Abstract
More than 76 million people worldwide are estimated to have diagnosable Alcohol Use Disorders (AUDs) (alcohol abuse or dependence), making these disorders a major global health problem. Pharmacotherapy offers promising means for treating AUDs, and significant progress has been made in the past 20 years. The U.S. Food and Drug Administration approved three of the four medications for alcoholism in the last two decades. Unfortunately, these medications do not work for everyone, prompting the need for a personalized approach to optimize clinical benefit or more efficacious medications that can treat a wider range of patients, or both. To promote global health, the potential reorganization of the National Institutes of Health (NIH) must continue to support the National Institute on Alcohol Abuse and Alcoholism’s (NIAAA’s) vision of ensuring the development and delivery of new and more efficacious medications to treat AUDs in the coming decade. To achieve this objective, the NIAAA Medications Development Team has identified three fundamental long-range goals: 1) to make the drug development process more efficient; 2) to identify more efficacious medications, personalize treatment approaches, or both, and 3) to facilitate the implementation and adaptation of medications in real-world treatment settings. These goals will be carried out through seven key objectives. This paper describes those objectives in terms of rationale and strategy. Successful implementation of these objectives will result in the development of more efficacious and safe medications, provide a greater selection of therapy options, and ultimately lessen the impact of this devastating disorder.
Keywords: alcohol, medications, drug development, Alcohol Use Disorders, alcohol dependence
Alcohol Use Disorders (AUDs) (alcohol abuse and dependence) affect 76 million adults worldwide, including 18 million Americans, and are responsible for a myriad of medical, psychological, social, economic, and personal problems (World Health Organization, 2004; Grant et al., 2004). AUDs rank among the leading causes of decrements in disability-adjusted life-years (DALYs) and rank third in preventable causes of death in the United States (Mokdad et al., 2004; Michaud et al, 2006). Tragically, more than 2.5 million individuals including 75,000 Americans die each year from alcohol-related events. The U.S. healthcare costs associated with AUDs are approximately 30 billion dollars annually, and the total economic cost to society is a staggering 235 billion dollars each year (Rehm et al., 2009).
AUDs are heterogeneous, complex disorders. They result from an interaction of genetic and environmental factors that differ from one drinker to another to produce a variety of phenotypes. Because of this great variability, it is unlikely that any single treatment intervention will be effective for all individuals with AUDs. Fortunately, during the past 20 years significant advances have been made. Currently, there are four medications approved by the U.S. Food and Drug Administration (FDA) to treat alcohol dependence: disulfiram, oral naltrexone, acamprosate, and a long-acting injectable naltrexone (O’Malley and O’Connor, 2011; Johnson, 2008; Litten et al., 2005). Unlike disulfiram, which causes an unpleasant aversive reaction when drinking, the medications approved most recently, naltrexone and acamprosate, appear to act by reducing craving or urge to drink (Litten et al., 2005). In addition to these medications, in a recent multi-site trial, topiramate, an FDA-approved medication for the treatment of epilepsy, has shown robust findings in reducing drinking in alcohol dependent patients (Johnson et al., 2007). Still, these medications do not work for everyone. Effect sizes generally are small and approximate those found in other psychiatric medications, such as antidepressants (Turner et al., 2008). The heterogeneity and behavioral complexities associated with alcohol dependence negate a simplistic one-size-fits-all approach to alcoholism treatment and therefore also to the development of medications for this complex disorder. Additional research is vital to develop more efficacious and safe alcohol medications. Similar to the paradigm used by physicians to treat depression, an arsenal of medication choices is optimal for the effective treatment of this heterogeneous disorder.
Although four medications have now been approved by the FDA for alcohol dependence, the process of drug development continues to be a challenge. For medications in general, the path to market for a successful candidate is long, costly, and inefficient. The average time to move a compound from discovery to market in the United States is 13.5 years (Paul et al., 2010; Munos, 2009). More than half of this time is spent after the Investigational New Drug (IND) paperwork has been filed. The process is also expensive, with costs averaging 1.8 billion dollars from discovery to launch (Paul et al., 2010; Munos, 2009). Finally, the probability of success is low—only 1 out of every 10,000 compounds screened will successfully make it into the pharmaceutical market. This high failure rate is a significant contributor to the high cost of drug development.
For central nervous system (CNS) compounds, clinical development has become even more challenging. It takes about 18 years to move a candidate compound in this category from discovery to market, 4.5 years longer than the average therapeutic compound (Kaitin and Milne, 2011). Furthermore, only 8 percent of new CNS compounds entering Phase 1 will reach the marketplace (Miller, 2010; Hurko and Ryan, 2005). Only 46 percent of CNS candidates succeeded in pivotal Phase 3 trials compared with 66 percent, on average, for all compounds (Kaitin and Milne, 2011). It is difficult to evaluate the drug development process of alcohol medications since the vast majority of compounds evaluated for alcohol treatment have been developed originally for other medical indications. Surprisingly, despite significant advances in the scientific discovery of new targets for medications, the likelihood of reaching FDA approval is no better now than it was 20 years ago (Miller, 2010). The FDA recently issued several white papers to address ways to increase the efficiency of the drug development process, including strategies to develop better evaluation tools as well as to streamline clinical trials (FDA, 2004 and 2006a).
To counter these problems in drug development, the NIAAA Medications Development Team has identified three long-term goals that will be crucial for moving research forward during the next 10 years. Those goals include 1) developing approaches to make the development of alcohol dependence medications more efficient—faster, more predictable, and less expensive; for example, by addressing the lengthy development of CNS compounds versus non-CNS compounds (Kaitin and Milne, 2011); 2) developing strategies to increase the effect size of candidate compounds in alcohol dependence clinical trials. Most of the multi-site trials for experimental medications have shown either no effect or a small effect (Johnson, 2008; Litten et al., 2005). Phase 3 trials are less likely to be funded when only small effect sizes are observed in proof-of-concept trials; and 3) facilitating the use of alcohol medications in real-world clinical practice. Currently, only 10 to 13 percent of patients with AUDs are routinely prescribed alcohol medications (Mark et al., 2009; McLellan, 2007). To accomplish these goals, seven key objectives have been identified: 1) to discover and validate new molecular targets for the treatment of alcohol dependence. This effort holds the promise of identifying novel therapeutics as well as more favorable side-effect profiles; 2) to develop and implement animal and human laboratory paradigms as screening models for drug development; 3) to bridge the often-discussed gaps in the drug development process (referred to as the “Valley of Death”) through a fully translational therapeutics development program; 4) to develop methodological approaches for conducting alcohol dependence clinical trials that are more efficient, both in terms of their economic and time costs; 5) to advance personalized medicine in the pursuit of new compounds, as a means of increasing the effect size in adequately selected patients; 6) to identify and remove barriers to the implementation and adoption of alcohol medications in real-world treatment settings; and 7) to facilitate the development of collaborative networks and partnerships among pertinent stakeholders seeking new therapeutics for addictive disorders, such as the Federal government, the pharmaceutical industry, academia, healthcare organizations, as well as patient and advocacy groups. Carrying out the first five objectives will help accomplish our first long-range goal of making the drug development process more efficient. Objectives 1, 4, and 5 likely will improve the signal detection problem in alcohol clinical trials, whereas objective 6 will facilitate the implementation and adoption of medication use. Finally, objective 7 will be essential in carrying out all three long-range goals. Each of these objectives is discussed in detail below in terms of rationale and corresponding strategy. While the purpose of this paper is to articulate these objectives, we acknowledge that this is not a comprehensive or critical review of the field.
