Abstract
Invasive micropapillary carcinoma (IMC) is generally an aggressive morphologic variant that has been described in the bladder, lung, breast, salivary gland, gastrointestinal tract, and ovary. Given the morphologic similarities between IMCs arising from different organ systems and the high propensity of this histologic subtype for lymphatic metastasis, it may be necessary to use immunohistochemical (IHC) markers to determine the primary site of an IMC. Few studies have compared the IHC profiles of IMCs originating from different sites. We tested a panel of 11 IHC markers for their ability to distinguish urothelial, lung, breast, and ovarian IMC using a tissue microarray constructed with primary tumor tissue from 47 patients with IMC (13 bladder, 6 lung, 16 breast, and 12 ovarian). For each tumor, correct classification as IMC was verified by reverse polarity MUC1 expression. We found that immunostaining for uroplakin, CK20, TTF-1, estrogen receptor (ER), WT-1 and/or PAX8, and mammaglobin was the best panel for determining the most likely primary site of IMC. The best markers to identify urothelial IMC were uroplakin and CK20, whereas p63, high molecular weight cytokeratin, and thrombomodulin were less sensitive and specific. Lung IMC was uniformly TTF-1 positive. Breast IMC was ER positive, mammaglobin positive, and PAX8/WT-1 negative, while ovarian IMC was ER positive, mammaglobin negative, and PAX8/WT-1 positive. In the metastatic setting, or when IMC occurs without an associated in situ or conventional carcinoma component, staining for uroplakin, CK20, TTF-1, ER and WT-1, and/or PAX8, and mammaglobin is the best panel for accurately classifying the likely primary site of IMC.
Keywords: micropapillary carcinoma, urothelial, bladder, breast, lung, ovary, immunohistochemistry
Invasive micropapillary carcinoma (IMC) is generally an aggressive morphologic variant of carcinoma that has been described in multiple anatomic sites, including the urinary bladder, lung, breast, salivary gland, gastrointestinal tract, and ovary.1,2,20,12,17,21 Characterized by avascular clusters of tumor cells floating in stromal clefts, IMC consistently demonstrates inverted cellular polarity with the apical membrane domain of tumor cells facing outward toward the surrounding stroma rather than inward toward a central lumen.9,14 Recent studies of breast IMC using high-resolution microarray, comparative genomic hybridization, and also expression profiling studies, support the concept that IMC represents a distinct genetic and clinicopathologic entity.10,23 Indeed, in almost all organ systems, with the exception of the ovary,21 IMC portends a poor prognosis with a greatly increased risk of lymph node metastases when compared with conventional carcinoma.1,2,4,9,16,22 In the bladder, IMC is considered to be so aggressive that some groups have advocated for radical cystectomy as a first-line therapy even for non-muscle invasive disease in place of conventional intravesicular therapies.5
Given the frequency of metastasis and also the morphologic similarities between IMC from different organ systems, it is often necessary to use immunohistochemical (IHC) stains to help identify the origin of a metastatic IMC of unknown primary. In addition, although IMC is typically associated with a component of conventional carcinoma in most organ systems, in the absence of this feature it may be difficult to determine whether one is dealing with a primary lesion or a metastasis, a distinction critical for appropriate clinical management. Although there are a number of studies that characterize the IHC profile of urothelial, lung, breast, and ovarian IMC individually, few studies have thoroughly compared the IHC profile of multiple IMC originating from different organ systems. In this study, we used a series of 47 carcinomas with micropapillary features to develop a minimal IHC panel of 6 markers that reliably distinguishes urothelial, lung, breast, and ovarian micropapillary carcinomas.
