor manipulation infringes the oncological principles[41]. To prevent the trocar-site seeding in the case of biliary malignancy, we now routinely extract the specimen intact with the use of an impermeable laparoscopic retrieval bag[39]. Furthermore, we began to perform hyperthermal intraperitoneal chemotherapy at the end of robotic surgery to annihilate potential intraabdominal disseminated tumor cells[42].
In conclusion, the incidence of PSMs after robotic surgery for biliary cancer is relatively low, and biliary malignancies can be selected as an indication of robotic surgery. Although robotic surgery seemed more elaborate than laparoscopic surgery in managing biliary cancer, we should be aware of the risk of PSMs when dealing with malignancies. Emphasis on adhering to strict oncological surgical principles is the best method of prevention. Further studies are mandatory to better determine the role of robotic biliary surgery on the long-term oncological outcomes.
COMMENTS
Background
In the past decade, laparoscopic and robotic techniques have rapidly expanded to the indication of various malignancies. However, reports of port-site metastases (PSMs), a phenomenon of tumor seeding or implantation at the port-site of entry of the laparoscopic trocars, remain an area of concern. It has presumed that gallbladder cancer is a contraindication of laparoscopic technique owing to extremely high incidence of PSMs. This study estimates the incidence of clinically detected PSM in patients undergoing robotic surgery for biliary malignancies, in order to investigate the indication of biliary malignancies for robotic surgery.
Research frontiers
The laparoscopic technique has been little used for biliary malignancies, because the incidence of PSMs after laparoscopic cholecystectomy for incidental gallbladder cancer was as high as 14%-29% within the first 2 years after the initial operation. However, there are very limited reports on the risk of PSMs occurring from robotic surgery for malignancies, especially with no data of PSM incidence after robotic biliary surgery.
Innovations and breakthroughs
This is the largest number of case reports from a single-institution worldwidely. Total to sixty-four patients with biliary tract cancers underwent robotic surgery, and sixty patients met the inclusion criteria. During a median 15-mo follow-up period, two female patients were detected solitary PSM after robotic surgery. The incidence of PSM was 3.3%. The relatively low incidence of PSMs after robotic surgery for biliary malignancies would arouse the interest of reappraisal the indication of laparoscopic or robotic procedures for biliary cancers.
Applications
Robotic surgery for biliary malignancies can be selected as an indication in selected patients with early or advanced biliary cancers, such as gallbladder cancer, Klatskin tumor and distal biliary cancer. Meanwhile, an emphasis on adhering to strict oncological surgical principles is the best method of prevention of PSMs, and further studies are mandatory to better determine the role of robotic biliary surgery on the long-term oncological outcomes.
Terminology
PSM is a phenomenon of tumor seeding or implantation at the port-site of entry of the laparoscopic trocars after laparoscopic or robotic surgery for malignancies. The high incidence would raise a question on the oncologic safety of laparoscopic or robotic procedures for malignancies.
Peer review
This is an interesting report of the author´s experience in the management of patients with biliary malignancies by using robot.
Footnotes
Supported by Eleven-five Special Subject of PLA Medicine and Health, No. 08Z016
Peer reviewers: De Aretxabala Xabier, Professor of Surgery, Universidad de Chile, Santos Dumont 999, Santiago 8380000, Chile; Juli Busquets, MD, PhD, Department of Surgery, Hospital Universitari de Bellvitge, C/Feixa Llarga, S/N, 08907 Barcelona, Spain
S- Editor Shi ZF L- Editor A E- Editor Xiong L
References
- 1.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97:56–64. doi: 10.1002/bjs.6788. [DOI] [PubMed] [Google Scholar]
- 4.Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, Sueda T. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol. 2007;95:207–212. doi: 10.1002/jso.20668. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg. 2010;97:1860–1866. doi: 10.1002/bjs.7228. [DOI] [PubMed] [Google Scholar]
- 6.West KL, Selim MA, Puri PK. Cutaneous metastatic cholangiocarcinoma: a report of three cases and review of the literature. J Cutan Pathol. 2010;37:1230–1236. doi: 10.1111/j.1600-0560.2010.01619.x. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno T, Ishizaki Y, Komuro Y, Yoshimoto J, Sugo H, Miwa K, Kawasaki S. Surgical treatment of abdominal wall tumor seeding after percutaneous transhepatic biliary drainage. Am J Surg. 2007;193:511–513. doi: 10.1016/j.amjsurg.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Polychronidis A, Tsaroucha AK, Perente S, Giatromanolaki A, Koukourakis M, Simopoulos C. Port-site metastasis of extrahepatic bile duct carcinoma after laparoscopic cholecystectomy without evidence of a primary tumour. Acta Chir Belg. 2008;108:768–770. doi: 10.1080/00015458.2008.11680336. [DOI] [PubMed] [Google Scholar]