Objective #1: Identify and Validate New Molecular Targets for the Treatment of Alcohol Use Disorders
A fundamental goal of medications development is to identify therapeutic targets and effective compounds. Research during the past three decades has enriched our understanding of biological mechanisms underlying alcohol dependence. In the late 1980’s, targets for drug development mainly were limited to serotonergic and dopaminergic neurotransmitter systems (Litten and Allen, 1991; Gorelick, 1989). Work over the subsequent 20 years, however, revealed that various neurotransmitter systems, neuromodulators, and intracellular signaling pathways have a role in alcohol dependence. Some of these targets have been validated, either in preclinical and/or clinical studies, as potential therapeutic targets for the treatment of alcohol dependence (Table 1).
Table 1.
Targets of Interest for Alcohol Dependence Medications
| Receptors | Representative Compounds | Status |
|---|---|---|
| 5–HT3* | ondansetron | Preclinical, clinical |
| 5–HT2* | olanzapine | Preclinical, clinical |
| adenosine A2a | DMPX | Preclinical |
| adrenergic α1* | prazosin | Preclinical, clinical |
| AMPA/GABAa receptor* | topiramate | Preclinical, clinical |
| CRF1 | antalarmin, MTIP, Pexacerfont, GSK561679 | Preclinical, clinical |
| GABA–A | RY 023 | Preclinical |
| GABA–B* | baclofen, CGP7930, GS39783, BHF177 | Preclinical, clinical |
| GLT1* | ceftriaxone | Preclinical |
| mGluRs | MTEP | Preclinical |
| nAChRs* | varenicline, CP–601932 | Preclinical, clinical |
| NK1* | LY686017, Aprepitant | Preclinical, clinical |
| NMDA Receptors* | neramexane, memantine | Preclinical, clinical |
| NOP | OS–462, UFP–102, UFP–112 | Preclinical |
| NPS receptor | NCG–0018568403 | Preclinical |
| NPY1, NPY2 | BIIE0246, JNJ–31020028, | Preclinical |
| opioid receptors* | nalmefene, naltrexone | Preclinical, clinical |
| DOR | SoRI–9409, TAN–67 | Preclinical |
| KOR | Nor–BNI | Preclinical |
| OX1, OX2 | SB–334867, JNJ–10397049 | Preclinical |
| P2X4* | Ivermectin | Preclinical |
| Vasopressin 1b | nelivaptan (SSR 149415) | Preclinical |
| Other Targets | ||
| ALDH–2 | CVT–10216 | Preclinical |
| GDNF | noribogaine | Preclinical |
| Glycine transporter, GlyT-1 | Org 25935 | Preclinical, clinical |
| HDAC* | SAHA, trichostatin A | Preclinical |
| L–type calcium channel auxiliary subunit, GABA modulator | gabapentin, pregabalin | Preclinical, clinical |
| mTORC1* | rapamycin, | Preclinical |
| Neuroimmune modulation | minocycline, ibudilast, pioglitazone | Preclinical |
| PDE4* | rolipram | Preclinical |
| PKC–epsilon | proprietary | Preclinical |
| Serotonin–norepinephrine transporter | duloxetine | Preclinical, clinical |
| Sigma–1 receptor | NE-100 | Preclinical |
5-HT, 5–Hydroxytryptamine ; ADLH, aldehyde dehydrogenase; AMPA, α–amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CB, cannabinoid; CRF, corticotrophin releasing factor; DOR, d-opioid receptor; GABA, gamma–aminobutyric acid; GDNF, glial derived neurotrophic factor; GLT1, glutamate transporter 1; HDAC, Histone deacetylase; KOR, δ–opioid receptor; mGluR, metabotropic glutamate receptor; MOR, μ–opioid receptor; mTOR1, mammalian target of rapamycin complex 1; nAChR, nicotinic acetylcholine receptors; NK, neurokinin; NOP, opioid receptor-like 1; NPS, Neuropeptide S; NPY, neuropeptide Y; OX, orexin receptor; P2X4, purinergic receptor P2X, ligand–gated ion channel, 4; PDE: phosphodiesterase; PKC, protein kinase C.
FDA-approved compounds available for this target.
Additional research is needed to identify targets that will produce more effective compounds with minimal side-effects. A better understanding of the biological mechanisms, including the different components underlying alcohol dependence, is vital. These components consist of reward, negative affect, stress, craving, incentive salience, impulsivity, compulsivity, habituation, executive function, and cognitive function (Redish et al., 2008; Koob and Volkow, 2009; George and Koob, 2010). Alcohol acts on systems in the brain, and causes adaptive changes in neurocircuitry, which contribute to the development of alcohol dependence (Redish et al., 2008; Koob and Volkow, 2009; George and Koob, 2010). The pathophysiology of alcoholism involves adaptations in a variety of ion channels, neurotransmitters, neuropeptides, and intracellular signaling systems (Johnson, 2008; Koob and Volkow, 2009; Spanagel, 2009; Johnson, 2008; Koob and Bloom, 1988). The efficacies of medications are influenced not only by actions on the targets but also by the modulatory systems. Finally, the pathophysiology of alcohol dependence will differ between and among individuals as a function of their pre-existing genetic vulnerabilities, their exposure to environmental risk factors, and during the progression of alcoholism.
The biological complexity of alcohol dependence suggests that focusing on a single target will not adequately address the clinical problem. It may prove necessary to target combinations of systems simultaneously, either through combined pharmacotherapy, or using compounds that act on multiple targets Johnson, 2008; Roth et al., 2004; Johnson et al., 2003). The rational for designing a combined pharmacology strategy faces considerable preclinical challenges, particularly with regard to the identification of the right combination of targets (Hopkins, 2008). The effectiveness of this approach in the treatment of other diseases, however, indicates its potential application in alcohol medication development. An empirically informed approach to this task hinges on the breakdown of the complex phenotype of clinical “alcoholism” into biologically defined intermediate component traits that may be found in varying combinations in different patients.
Exploring genetic and environmental factors involved in the pathophysiology of alcoholism, as well as the interactions between them, may aid the discovery of novel targets for medications development. A mechanistic understanding of addictive processes requires studies at multiple levels of complexity, from genes, transcriptional/translational processes, proteins, intracellular pathways, cellular connections, and circuits, to behavior. Understanding genes to behavioral pathways requires interdisciplinary studies. Ultimately, this will lead to a better understanding of alcohol dependence (Kendler, 2005). Often the serendipitous discovery of effective medications provides a better understanding of disease pathophysiology in general, regardless of how those new targets were identified. Moreover, because of the high prevalence of psychiatric/substance abuse comorbidity, identifying neural systems involved in comorbid disorders (Haber and Rauch, 2010 ; Insel, 2010) and their relationship to those associated with alcohol addiction may yield additional targets, which could be especially important for alcohol dependent patients with psychiatric comorbidity.
Exploring different components of alcohol dependence and the corresponding individual vulnerabilities to these components also could lead to different clinical profiles of dependent patients. This differentiation among the dependent patient clinical profiles will assist in developing specific treatment strategies, both with regard to pharmacological and nonpharmacological interventions within each component of alcohol dependence. Targeting medications to the specific additive components will, most likely, advance personalized medicine, an objective discussed in detail below.