MATERIALS AND METHODS
Tissue Selection
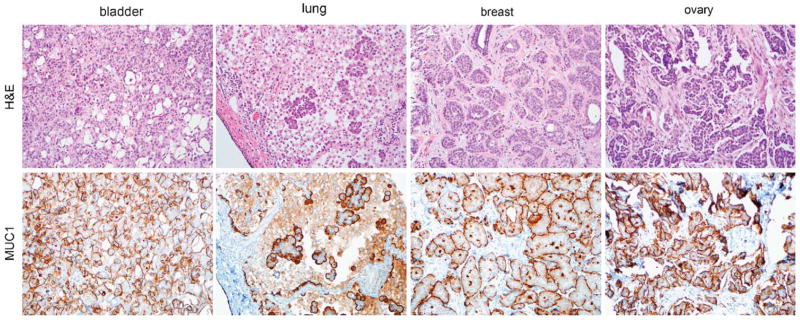
Formalin-fixed paraffin-embedded primary tumor tissue from 47 IMC cases was collected from the files of the Johns Hopkins Hopspital and arrayed in triplicate on 2 tissue microarrays with protocol approval from the Institutional Review Board. The group included 13 cases of micropapillary variant of urothelial carcinoma, 6 cases of micropapillary lung carcinoma, 16 cases of micropapillary breast carcinoma, and 12 cases of invasive micropapillary serous carcinoma originating in the ovary (also known as low-grade serous carcinoma). Micropapillary tumors consisting exclusively of an in situ component were excluded. To verify the diagnosis, all IMC cases were immunostained for MUC1 expression. As expected, all cases showed inverted polarity, with MUC1 expression along the membrane segment facing the stroma, a previously reported feature of micropapillary carcinomas in most organ systems (Fig. 1).14 In 31% (4 of 13) of the urothelial cases, 33% (2 of 6) of lung cases, and 6% (1 of 16) of breast cases, a concurrent component of in situ or invasive conventional carcinoma was also included in the array as a control.
FIGURE 1.
MUC1 immunostaining of micropapillary tumors. MUC1 localization is inverted in micropapillary tumors and expressed along the membrane segment facing the stroma. This inverted expression of MUC1 was used to verify the micropapillary diagnosis for bladder, lung, breast, and ovarian tumors used in this study (all photomicrographs at ×200). H&E indicates hematoxylin and eosin.
IHC and Scoring
After deparaffinization and rehydration, 4-μm tissue sections were subjected to antigen retrieval and primary antibody incubation with 12 different antibodies and appropriate positive controls as detailed in Table 1. Primary antibody was omitted for negative controls. All immunostaining, with the exception of mammaglobin, PAX8, and uroplakin was performed on a Benchmark XT automated stainer (Ventana Medical Systems, Tucson, AZ) with primary antibody incubations around 30 minutes at room temperature. Mammaglobin and PAX8 primary antibodies were incubated at room temperature for 15 minutes. Uroplakin was incubated at 4°C overnight.3 For each antibody, the number of cases with ≥10% of cells showing 1+ or greater intensity staining (on a 0 to 3+ scale) was recorded by a pathologist (T.L.). Only nuclear localization was scored for estrogen receptor (ER), p63, PAX8, TTF-1, and WT-1, whereas cytoplasmic/membranous staining was scored for all others.
TABLE 1.
Immunohistochemical Staining Details
| Marker | Company | Clone | Species | Retrieval | Dilution |
|---|---|---|---|---|---|
| CK20 | Ventana | Ks20.8 | Mouse | None/protease | Prediluted |
| CK7 | Dako | OV-TL 12/30 | Mouse | None | 1:50 |
| CK903 | Ventana | 34betaE12 | Mouse | None | Prediluted |
| ER | Novacastro | F11 | Mouse | EDTA | Prediluted |
| Mammaglobin | Dako | 304-1A5 | Mouse | Citrate | 1:100 |
| MUC1 | Vector | Ma695 | Mouse | EDTA | 1:100 |
| P63 | Cell Marque | 4A4 | Mouse | EDTA | Prediluted |
| PAX8 | Protien Tech Inc | Polyclonal | Rabbit | EDTA | 1:100 |
| Thrombomodulin | Dako | 1009 | Mouse | Pepsin | 1:1000 |
| TTF-1 | Ventana | 8G7G3/1 | Mouse | EDTA | Prediluted |
| Pan-uroplakin | See Ref. 3 | See Ref. 3 | Rabbit | Citrate | 1:20,000 |
| WT-1 | Cell Marque | 6F-H2 | Mouse | EDTA | Prediluted |
ER indicates estrogen receptor; EDTA, ethylenediaminetetraacetic acid.