- 9.Ohmura Y, Yokoyama N, Tanada M, Takiyama W, Taka shima S. Port site recurrence of unexpected gallbladder carcinoma after a laparoscopic cholecystectomy: report of a case. Surg Today. 1999;29:71–75. doi: 10.1007/BF02482974. [DOI] [PubMed] [Google Scholar]
- 10.Baer HU, Metzger A, Glättli A, Klaiber C, Ruchti C, Czerniak A. Subcutaneous periumbilical metastasis of a gallbladder carcinoma after laparoscopic cholecystectomy. Surg Laparosc Endosc. 1995;5:59–63. [PubMed] [Google Scholar]
- 11.Ricardo AE, Feig BW, Ellis LM, Hunt KK, Curley SA, MacFadyen BV, Mansfield PF. Gallbladder cancer and trocar site recurrences. Am J Surg. 1997;174:619–622; discussion 622-623. doi: 10.1016/s0002-9610(97)00178-5. [DOI] [PubMed] [Google Scholar]
- 12.Paolucci V. Port site recurrences after laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2001;8:535–543. doi: 10.1007/s005340100022. [DOI] [PubMed] [Google Scholar]
- 13.Paolucci V, Schaeff B, Schneider M, Gutt C. Tumor seeding following laparoscopy: international survey. World J Surg. 1999;23:989–995; discussion 996-997. doi: 10.1007/s002689900613. [DOI] [PubMed] [Google Scholar]
- 14.Z’graggen K, Birrer S, Maurer CA, Wehrli H, Klaiber C, Baer HU. Incidence of port site recurrence after laparoscopic cholecystectomy for preoperatively unsuspected gallbladder carcinoma. Surgery. 1998;124:831–838. [PubMed] [Google Scholar]
- 15.Ceulemans R, Henri M, Leroy J, Marescaux J. Laparoscopic surgery for cancer: are we ready? Acta Gastroenterol Belg. 2003;66:227–230. [PubMed] [Google Scholar]
- 16.Yoshimura F, Inaba K, Kawamura Y, Ishida Y, Taniguchi K, Isogaki J, Satoh S, Kanaya S, Sakurai Y, Uyama I. Clinical outcome and clinicopathological characteristics of recurrence after laparoscopic gastrectomy for advanced gastric cancer. Digestion. 2011;83:184–190. doi: 10.1159/000322032. [DOI] [PubMed] [Google Scholar]
- 17.Kanji A, Gill RS, Shi X, Birch DW, Karmali S. Robotic-assisted colon and rectal surgery: a systematic review. Int J Med Robot. 2011;7:401–407. doi: 10.1002/rcs.432. [DOI] [PubMed] [Google Scholar]
- 18.Huang MJ, Liang JL, Wang H, Kang L, Deng YH, Wang JP. Laparoscopic-assisted versus open surgery for rectal cancer: a meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Colorectal Dis. 2011;26:415–421. doi: 10.1007/s00384-010-1091-6. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 20.Döbrönte Z, Wittmann T, Karácsony G. Rapid development of malignant metastases in the abdominal wall after laparoscopy. Endoscopy. 1978;10:127–130. doi: 10.1055/s-0028-1098280. [DOI] [PubMed] [Google Scholar]
- 21.Zivanovic O, Sonoda Y, Diaz JP, Levine DA, Brown CL, Chi DS, Barakat RR, Abu-Rustum NR. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol Oncol. 2008;111:431–437. doi: 10.1016/j.ygyno.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Freeman RK, Wait MA. Port site metastasis after laparoscopic staging of esophageal carcinoma. Ann Thorac Surg. 2001;71:1032–1034. doi: 10.1016/s0003-4975(00)02435-8. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179–189. doi: 10.1016/s0090-8258(03)00507-9. [DOI] [PubMed] [Google Scholar]
- 24.Chen YY, Yen HH. Subcutaneous metastases after laparoscopic-assisted partial hepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2011;21:e41–e43. doi: 10.1097/SLE.0b013e3182078ac3. [DOI] [PubMed] [Google Scholar]
- 25.Micali S, Celia A, Bove P, De Stefani S, Sighinolfi MC, Kavoussi LR, Bianchi G. Tumor seeding in urological laparoscopy: an international survey. J Urol. 2004;171:2151–2154. doi: 10.1097/01.ju.0000124929.05706.6b. [DOI] [PubMed] [Google Scholar]
- 26.Rassweiler J, Tsivian A, Kumar AV, Lymberakis C, Schulze M, Seeman O, Frede T. Oncological safety of laparoscopic surgery for urological malignancy: experience with more than 1,000 operations. J Urol. 2003;169:2072–2075. doi: 10.1097/01.ju.0000067469.01244.5c. [DOI] [PubMed] [Google Scholar]
- 27.El-Tabey NA, Shoma AM. Port site metastases after robot-assisted laparoscopic radical cystectomy. Urology. 2005;66:1110. doi: 10.1016/j.urology.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Ndofor BT, Soliman PT, Schmeler KM, Nick AM, Frumovitz M, Ramirez PT. Rate of port-site metastasis is uncommon in patients undergoing robotic surgery for gynecological malignancies. Int J Gynecol Cancer. 2011;21:936–940. doi: 10.1097/IGC.0b013e3182174609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines ®): Hepatobliary Cancers Version 2. 2012. Available from: http: //www.nccn.org/professionals/physician_gls/f_guidelines.asp#hepatobiliary.