Objective #2: Developing and Implementing Screening Models Using Animal and Human Laboratory Paradigms
Many animal and human laboratory paradigms are used to study different facets of alcohol dependence (Crabbe et al., 2010; Heilig et al., 2010; Leeman et al., 2010; Stephens et al., 2010; Koob et al., 2009; Mason et al., 2008; Egli, 2005; Anton et al., 2004; O’Malley et al. 2002). These models also are used for evaluating medications with the ultimate aim of informing decisions to carry out clinical trials (Egli, 2005, Koob et al. 2009). To gauge the predictive value of animal and human laboratory models, medications which have been evaluated clinically should also be tested in preclinical animal and human laboratory models (Koob et al. 2009; Mello, 1992; Johnson et al., 2005). The subsequent concordance between preclinical models and clinical studies reveals the extent to which the preclinical data may predict clinical outcomes. This strategy includes testing FDA approved medications for alcohol dependence, as well as medications such as baclofen, ondansetron, and topiramate, which have been extensively evaluated in clinical studies (Heilig and Egli, 2006). Table 2 shows the effects of naltrexone, acamprosate, and topiramate in tests modeling various aspects of alcohol dependence. As a negative control, notable failures, such as bromocriptine and ritanserin (Naranjo et al., 1997; Johnson et al., 1996; Powell et al., 1995), also are available; the numbers of compounds falling into this category are likely to grow in future years as more medications for alcohol dependence are evaluated clinically. It is essential that standard methods be used to evaluate, reference, and test medications because changes to the protocol or animal species or strain can affect the results. This approach has been used successfully by the National Institute of Neurological Disorders and Stroke. It remains to be determined whether a common signal or set of signals from the models will emerge for the diverse medications that are likely to show clinical efficacy for alcohol dependence.
Table 2.
Effects of Clinically Effective Medications on Animal Models of Alcohol Addiction
| Animal Model | Naltrexone | Acamprosate | Topiramate | |
|---|---|---|---|---|
| Acute Reinforcing Effects of Alcohol | Alcohol self-administration | ↓ | No effect | ↓ |
| Conditioned place preference | ↓ | ↓ | No effect | |
| Alcohol deprivation effect | ↓ | ↓ | ↓ | |
| Increased alcohol self- administration (dependent animals) | ↓ | ↓ | ↓ | |
| Withdrawal/Negative Affect | Anxiety-like responses | No effect | ↓ | ↓ |
| Conditioned place aversion | No effect* | Not reported | Not reported | |
| Relapse to Heavy Drinking | Stress-induced reinstatement | No effect | Not reported | ↓† |
| Alcohol-induced reinstatement | ↓ | Not reported | Not reported | |
| Cue-induced reinstatement | ↓ | ↓ | Not reported |
Data based on Egli, 2005 and Koob et al., 2009.
Naloxone had no effect on expression of conditioned place aversion, but enhanced acquisition.
Topiramate reduced stress enhanced ethanol drinking in C57 mice.
A review of the literature supports the plausibility of using animal models to evaluate medications for alcohol dependence, particularly those that model biological factors contributing to excessive drinking (Egli, 2005). Consequently, NIAAA’s Preclinical Medication Efficacy Testing Program was initiated to test medications in three different animal models of alcohol dependence. These models consist of limited access alcohol drinking in P (i.e., alcohol preferring) and HAD1 (i.e., high-alcohol drinking) rats selectively bred for alcohol preference (Li et al. 1987, 1993) and in mice that drink large volumes of alcohol after they are made dependent through chronic-intermittent alcohol vapor exposure (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005). The latter model is similar to work that originally established this approach in rats (Roberts et al., 2000; Rimondini et al., 2002; Heilig and Koob, 2007). An ongoing component of this program is to evaluate reference compounds and then compare the results with those of test compounds. This includes examining medications that already have undergone clinical evaluation. In these evaluations, naltrexone and topiramate, medications that showed the most consistent effects in clinical studies (Johnson, 2008; Litten et al., 2005), displayed robust dose-dependent effects in these animal models. This data is then used to compare the effects of novel, proprietary compounds submitted to NIAAA by pharmaceutical companies and other sources. These models are used to evaluate medications that already have gone through clinical testing enabling us to further refine the results and to better determine the clinical utility of the test compounds.
NIAAA’s Integrative Neuroscience Initiative on Alcoholism (INIA) West consortium is performing a complementary effort to validate and identify targets for medication development for the treatment of alcoholism. Primary in vivo screens are implemented in three stages using models representing distinct aspects of alcohol dependence as illustrated in Table 2. Stage I tests reflect the acute reinforcing effects of alcohol as assessed by operant self-administration of alcohol by rats and by continuous and limited access to drinking among mice and alcohol-preferring P and HAD rats. INIA West also has developed two additional lines of mice (High Drinking in the Dark [HDID1, 2]) that display high consumption during limited access to alcohol. Stage II tests include dependence-induced excessive drinking and motivational withdrawal measures. Stage III tests focus on relapse and measure a drug’s influence on alcohol- and stress-induced reinstatement of animals’ self-administration as well as their response to a conditioned approach. It is anticipated that all drugs will undergo Stage I and Stage II testing, whereas Stage III testing and additional secondary screens will be reserved for compounds that yield positive results in Stages I and II. A major goal of the INIA West target validation effort is to use the extensive gene expression databases to select novel molecules in concert with molecules that already have obtained FDA approval for human use to expedite confirmation of preclinical findings in human clinical studies.
A similar research program is being planned for human laboratory paradigms. So far, significant progress has been made in developing a variety of human laboratory models (Roache, 2010; Chaplin et al., 2008; Mason et al., 2008; Miranda et al., 2008; Ramchandani et al., 2006; Anton et al, 2004; O’Malley et al., 2002). Three major human laboratory paradigms are used—cue-induced (e.g., alcohol, positive and negative images) reactivity, alcohol-self administration, and alcohol administration—each with several variations. An important aspect when using human lab paradigms as screening models for medications is the target population. Because subtypes of alcohol dependent patients may respond differently to alcohol medications, the subject population used in the human laboratory (as well as subsequent clinical trials) should reflect the patient population most likely to benefit. This strategy was used by NIAAA Intramural investigators to study the effects of a neurokinin-1 (NK1) antagonist on alcohol craving in recently detoxified alcoholics (George et al., 2008). Because NK1 receptors modulate stress and anxiety responses, the researchers recruited subjects with trait anxiety disorder. One challenge in enrolling such similar populations is that non-treatment seeking alcohol dependent patients usually are recruited for human laboratory studies, whereas treatment-seeking alcoholics are enrolled in clinical trials. The non-treatment population generally is younger and drinks less than those in the treatment-seeking population (Anton et al 2004 and 2006). Moreover, in nicotine-dependence studies, these two populations appear to differ greatly in their response to medications (Perkins et al., 2008). Again, the best validation method would be to use the same subjects for both human laboratory studies and clinical trials, an approach currently being investigated. Using this method, we are able to compare outcomes of those who respond favorably to the medication in the trial (e.g., reduction in heavy drinking) with their response in the human laboratory paradigm (e.g., reduced cue-induced craving and decreased self-administration), thus helping to determine the predictive validity of the laboratory model under investigation. Disadvantages of this approach, however, include a possible diminution of the ecological validity of the clinical trial as well as exposing treatment-seeking patients to possible further deterioration resulting from alcohol self-administration in certain human laboratory paradigms.
An essential step in medications development over the next decade will be to further refine animal and human laboratory paradigms that can be used as reliable screening models, making the drug development process both faster and more predictable.
Objective #3: Bridge Gaps in the Drug Development Process
Drug development for alcohol dependence is a relatively young field compared with other fields, such as cancer, cardiovascular diseases, and psychiatric disorders. Alcohol researchers have explored many potential targets in the brain for drug development during the past 20 years (examples in Table 1). Most of these targets have been tested in animal and human studies using medications that currently are approved by the FDA for other medical indications (Litten et al., 2005). Recently, however, a number of novel compounds developed for other medical indications also have been tested in Phase 2 alcohol clinical trials by pharmaceutical companies, such as Lilly, Alkermes, and Merck.