RESULTS
The results of the IHC staining for the 47 cases are summarized in Tables 2 and 3. Nearly all urothelial, lung, breast, and ovarian micropapillary cases labeled with CK7. For the urothelial micropapillary carcinoma, the most sensitive marker was pan-uroplakin, which showed membranous and/or cytoplasmic staining in 92% (11 of 12) of evaluable cases (Fig. 2). In contrast, uroplakin immunostaining was entirely absent from breast, lung, and ovarian IMC making it specific for urothelial IMC. CK20 also labeled 54% (7 of 13) of urothelial IMC cases, but was less specific as it was also expressed by 17% (1 of 6) of lung IMC cases. All CK20-positive urothelial IMC cases were also uroplakin positive. High molecular weight cytokeratin (34βE12) and p63, typically expressed in about two-thirds of conventional urothelial carcinomas were expressed in only 15% (2 of 13) and 27% (3 of 11) of urothelial IMC cases. Similarly, thrombomodulin was expressed by only 23% (3 of 13) of urothelial IMC cases and was not specific, labeling 19% (3 of 16) of breast and 17% (1 of 6) of lung IMC cases as well. For lung IMC, TTF-1 was the most sensitive and specific marker, labeling 100% (6 of 6) of lung cases and absent from all other IMC cases (Fig. 2).
TABLE 2.
Recommended Immunohistochemical Panel for Micropapillary Carcinomas
| n | Uroplakin (%) | CK20 (%) | TTF-1 (%) | ER (%) | Mammaglobin (%) | WT-1 (%) | PAX8 (%) | |
|---|---|---|---|---|---|---|---|---|
| Bladder | 11–13 | 92 | 54 | 0 | 0 | 0 | 0 | 0 |
| Breast | 16 | 0 | 0 | 0 | 88 | 56 | 0 | 0 |
| Lung | 6 | 0 | 17 | 100 | 17 | 0 | 0 | 0 |
| Ovary | 11–12 | 0* | 0* | 0* | 92 | 0* | 91 | 100 |
n = 2.
ER indicates estrogen receptor.
TABLE 3.
Additional Immunohistochemical Markers for Micropapillary Carcinomas
| n | CK7 (%) | p63 (%) | HMWCK (%) | Thrombomodulin (%) | |
|---|---|---|---|---|---|
| Bladder | 11–13 | 100 | 27 | 15 | 23 |
| Breast | 16 | 94 | 6 | 6 | 19 |
| Lung | 6 | 100 | 50 | 33 | 17 |
| Ovary | 11–12 | 100 | 0* | 100* | 0* |
n = 2.
HMWCK indicates high molecular weight cytokeratin.
FIGURE 2.
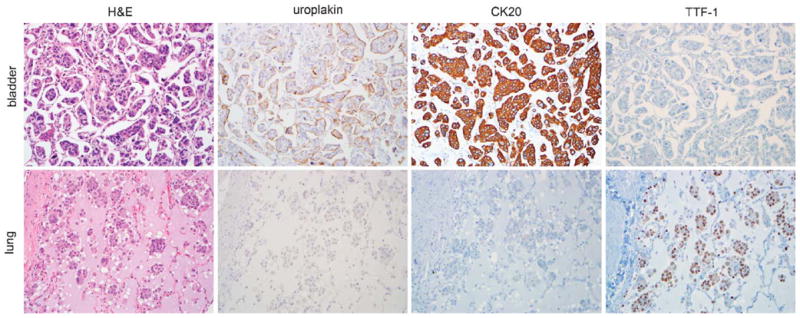
Bladder invasive micropapillary carcinoma (IMC) cases expressed uroplakin and CK20, but were negative for TTF-1, whereas lung IMC cases expressed TTF-1 and variable CK20 but were negative for uroplakin (all photomicrographs at ×200). H&E indicates hematoxylin and eosin.