- 30.Rané A, Eng MK, Keeley FX. Port site metastases. Curr Opin Urol. 2008;18:185–189. doi: 10.1097/MOU.0b013e3282f4ab73. [DOI] [PubMed] [Google Scholar]
- 31.Pemberton RJ, Tolley DA, van Velthoven RF. Prevention and management of complications in urological laparoscopic port site placement. Eur Urol. 2006;50:958–968. doi: 10.1016/j.eururo.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 32.Steinert R, Lippert H, Reymond MA. Tumor cell dissemination during laparoscopy: prevention and therapeutic opportunities. Dig Surg. 2002;19:464–472. doi: 10.1159/000067598. [DOI] [PubMed] [Google Scholar]
- 33.Ziprin P, Ridgway PF, Peck DH, Darzi AW. The theories and realities of port-site metastases: a critical appraisal. J Am Coll Surg. 2002;195:395–408. doi: 10.1016/s1072-7515(02)01249-8. [DOI] [PubMed] [Google Scholar]
- 34.Sooriakumaran P, Kommu SS, Anderson C, Rane A. Port-site metastasis after laparoscopic surgery: what causes them and what can be done to reduce their incidence? BJU Int. 2009;103:1150–1153. doi: 10.1111/j.1464-410X.2009.08363.x. [DOI] [PubMed] [Google Scholar]
- 35.Jurczok A, Schneider A, Fornara P. Inhibition of tumor implantation after laparoscopy by specific oligopeptides: a novel approach to adjuvant intraperitoneal therapy to prevent tumor implantation in an animal model. Eur Urol. 2007;52:590–595. doi: 10.1016/j.eururo.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 36.Wittich P, Mearadji A, Marquet RL, Bonjer HJ. Irrigation of port sites: prevention of port site metastases? J Laparoendosc Adv Surg Tech A. 2004;14:125–129. doi: 10.1089/1092642041255423. [DOI] [PubMed] [Google Scholar]
- 37.Schneider C, Jung A, Reymond MA, Tannapfel A, Balli J, Franklin ME, Hohenberger W, Köckerling F. Efficacy of surgical measures in preventing port-site recurrences in a porcine model. Surg Endosc. 2001;15:121–125. doi: 10.1007/s004640010069. [DOI] [PubMed] [Google Scholar]
- 38.Gerhards MF, Gonzalez DG, ten Hoopen-Neumann H, van Gulik TM, de Wit LT, Gouma DJ. Prevention of implantation metastases after resection of proximal bile duct tumours with pre-operative low dose radiation therapy. Eur J Surg Oncol. 2000;26:480–485. doi: 10.1053/ejso.1999.0926. [DOI] [PubMed] [Google Scholar]
- 39.Goetze TO, Paolucci V. Use of retrieval bags in incidental gallbladder cancer cases. World J Surg. 2009;33:2161–2165. doi: 10.1007/s00268-009-0163-7. [DOI] [PubMed] [Google Scholar]
- 40.Lee JM, Kim BW, Kim WH, Wang HJ, Kim MW. Clinical implication of bile spillage in patients undergoing laparoscopic cholecystectomy for gallbladder cancer. Am Surg. 2011;77:697–701. [PubMed] [Google Scholar]
- 41.Mutter D, Hajri A, Tassetti V, Solis-Caxaj C, Aprahamian M, Marescaux J. Increased tumor growth and spread after laparoscopy vs laparotomy: influence of tumor manipulation in a rat model. Surg Endosc. 1999;13:365–370. doi: 10.1007/s004649900991. [DOI] [PubMed] [Google Scholar]
- 42.Ba MC, Cui SZ, Lin SQ, Tang YQ, Wu YB, Wang B, Zhang XL. Chemotherapy with laparoscope-assisted continuous circulatory hyperthermic intraperitoneal perfusion for malignant ascites. World J Gastroenterol. 2010;16:1901–1907. doi: 10.3748/wjg.v16.i15.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]