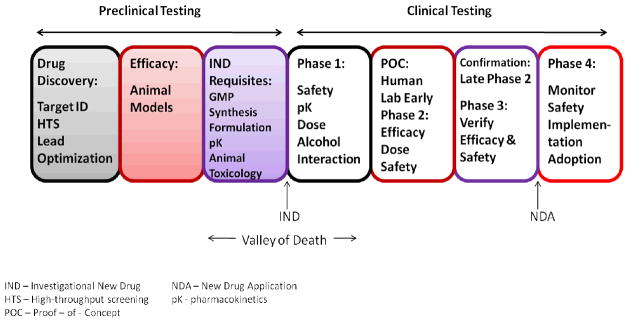
As the alcohol field accelerates its development of novel compounds, it is important to have an infrastructure to move these compounds efficiently along the pipeline from drug discovery through preclinical testing to clinical trials (Figure 1). Currently, the National Institutes of Health (NIH) has several programs to advance drug discovery and novel compound development during the early stages of drug development. For example, the NIH Common Fund Molecular Libraries and Imaging program (http://commonfund.nih.gov/molecularlibraries/) offers researchers access to large scale screening assays to identify small molecules that can be optimized as chemical probes in the study of gene and cell function and the biochemical pathways that serve as targets for new drug development. This resource already is being used in search of alcoholism therapeutics (McCoy et al., 2010). The NIH Rapid Access to Interventional Development (NIH–RAID) program (http://commonfund.nih.gov/raid/) also is available, on a competitive basis, to move novel compounds through the preclinical development phase. Available services include access to bulk supplies, Good Manufacturing Practice (GMP), formulations, pharmacokinetic testing, and animal toxicology reports. (This program will be re-launched soon under a new name Bridging Interventional Development Gaps). NIH also intends to establish a new entity, the National Center for Advancing Translational Sciences (NCATS), to accelerate the development, testing, and implementation of candidate compounds across a wide range of diseases (Collins, 2011). This Institute will incorporate the programs described above as well as other approaches to expedite the drug development process. In addition, the NIH Clinical Center has been designated as a national resource, and will be making its resources available on a competitive basis to academic investigators. Of note, one of the first resources in this category are the Clinical Center Pharmaceutical Development Services, which can formulate clinical trials materials, manufacture placebo, and monitor stability of materials—functions often not available to individual academic investigators.
Figure 1.
Phases of Alcohol and Drug Development
The “Valley of Death” for alcohol drug development—the gap between preclinical development and clinical testing—is moving novel compounds through IND requirements and human Phase 1 testing after the compound has demonstrated efficacy in animal models. This gap in development has impeded several promising novel compounds from moving forward along the drug development pipeline (Barron and Littleton, 2011; Wang et al., 2002). To successfully navigate this obstacle, in addition to NIH-wide programs, NIAAA may need to contract with Clinical Research Organizations (CROs) and Clinical Manufacturing Organizations (CMOs) to facilitate the timely development of candidate compounds. Finally, future partnerships between NIAAA and the pharmaceutical/biotechnology industry offer another resource for successfully moving promising compounds through development.
Objective #4: Conduct Clinical Trials More Efficiently Using Enhanced Methodology and Facilitation of Proof of Concept Trials
Proof-of-concept trials (early Phase 2) and large confirmatory Phase 3 trials are essential components of the drug development process. Currently, NIAAA supports proof-of-concept trials and relies on pharmaceutical companies to conduct the larger confirmatory trials. Since 1992, NIAAA has made medications development a high priority area and currently supports more than 30 pharmacotherapy clinical trials through a variety of grant mechanisms. Medications under investigation include baclofen, pregabalin, zonisamide, gabapentin, ondansetron, duloxetine, olanzapine, and prazosin. In addition, medications are being tested in understudied populations, such as those with comorbid psychiatric disorders (depression, anxiety disorders, bipolar, schizophrenia), comorbid substance abuse (tobacco, cocaine), comorbid medical disorders (HIV/AIDS, Hepatitis C), as well as in adolescents and young adults.
A drawback to proof-of-concept projects is that they take on average 5 to 6 years to complete. In response, the NIAAA Medications Team recently created a new proof-of-concept trial system that trims this time to 1 to 1.5 years. This initiative, called the NIAAA Clinical Investigation Group (NCIG), was approved by the NIAAA External Advisory Board and NIAAA Council, and utilizes a contract mechanism to achieve the desired efficiency. NCIG’s organization includes a steering committee (the NIAAA Medications Team), a CRO, multiple academic clinical sites, a pharmacy, and a monitoring group. The basic NCIG trial model stipulates that each clinical research site must enroll four subjects per recruitment month and adhere to FDA regulations and International Conference on Harmonisation (ICH) Good Clinical Practices (GCP). In addition to a thorough review by site Institute Review Boards (IRBs), the clinical trial protocol is reviewed by the FDA and an independent Data and Safety Monitoring Board (DSMB).
Currently, two multi-site trials have been completed and a third is underway. Results of the first NCIG trial demonstrated a lack of efficacy of quetiapine in very heavy drinking alcohol dependent patients (Litten et al., 2011), while the results of NCIG 2 evaluating levetiracetam currently is being analyzed. A one year follow-up study of subjects in all NCIG trials also is underway to determine why some subjects remain abstinent whereas others relapse to drinking. During the next decade, as more promising agents become available, the NCIG operation may be expanded to test multiple compounds simultaneously.
With regard to methodology, CNS trials, including alcohol trials, are considered more challenging than non CNS trials (Kaitin and Milne, 2011). For example, in CNS trials, long-term testing is required, treatment endpoints are difficult to measure, and behavioral changes can occur during treatment independent of the effects of the medication. The methodological issues of alcohol pharmacotherapy trials are complex, consisting of several major components that include study design, population selection, recruitment, adherence, retention, psychosocial platform, outcome measures, and safety.
Throughout the past two decades, alcohol researchers have sought ways to improve the methodology of alcohol clinical trials. In particular, NIAAA supported several workshops on methodology and conducted two large multi-site trials, Project MATCH and COMBINE, resulting in a dozen manuals on various aspects of conducting alcohol trials. NIAAA currently is supporting and conducting secondary analysis on datasets from several multi-site clinical trials on alcoholism to inform optimal selection of treatment endpoints, grace periods, duration of trial, methods for handling missing data, as well as understanding the effect of the placebo response. To facilitate this activity, NIAAA has joined a working group called Alcohol Clinical Trials Initiative (ACTIVE), which identifies and clarifies alcohol clinical trials methodology (Anton et al., 2011). Members of ACTIVE include representatives from the FDA, the European Medical Agency (EMA), NIAAA, the National Institute on Drug Abuse (NIDA), academia, and the pharmaceutical industry.
Efforts also are underway to develop alcohol biomarkers and alcohol sensor devices (Litten et al., 2010). Alcohol biomarkers may play an important role in alcohol clinical trials. They could be used for determining inclusion/exclusion criteria, as primary or secondary endpoint measurements, and to assist in identifying subtypes of patients. In particular, progress has been made in developing transdermal alcohol sensors (Barnett, 2011). These devices measure alcohol vapor through the skin and offer objective assessments of abstinence or drinking as well as the amount of alcohol consumed, an advantage over subjective self-reports. Goals for the next decade include making these sensors more precise in measuring alcohol intake, less expensive, and more comfortable to wear. Furthermore, additional research is needed to determine inter-individual variability and its utility in various settings (Litten et al., 2010). The development of biomarkers to serve as a surrogate endpoint for efficacy and safety also would make the drug development process more efficient.