For breast and ovarian IMC, ER-labeled 88% (14 of 16) and 92% (11 of 12) of cases, respectively. Including WT-1 and PAX8 in the panel distinguished the ovarian IMC cases as 91% (10 of 11) and 100% (11of 11) of ovarian cases expressed these markers, and they were both entirely specific for ovarian IMC (Fig. 3). Mammaglobin was the only specific breast marker labeling 56% (9 of 16) of breast IMC cases (Fig. 3). Given the high frequency of ER positivity in the IMC breast cases, mammaglobin was generally positive in cases that were also ER positive, although there was 1 ER-negative, mammaglobin-positive case.
FIGURE 3.
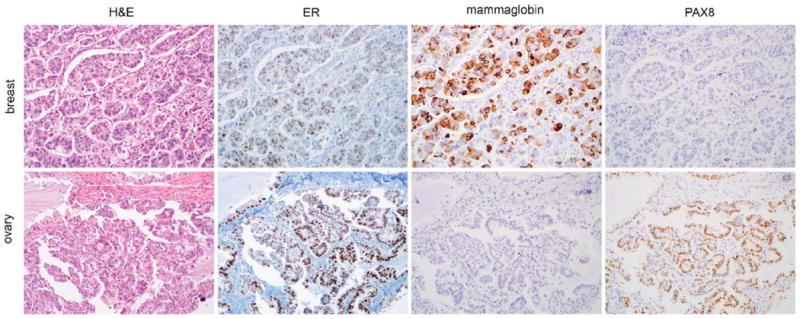
Both breast and ovarian invasive micropapillary carcinoma (IMC) cases expressed estrogen receptor (ER), whereas breast cases expressed mammaglobin and were negative for PAX8, and ovarian IMC cases were negative for mammaglobin and expressed PAX8 (all photomicrographs at ×200). H&E indicates hematoxylin and eosin.
DISCUSSION
IMC in most organ systems typically presents with high-stage disease and a high proportion of patients develop lymph node metastases. When presenting as a metastasis with an unknown primary or as an apparent primary tumor without associated in situ or conventional carcinoma component, it is prudent to utilize immunohistochemistry to help localize the site of origin. Although a number of reviews and case reports in the literature have highlighted the pitfalls of diagnosis associated with metastatic micropapillary carcinomas, no earlier study has systematically examined the immunoprofile of a large series of IMC cases originating in different organ systems. In this study, we found that an IHC panel composed of uroplakin, CK20, TTF-1, ER, WT-1/PAX8, and mammaglobin is the best combination to accurately classify the primary site of IMC. In addition, because CK20-positive urothelial IMC cases were a subset of the uroplakin-positive cases, and the mammaglobin-positive breast cases were generally also ER positive, our recommended panel could theoretically be reduced to only 4 markers: uroplakin, TTF-1, ER, and WT-1 or PAX8. Markers that were less sensitive/specific and that we do not recommend included p63, high molecular weight cytokeratin, thrombomodulin, and CK7.
The IHC profiles of bladder, lung, breast, and ovarian IMC have been separately studied in a handful of earlier reports. Samaratunga and Khoo18 looked at 7 cases of micropapillary urothelial carcinoma and found them to be uniformly CK7 positive and focally CK20 positive. In addition, 57% showed focal 34βE12 reactivity, a somewhat higher percentage than we found in this study. More specific urothelial markers such as thrombomodulin and uroplakin have not been previously investigated in bladder IMC. Similar to our current findings, Amin et al1 reported that CK20 positivity is not specific to bladder IMC. Of 15 cases of lung IMC, they found that 13% were positive for CK20. Fortunately, TTF-1 is specific for lung IMC, and as in our study, Amin et al2 reported that the majority (80%) of their lung cases expressed the transcription factor.