Priorities over the next several years, as determined from discussions with regulatory agencies, pharmaceutical industry, and academia, are to look closely at treatment endpoints and placebo responses—two areas that are particularly important in terms of improving the methodological aspects of drug development.
Treatment Endpoints
The most commonly used outcome measures in alcohol trials are percent days abstinent, drinks per day, drinks per drinking day, and the number (or percent) of heavy drinking days. During the past decade, NIAAA has conducted analyses using data from various alcohol studies to provide information to the FDA for use in identifying primary endpoints for alcohol pharmacotherapy trials. The FDA recently moved from using total abstinence as the primary endpoint for pivotal Phase 3 trials to using percent subjects with no heavy drinking (PSNHDDs) (FDA, 2006b). This shift was based on the relationship between heavy drinking (5 or more drinks/day for males and 4 or more drinks/day for females) and alcohol-related consequences (Breslow and Graubard, 2008; Dawson et al., 2008; Jackson, 2008; Li, 2007; Greenfield et al., 2005). The NIAAA Medications Development Team (Falk et al., 2010) recently examined the validity of PSNHDDs as an endpoint using data from two large and very diverse alcohol trials, COMBINE (Anton et al., 2006) and a multi-site topiramate trial (Johnson et al., 2007). In these data sets, PSNHDDs appeared as sensitive as more traditional outcome measures, though confirmation is needed in other datasets. Still, other approaches are encouraged that include both continuous and categorical endpoints to measuring treatment outcomes. For example, the cumulative proportion of responder analysis, which shows the proportion of responders over the entire range of possible cut-off points for a given outcome measure, is promising for the alcohol field (Farrar et al., 2006). Analyses, so far, have indicated that allowing one or more heavy drinking days over the treatment period can increase the effect size (Falk et al., 2010). However, regardless of the selected outcome measure, future studies should validate drinking endpoints against clinically relevant markers, such as measurements of alcohol-related consequences, treatment utilization, and costs.
Placebo Effects
The placebo effect is a complex, psychobiological event associated with a variety of medical conditions, including pain, Parkinson’s disease, irritable bowel syndrome, depression, anxiety, and addiction. It also is linked to physiological systems, such as the cardiovascular, respiratory, immune, and endocrine systems (Finnis et al., 2010; Kaptchuk et al., 2008; Benedetti et al., 2005). From a psychological perspective, many mechanisms have been proposed to explain the placebo effect, including expectations, conditioning, memory, motivation, somatic focus, reward, and anxiety reduction (Finniss et al., 2010). The biological mechanism remains unclear, with several neurotransmitter systems being implicated, including the opioid, dopamine, adrenergic, and serotonin systems (Finniss et al., 2010). Regardless of its mechanism, the placebo effect observed in clinical trials appears to involve a complex interaction among patient, clinical staff, and treatment environment.
The placebo response makes it difficult to determine if the positive outcomes obtained in a clinical trial are attributable solely to the test medication. For example, in recent meta-analyses of depression clinical trials, a higher placebo rate was associated with a smaller effect size of the experimental antidepressant than those trials with a lower placebo response (Kirsch et al., 2008; Nunes and Levin, 2004). Currently, little information exists on characterizing the placebo effect in alcohol trials. Weiss et al. (2008) reported that in the COMBINE trial, the act of taking a pill and receiving a low-level intense behavioral therapy caused a small but significant decrease in drinking beyond that of a group that never took pills. However, there is dramatic improvement in drinking from baseline to treatment that, for the most part, is not explained by the act of taking a placebo tablet. For example, in the COMBINE trial, Anton et al. (2006) reported that the percent days abstinent in the placebo group (which received low intensity medical management and placebo) increased from 24 percent at baseline to 74 percent during treatment, a change just slightly greater than that found in a group that did not take pills during treatment (24 percent at baseline versus 67 percent during treatment). The vast difference between baseline and treatment values is observed in almost all the alcohol clinical trials, including pharmacological and nonpharmacological (behavioral) trials (Project MATCH Research Group, 1997). Interestingly, there appears to be as much reduction in drinking before the start of the study as during treatment itself. For example, in a recently-completed NIAAA multi-site trial of quetiapine, where subjects had a choice of reducing, stopping, or continuing to drink before randomization, subjects decreased their average daily alcohol consumption by 56 percent during the 30-day period prior to randomization (NCIG, unpublished results).
The exact causes for these nonspecific decreases in drinking before and during an alcohol clinical trial are unclear. For example, nonspecific causes could include patient contact with staff, expectations, conditioning, motivation, and reward (Finniss et al., 2010). Mitigating the placebo effect, most likely, will help to improve sensitivity in the detection of treatment differences between the experimental medication and placebo groups.
Objective 5: Advance Personalized Medicine in Pursuit of New Therapeutics
Personalized medicine is likely to have a prominent role in healthcare over the next several decades (Burrill et al., 2010; Schadt, 2010). Within the next 5 to 10 years, it will be possible to determine a person’s entire genome (DNA), transcriptome (RNA), and chemical epigenetic modifications of that genome within minutes and for less than $100 (Schadt, 2010). This new information will help fuel a movement toward specialized treatment of disease based on the presence or absence of specific genetic and other biological risk factors. Even today pharmacogenomic testing is being conducted to evaluate the safety of various medications for the treatment of numerous medical disorders, including cancer, bipolar disorder, epilepsy, depression, and hypertension (Lazary et al., 2011).
In the treatment of alcoholism, it is clear that no single treatment is effective for every individual. A more efficient approach is to personalize the treatment of each individual by matching specific interventions to his or her profile. A profile may consist of individual patients’ demographic characteristics; physiological/biochemical indicators; genome/transcriptome/epigenetic characteristics; cultural indices; and behavioral experience. These individual profiles eventually could be linked to different components of alcohol dependence (e.g., reward, negative affect, craving), as addressed earlier. During the next decade, development of an algorithm that combines these various elements may be the next step in predicting patient response to specific treatments.
Although the application of pharmacogenetics to the treatment of alcohol dependence has only just begun, it represents a fruitful area of research for the next decade (Hutchison, 2010; Kranzler and Edenberg, 2010; Heilig et al., 2011). For example, Oslin et al. (2003) first suggested that the A118G variant of the mu-opioid receptor gene (OPRM1) is associated with greater reductions in drinking in alcohol dependent patients treated with naltrexone. This finding was then replicated in a secondary analysis of the large NIAAA sponsored COMBINE trial (Anton et al. 2008), although replication has not been consistent (Gelernter et al. 2007). The OPRM1 variant’s ability to influence alcohol reward and boost naltrexone’s effects also is supported by elegant human laboratory studies (Ray and Hutchison 2007; Ray and Hutchison 2004). The mechanisms underlying this variant’s ability to moderate naltrexone’s effects have been suggested by a series of translational studies in non-human primates (Barr et al., 2007 and 2010), humans, and genetically modified mice (Ramchandani et al., 2011). Collectively, these studies suggest that naltrexone acts on the dopaminergic brain reward systems, which are preferentially activated by alcohol in OPRM1 118G carriers. These findings raise the possibility that what seems to be a small effect size for naltrexone in the general patient population may, in fact, be particularly efficacious in this genetically defined subpopulation (Heilig et al., 2011).
Genetic moderators of treatment efficacy are likely to become the rule rather than the exception. For instance, Johnson et al. (2011) recently found that two polymorphisms within the serotonin transporter (5-HTT) gene can alter an individual’s response to ondansetron. The two sites include the 5′-regulatory region with a long form (L) that possesses 44 additional base pairs versus the short (S) form (LL versus LS/SS) and another site, rs 1042173 (TT versus TG/GG), in the 3′-untranslated region of the 5-HTT gene. Interestingly, the investigators reported an interaction between these two sites, whereby the LL and TT significantly increased the effect size of ondansetron. Kranzler et al. (2011) reported that the LL variant of the 5-HTT gene had an effect on the responsiveness to sertraline in alcohol dependent patients. The late-onset alcoholics with the LL variant reported fewer drinking and heavy drinking days with sertraline, whereas the LL early-onset alcoholics experienced heavier drinking than that of the matched placebo. In a recent study, Kiefer et al. (2010) found that a variation of the GATA binding protein 4 (GATA4) gene influences an individual’s response to acamprosate. In another study, Hutchison et al. (2006) showed that olanzapine-treated alcohol dependent subjects with the seven-repeat allele of the dopamine 4 receptor (DRD4) displayed greater reductions in alcohol craving and decreased alcohol consumption than olanzapine-treated individuals without the seven-repeat allele. Finally, pharmacogenomics is not only important in determining efficacy, but also in identifying those who might suffer from adverse side effects. In a recent study, a single nucleotide polymorphism (SNP) variant in the intron 9 of the glutamate receptor GluR5 gene (GRIK1) was associated with a higher severity of topiramate-induced side effects (Ray et al., 2009).
Research that focuses on matching patients to specific treatments during the next decade will, without a doubt, be a high priority. It also carries some of the greatest challenges. Identifying treatment effects that are restricted to carriers of minor (less common) alleles raises particular challenges for the design and analysis of clinical trials. Nonetheless, the progress made thus far in the field of pharmacogenomics is encouraging. Laboratory-based studies that use a priori genetically defined populations and surrogate biomarkers of efficacy, such as brain activations responding to alcohol-associated stimuli or alcohol-induced dopamine release, are particularly well positioned to guide later stage clinical development. During the next decade, both pharmacogenomics and pharmacogenetics will play a prominent role in developing newer and safer medications for alcohol dependence.
Objective 6: Facilitate adaptation of Alcohol Dependence Medications in Treatment Settings
One of the greatest hurdles facing medications development may be the fact that while four medications have been approved by the FDA to treat alcoholism, their use, so far, has been limited (Knudsen et al., 2011; Mark et al., 2009). For example, in 2007, in the United States, approximately 720,000 prescriptions were written for AUD medications, representing a sales volume of 78 million dollars (Mark et al., 2009). This is quite low considering that there are approximately 18 million Americans suffering from AUDs. To put this in perspective, the antidepressant Lexapro had a sales volume of 1.7 billion dollars in 2004 even though the number of U.S. adults suffering from major depression is similar to those suffering from AUDs (Mark et al., 2009). Despite the relatively low uptake of alcohol medications, their use is growing. For instance, compared to 2003, the number of prescriptions of alcohol medications increased 83 percent during the following four years and the sales volumes increased 2.5 fold. This increase was attributed to the introduction of acamprosate, which has become the market leader for alcohol medications (Mark et al., 2009).
There are many reasons why medications are not used more frequently to treat alcohol dependence in the United States, including a simple lack of awareness about the medication, weak marketing efforts, perceived lack of efficacy, refusal of patients to take medication, high cost, side effects, lack of patient demand, shortage of physicians in addiction treatment settings, and lack of organizational support in promoting medications for alcohol treatment (Mark et al., 2003a and b; Thomas et al., 2003 and 2008; Knudsen et al., 2011).
Accordingly, one of the long-range goals for the next decade is to develop an infrastructure to facilitate medication use in real-world practice settings. This includes educating the public and health care professionals, emphasizing the benefits of alcohol medications, and de-stigmatizing the illness of alcohol dependence. Other goals include developing a rapid and easy screening measure for detecting alcohol problems (Smith et al., 2009) and providing parity of insurance coverage. The 2010 Patient Protection and Affordable Care Act (part of the health care reform) should provide numerous opportunities to make treatment more accessible and affordable. Finally, medication use to treat alcohol dependence will, undoubtedly, increase as the number of FDA-approved medications and their marketing levels increase. It also is vital that research continues to further define the barriers as well as effective strategies for offsetting those barriers in specialty addiction, primary care, and mental health care settings.
Ultimately, development of therapeutics that are highly effective and well tolerated will be the main driver of expanding pharmacotherapy of alcohol dependence. In the age of the Internet, once such medications are available, well-informed patients will create a demand for their use that simply will have to be met.
Objective 7: Facilitate Collaborative Networks and Partnerships among Government, Academia, Pharmaceutical/Biotechnology Companies, Healthcare Organizations, and Advocacy Groups
Building an effective medications development program is too big a task for any one government agency, pharmaceutical/biotechnology company, or other stakeholder organization. Establishing private-public partnerships is vital for accomplishing the objectives outlined here. For example, involvement of pharmaceutical companies is necessary for carrying out the large confirmatory trials required for obtaining FDA approval; NIAAA and academia are essential for discovering targets and validating screening models; and healthcare organizations and advocacy groups play an important role in getting medications into mainstream medicine.
Private-public partnerships also can fill in the gaps in the drug development process. For example, several academic alcohol researchers have developed novel compounds that have shown promise in animal models (Barron and Littleton, 2011; Overstreet et al., 2003; Wang et al., 2002; Ningaraj et al, 2001). However, most of these researchers do not have the resources to carry out the studies required to obtain an IND for testing in human studies. As a possible scenario, a pharmaceutical company could step in, agree to share intellectual property rights, and use their resources to obtain an IND and begin Phase 1 safety studies. NIAAA then could agree to conduct the proof-of-concept trial through the NCIG program, taking some of the risks that would, if the results are positive, provide additional incentive for the company to proceed to larger trials.
Such private-public partnerships also offer advantages for the pharmaceutical industry, which recently has begun to remodel its structure for drug development (Agres and Gwynne, 2010). Under the current system, the cost of drug development has increased dramatically, innovation of new compounds has stalled, and sales growth has remained flat (Munos, 2009). New strategies among the pharmaceutical companies include developing collaborative research projects with government and academia (Munos and Chin, 2009 and 2011). Undoubtedly, developing collaborative networks and partnerships will provide more opportunities and choices for alcohol drug development. In the end, the ability of everyone to work together efficiently will determine how quickly we are able to overcome the many challenges of drug development and, most importantly, how quickly we are able to provide more effective medications to the public.
Final Thoughts
Over the last two decades, significant advances have been made in medications development to treat alcoholism. Nonetheless, much work remains and with many exciting opportunities. These include discovering new molecular targets for drug development, validating animal and human lab screening models, exploring pharmacogenomics for personalized medicine, improving the methodology of alcohol clinical trials, building an infrastructure for research, and engaging public support for the use of medications in the treatment of alcoholism.
The future holds considerable promise. To be successful, however, we must make the process of drug development more efficient, in terms of time, cost, and predictability—from partnering with pharmaceutical companies and improving signal detection in alcohol clinical trials, to developing effective approaches for facilitating the use of medications in specialized, primary, and mental health settings. To carry out these goals, the NIAAA Medications Team has identified seven objectives for the coming decade. These objectives will enable us to realize our vision of producing several new alcohol medications during the course of the next decade. While these goals face considerable challenges, as outlined above, it is vital that we remain focused, make rational decisions and, perhaps most importantly, work together as a team. By doing so, we will improve treatment for patients with alcohol dependence, their families, and the public health in general, both in the United States and throughout the world.