A small number of studies have compared the IHC profiles of breast and ovarian micropapillary carcinoma. The majority of studies looking at the immunoprofile of breast IMC have focused on hormone receptor and Her2-neu status. Similar to the current findings, ER positivity has been reported in the majority of breast IMCs, with some studies showing as many as 91% of tumors positive (reviewed in Ref. 13). Mammaglobin was recently studied by Kanner et al6 in 10 breast IMC cases, with 70% of them expressing the protein. Consistent with our results, in the same study, 12 serous ovarian carcinomas were entirely negative for mammaglobin. Moritani et al11 looked at the nuclear expression of WT-1 in 37 cases of breast IMC and found that 3% of tumors were focally weakly positive for this marker. In a similar study, Lee et al8 found that of 34 cases, 26% were positive for WT-1. Some cross-reactivity with usual-type ductal breast carcinoma has been seen with WT-1 immunostaining, leading some to suggest that PAX8 is a more specific marker for ovarian carcinoma.15 However, PAX8 has not previously been studied in micropapillary breast or ovarian carcinoma. In this study, we found that WT-1 nuclear positivity was specific for ovarian IMC, but PAX8 immunostaining may be useful as an additional specific adjunct stain.
In addition to the bladder, lung, breast, and ovary, IMC has been rarely reported in the salivary glands and gastrointestinal tract, although we did not address such cases in this study because of their infrequent occurrence. A micropapillary variant of salivary ductal carcinoma was reported to be positive for CK7 and negative for CK20 in a small case series.12 Several case reports describe IMC occurring in the stomach and colon17,19 and a recent case series has documented IMC occurring in the ampullo-pancreatobiliary region.7 Unfortunately, because of the scarcity of such cases, the IHC profile of gastrointestinal IMC remains anecdotal. Given the ubiquitous expression of CK7 seen in bladder, lung, breast, and ovarian IMC, CK7 negativity would be predicted to be helpful in distinguishing colonic IMC; however, only scattered case reports document this fact.24 Similarly, CDX2 expression has not been studied extensively in these tumors as a specific marker of colonic micropapillary adenocarcinoma. As more cases of gastrointestinal IMC are recognized, future studies will be helpful to establish a more specific immunoprofile for these tumors.
In addition to identifying an optimal IHC panel, our study is the first to utilize a number of relatively newly available immunostains to specifically label micropapillary carcinomas from different sites. Uroplakin has been reported as a sensitive and specific marker for conventional urothelial carcinomas, however, it has not been previously studied in the micropapillary variant of urothelial carcinoma. Similarly, mammaglobin has been shown to be a sensitive and specific marker of conventional ductal carcinomas, but our study is among the first to look at its expression in breast IMC. Finally, in the ovary, PAX8 is emerging as an alternative to WT-1 immunostaining as it is specifically expressed by tissues of renal, thyroid, or Mullerian origin, however, we are the first to study it in ovarian IMC. We found that use of additional organ system-specific markers such as uroplakin, mammaglobin, and PAX8 added specificity to our IHC panel and helped us to more confidently distinguish urothelial, lung, breast, and ovarian micropapillary carcinomas. Ultimately, the use of these markers allowed us to develop a relatively small IHC panel (6 markers) that we feel reliably identified the site of origin of the majority of IMCs.