Acknowledgments
The authors thank Barbara Vann of CSR, Incorporated for her excellent editorial comments. The authors also thank Dr. George Koob for his review of this manuscript and his thoughtful comments.
Footnotes
Author Contribution
All authors were responsible for the development of this manuscript. In addition, all authors critically reviewed the content and approved the final version for publication.
Disclosure/Conflict of Interest
The authors declare that, except for M.R. who is a government contractor and has received income from Merck for support of one of their trials, they have not received compensation from any individual or corporate entity over the past 3 years for research or professional service. In addition, there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Agres T, Gwynne P. Scientific American Pathways: 2010. New York: Scientific American; 2010. Remodeling pharma; pp. 66–76. [Google Scholar]
- Anton RF, Litten RZ, Falk DE, Palumbo JM, Bartus RT, Robinson RL, Kranzler HR, Kosten TR, Meyer RE, O’Brien CP, Mann K, Meulien D. The Alcohol Clinical Trials Initiative (ACTIVE): Purpose and goals for assessing important and salient issues for medications development alcohol use disorders. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.182. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Kranzler H, Breder C, Marcus RN, Carson WH, Jian Han. A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J Clin Psycholpharmacol. 2008;28:5–12. doi: 10.1097/jcp.0b013e3181602fd4. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Duraxo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Barnett N. Symposium: New Developments in Research with Transdermal Alcohol Sensors: Laboratory, Field, and Clinical Applications. 34th Annual Scientific Meeting of the Research Society on Alcoholism; Atlanta, GA. 2011. [Google Scholar]
- Barr CS, Chen SA, Schwandt ML, Lindell SG, Sun H, Suomi SJ, Heilig M. Suppression of alcohol preference by naltrexone in the Rhesus Macaque: A critical role of genetic variation at the μ-opioid receptor gene locus. Biol Psychiatry. 2010;67:78–80. doi: 10.1016/j.biopsych.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley D, Heilig M. Association of a functional polymorphism in the μ-opioid receptor gene with alcohol response and consumption in male Rhesus Macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Barron S, Littleton J. Polyamines and the NMDA receptor in medications development for alcohol abuse/dependence. Recent Patents CNS Drug Discov. 2011 (in press) [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Graubard BI. Prospective study of alcohol consumption in the United States: quantity, frequency, and cause-specific mortality. Alcohol Clin Exp Res. 2008;32:513–521. doi: 10.1111/j.1530-0277.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Burrill GS. Scientific American Pathways: 2010. New York: Scientific American; 2010. Healthcare on the threshold of innovation; pp. 18–19. [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: An assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS. Reengineering translational science: The time is right. Sci Translat Med. 2011;3:1–6. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: Is better consilience possible? Addict Biol. 2010;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95:62–72. doi: 10.1016/j.drugalcdep.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Dworkin RH, Mitchell BM. Use of the cumulative proportion of responders analysis graph to present pain data over a range of cut-off points: Making clinical trial data more understandable. J Pain Symptom Manage. 2006;31:369–377. doi: 10.1016/j.jpainsymman.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Challenge and Opportunity on the Critical Path to New Medical Products. U.S. Department of Health and Human Services; Rockville, Maryland: 2004. [Google Scholar]
- FDA. Critical Path Opportunities Report. U.S. Department of Health and Human Services; Rockville, Maryland: 2006a. [Google Scholar]
- FDA. Medical Review of Vivitrol: 21–897. U.S. Department of Health and Human Services; Rockville, Maryland: 2006b. [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA. Serotonin uptake blockers and the treatment of alcoholism. In: Galanter M, editor. Recent Developments in Alcoholism: Treatment. Vol. 7. New York: Plenum Press; 1989. pp. 267–281. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Greenfield TM, Bond J, Kerr WC, Nayak MB, Anton RF, Kranzler HR, Kaskutas LA. Risks of Alcohol Use Disorders for treated and concerned individuals are higher than for others at given patterns of drinking. Presented at Annual Epidemiological Symposium of the Kettil Bruun Society for the Epidemiological and Social Research on Alcohol; Riverside, CA. May 30.2005. [Google Scholar]
- Griffin WC, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Rauch SL. Neurocircuitry: A window into the networks underlying neuropsychiatric disease. Neuropsychopharmacology. 2010;35:1–3. doi: 10.1038/npp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: Are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotrophin-releasing factor in alcohol dependence. TRENDS Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hopkins AL. Network pharmacology: The next paradigm in drug discovery. Nature Chem Biol. 2008;4:682–6690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Hurko O, Ryan JL. Translational research in central nervous system drug discovery. J Am Soc Exp Neuro Therap. 2005;2:671–682. doi: 10.1602/neurorx.2.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Ann Rev Clin Psychol. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, Swift R. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Insel TR. Faulty circuits. Sci Am. 2010;302:44–51. doi: 10.1038/scientificamerican0410-44. [DOI] [PubMed] [Google Scholar]
- Jackson KM. Heavy episodic drinking: determining the predictive utility of five or more drinks. Psychol Addict Behav. 2008;22:68–77. doi: 10.1037/0893-164X.22.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K. Oral topiramate for treatment of alcohol dependence: A randomized controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Seneviratne C, Roache JD, Javors MA, Wang XQ, Liu L, Penberthy JK, DiClemente CC, Li MD. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatry. 2011;168:265–275. doi: 10.1176/appi.ajp.2010.10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Jasinski DR, Galloway GP, Kranzler H, Weinreib R, Anton RF, Mason BJ, Bohn MJ, Pettinati HM, Rawson R, Clyde C. Ritanserin in the treatment of alcohol dependence – A multi-center clinical trial. Psychopharmacology. 1996;128:206–215. doi: 10.1007/s002130050126. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Mann K, Willenbring ML, Litten RZ, Swift RM, Lesch OM, Berglund M. Challenges and opportunities for medications development in alcoholism: An international perspective on collaborations between academia and industry. Alcohol Clin Exp Res. 2005;29:1528–1540. doi: 10.1097/01.alc.0000174690.63787.fc. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM. Topiramate for treating alcohol dependence. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kaptchuk T, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AJ. Components of placebo effect: randomized controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitin KI, Milne CP. A dearth of new meds. Sci Am. 2011;305:16. doi: 10.1038/scientificamerican0811-16. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Toward a philosophical structure for psychiatry. Am J Psychiatry. 2005;162:433–440. doi: 10.1176/appi.ajp.162.3.433. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Witt SH, Frank J, Richter A, Treutlein J, Lemenager T, Nothen MM, Cichon S, Batra A, Berner M, Wodarz N, Zimmermann US, Spanagel R, Wiedermann K, Smolka MN, Heinz A, Rietschel M, Mann K. Involvement of the atrial natriuretic peptide transcription factor GATA4 in alcohol dependence, relapse risk and treatment response to acamprosate. Pharmacogenomics J. 2010 doi: 10.1038/tpj.2010.51. online. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. PLoS Medicine. 2008;5:260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: A Rosetta Stone approach. Nature Reviews Drug Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias A, Pettinati H, Oncken C. A double-blind, randomized trial of sertraline for alcohol dependence. J Clin Psychopharmacol. 2011;31:22–30. doi: 10.1097/JCP.0b013e31820465fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Edenberg HJ. Pharmacogenetics of alcohol and alcohol dependence treatment. Curr Pharmaceutical Design. 2010;16:2141–2148. doi: 10.2174/138161210791516387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5:21–27. doi: 10.1097/ADM.0b013e3181d41ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J, Juhasz G, Hunyady L, Bagdy G. Personalized medicine can pave the way for the safe use of CB1 receptor antagonists. Trends Pharmacol Sci. 2011;32:270–280. doi: 10.1016/j.tips.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS. Ethanol consumption: How should we mesure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: Present findings and future research needs. J Gastroenterol Hepatolol. 2007;23(Suppl 1):S2–S8. doi: 10.1111/j.1440-1746.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–96. [PubMed] [Google Scholar]
- Litten RZ, Fertig J, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson B, Pettinati H, Swift R, Afshar M, Brunette MF, Tiouririne NAD, Kampman K, Stout R. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01649.x. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: Recent advances and future research opportunities. Alcohol Clin Exp Res. 2010;34:955–967. doi: 10.1111/j.1530-0277.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig J, Mattson ME, Egli M. Development of medications for alcohol use disorders: Recent advances and ongoing challenges. Expert Opin Emerging Drugs. 2005;10:323–343. doi: 10.1517/14728214.10.2.323. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Allen JP. Pharmacotherapies for alcoholism: Promising agents and clinical issues. Alcohol Clin Exp Res. 1991;15:620–633. doi: 10.1111/j.1530-0277.1991.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kassed CA, Vandivort-Warren R, Levit KR, Kranzler HR. Alcohol and opioid dependence medications: Prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009;99:345–349. doi: 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003a;71:219–228. doi: 10.1016/s0376-8716(03)00134-0. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X, Bransberger P, Poole VH, Crosse S. Physicians’ opinions about medications to treat alcoholism. Addiction. 2003b;98:617–626. doi: 10.1046/j.1360-0443.2003.00377.x. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology. 2008;200:141–150. doi: 10.1007/s00213-008-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, Marugan JJ, Liu K, Zheng W, Southall N, Huang W, Heilig M, Austin CP. Selective modulation of Gq/Gs pathways by naphtha pyrano pyrimidines as antagonists of the neuropeptide S receptor. ACS Chem Neurosci. 2010;1:559–574. doi: 10.1021/cn100040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT. Reducing heavy drinking: A public health strategy and a treatment goal? J Subst Abuse Treat. 2007;33:81–83. doi: 10.1016/j.jsat.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Mello NK. Behavioral strategies for the evaluation of new pharmacotherapies for drug abuse treatment. In: Harris LS, editor. Problems of Drug Dependence 1991. DHHS(ADM) 92-1888. Washington DC: US Government Printing Office; 1992. pp. 150–154. [PubMed] [Google Scholar]
- Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, Ebrahim S, Ezzati M, Salomon JA, Kreiser JG, Hogan M, Murray CJL. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:1–49. doi: 10.1186/1478-7954-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Is pharma running out of brainy ideas? Science. 2010;329:502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- Miranda R, MacKillop J, Monit PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effectsd of topiramate on urge to drink and the subject effects of alcohol: A preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Munos BH, Chin WW. How to revive breakthrough innovation in the pharmaceutical industry. Sci Translat Med. 2011;3:1–3. doi: 10.1126/scitranslmed.3002273. [DOI] [PubMed] [Google Scholar]
- Munos BH, Chin WW. A call for sharing: Adapting pharmaceutical research to new realities. Sci Translat Med. 2009;1:1–4. doi: 10.1126/scitranslmed.3000155. [DOI] [PubMed] [Google Scholar]
- Munos B. Lessons from 60 years of pharmacetucial innovation. Nature Rev. 2009;8:959–968. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Dongier M, Bremner KE. Long-acting injectable bromocriptine does not reduce relapse in alcoholics. Addiction. 1997;92:969–978. [PubMed] [Google Scholar]
- Ningaraj NS, Chen W, Schloss JV, Faiman MD, WJY S-methyl-N,N-diethylthiocarbamate sulfoxide elicits neuroprotective effect against N-methyl-D-aspartate receptor-medicated neurotoxicity. J Biomed Sci. 2001;8:104–113. doi: 10.1007/BF02255978. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, O’Connor PG. Medications for unhealthy alcohol use. Alcohol Res Health. 2011;33:300–312. [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neurpsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kralic JE, Morrow AL, Ma ZZ, Zhang YW, Lee DYW. NPI-031G (puerarin) reduces anxiogenic effects of alcohol withdrawal or benzodiazepine inverse or 5-HT2c agonists. Pharmacol Biochem Behav. 2003;75:619–625. doi: 10.1016/s0091-3057(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nature Rev. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer ML, Fonte CA, Briski JL, Scott JA, Chengappa KNR. Development of procedures from early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2008;84:216–221. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Powell BJ, Campbell JL, Landon JF, Liskow BI, Thomas HM, Nickel EJ, Dale TM, Penick EC, Samuelson SD, Lacoursiere RB. A double-blind, placebo-controlled study of nortriptyline, and bromocriptine in male alcoholics subtyped by comorbid psychiatric disorders. Alcohol Clin Exp Res. 1995;19:462–468. doi: 10.1111/j.1530-0277.1995.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Neumark Y, Zimmermann US, Morzorati SL, de Wit H. The alcohol clamp: Applications, challenges, and new directions – An RSA 2004 Symposium summary. Alcohol Clin Exp Res. 2006;30:155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, MacKillop J, McGeary J, Tidey JW, Rohsenow DJ, Gwaltney C, Swift RW, Monti PM. A preliminary pharmacogenetic investigation of adverse events from topiramate in heavy drinkers. Exp Clin Psychopharmacol. 2009;17:122–129. doi: 10.1037/a0015700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the μ-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: Vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–487. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Roache JD. Role of the human laboratory in the development of medications for alcohol and drug dependence. In: Johnson BA, editor. Addiction Medicine: Science and Practice. Springer Science+Business Media; New York: 2010. pp. 129–157. [Google Scholar]
- Roberts AJ, Heyser C, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: Animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler Dj, Kroeze WK. Magic shotguns versus magic bullets: Selectively non-selective drugs for mood disorders and schizophrenia. Nature Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Schadt EE. Scientific American Pathways: 2010. New York: Scientific American; 2010. Mastering information for personal medicine; pp. 64–65. [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783–788. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T, Crombag HS, Cunningham CL, Heilig M, Crabbe JC. Reward sensitivity: Issues of measurement, and achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:145–168. doi: 10.1111/j.1369-1600.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Wallack SS, Lee S, McCarty D, Swift R. Research to practice: Adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24:1–11. [PubMed] [Google Scholar]
- Thomas SE, Miller PM, Randall PK, Book SW. Improving acceptance of naltrexone in community addiction treatment centers: A pilot study. J Subst Abuse Treat. 2008;35:260–268. doi: 10.1016/j.jsat.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Snell LD, Tabakoff B, Levinson SR. Inhibition of neuronal Na+ channels by the novel antiepileptic compound DCUKA: Identification of the diphenylureido moiety as an inactivation modifier. Exp Neurol. 2002;178:129–138. doi: 10.1006/exnr.2002.8029. [DOI] [PubMed] [Google Scholar]
- Weiss RD, O’Malley SS, Hosking JD, LoCastro JS, Swift R. Do patients with alcohol dependence respond to placebo? Results from the COMBINE study. J Stud Alcohol Drugs. 2008;69:878–884. doi: 10.15288/jsad.2008.69.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Status Report on Alcohol 2004. WHO; Geneva: 2004. [Google Scholar]