References
- 1.Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 1994;18:1224–1232. doi: 10.1097/00000478-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Huang HY, Shariat SF, Sun TT, et al. Persistent uroplakin expression in advanced urothelial carcinomas: implications in urothelial tumor progression and clinical outcome. Hum Pathol. 2007;38:1703–1713. doi: 10.1016/j.humpath.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson SL, Borghede G, Holmang S. Micropapillary bladder carcinoma: a clinicopathological study of 20 cases. J Urol. 1999;161:1798–1802. doi: 10.1016/s0022-5347(05)68807-6. [DOI] [PubMed] [Google Scholar]
- 5.Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M.D. Anderson Cancer Center experience with 100 consecutive patients. Cancer. 2007;110:62–67. doi: 10.1002/cncr.22756. [DOI] [PubMed] [Google Scholar]
- 6.Kanner WA, Galgano MT, Stoler MH, et al. Distinguishing breast carcinoma from Mullerian serous carcinoma with mammaglobin and mesothelin. Int J Gynecol Pathol. 2008;27:491–495. doi: 10.1097/PGP.0b013e31817d5340. [DOI] [PubMed] [Google Scholar]
- 7.Khayyata S, Basturk O, Adsay NV. Invasive micropapillary carcinomas of the ampullo-pancreatobiliary region and their association with tumor-infiltrating neutrophils. Mod Pathol. 2005;18:1504–1511. doi: 10.1038/modpathol.3800460. [DOI] [PubMed] [Google Scholar]
- 8.Lee AH, Paish EC, Marchio C, et al. The expression of Wilms’ tumour-1 and Ca125 in invasive micropapillary carcinoma of the breast. Histopathology. 2007;51:824–828. doi: 10.1111/j.1365-2559.2007.02884.x. [DOI] [PubMed] [Google Scholar]
- 9.Luna-More S, Gonzalez B, Acedo C, et al. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract. 1994;190:668–674. doi: 10.1016/S0344-0338(11)80745-4. [DOI] [PubMed] [Google Scholar]
- 10.Marchio C, Iravani M, Natrajan R, et al. Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J Pathol. 2008;215:398–410. doi: 10.1002/path.2368. [DOI] [PubMed] [Google Scholar]
- 11.Moritani S, Ichihara S, Hasegawa M, et al. Serous papillary adenocarcinoma of the female genital organs and invasive micropapillary carcinoma of the breast. Are WT1, CA125, and GCDFP-15 useful in differential diagnosis? Hum Pathol. 2008;39:666–671. doi: 10.1016/j.humpath.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Nagao T, Gaffey TA, Visscher DW, et al. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol. 2004;28:319–326. doi: 10.1097/00000478-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004;11:297–303. doi: 10.1097/01.pap.0000138142.26882.fe. [DOI] [PubMed] [Google Scholar]
- 14.Nassar H, Pansare V, Zhang H, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004;17:1045–1050. doi: 10.1038/modpathol.3800166. [DOI] [PubMed] [Google Scholar]
- 15.Nonaka D, Chiriboga L, Soslow RA. Expression of Pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32:1566–1571. doi: 10.1097/PAS.0b013e31816d71ad. [DOI] [PubMed] [Google Scholar]
- 16.Paterakos M, Watkin WG, Edgerton SM, et al. Invasive micropapillary carcinoma of the breast: a prognostic study. Hum Pathol. 1999;30:1459–1463. doi: 10.1016/s0046-8177(99)90168-5. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto K, Watanabe M, De La Cruz C, et al. Primary invasive micropapillary carcinoma of the colon. Histopathology. 2005;47:479–484. doi: 10.1111/j.1365-2559.2005.02241.x. [DOI] [PubMed] [Google Scholar]
- 18.Samaratunga H, Khoo K. Micropapillary variant of urothelial carcinoma of the urinary bladder: a clinicopathological and immunohistochemical study. Histopathology. 2004;45:55–64. doi: 10.1111/j.1365-2559.2004.01895.x. [DOI] [PubMed] [Google Scholar]
- 19.Shimoda M, Okada Y, Hayashi Y, et al. Primary invasive micropapillary carcinoma of the stomach. Pathol Int. 2008;58:513–517. doi: 10.1111/j.1440-1827.2008.02265.x. [DOI] [PubMed] [Google Scholar]
- 20.Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660–662. [PubMed] [Google Scholar]
- 21.Smith Sehdev AE, Sehdev PS, Kurman RJ. Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: a clinicopathologic analysis of 135 cases. Am J Surg Pathol. 2003;27:725–736. doi: 10.1097/00000478-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol. 2001;32:583–589. doi: 10.1053/hupa.2001.24988. [DOI] [PubMed] [Google Scholar]
- 23.Weigelt B, Horlings H, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 24.Wen P, Xu Y, Frankel WL, et al. Invasive micropapillary carcinoma of the sigmoid colon: distinct morphology and aggressive behavior. Int J Clin Exp Pathol. 2008;1:457–460. [PMC free article] [PubMed] [Google Scholar]