Abstract
Sodium channel inhibitor (SCI) insecticides are hypothesized to inhibit voltage-gated sodium channels by binding selectively to the slow-inactivated state. Replacement of valine at position 787 in the S6 segment of homology domain II of the rat Nav1.4 sodium channel by lysine (V787K) enchances slow inactivation of this channel whereas replacement by alanine or cysteine (V787A, V787C) inhibits slow inactivation. To test the hypothesis that SCI insecticides bind selectively to the slow-inactivated state, we constructed mutated Nav1.4/V787A, Nav1.4/V787C, and Nav1.4/V787K cDNAs, expressed wildtype and mutated channels with the auxiliary β1 subunit in Xenopus oocytes, and used the two-electrode voltage clamp technique to examine the effects of these mutations on channel inhibition by four SCI insecticides (indoxacarb, its bioactivated metabolite DCJW, metaflumizone, and RH3421). Mutations at Val787 affected SCI insecticide sensitivity in a manner that was independent of mutation-induced changes in slow inactivation gating. Sensitivity to inhibition by 10 μM indoxacarb was significantly increased in all three mutated channels, whereas sensitivity to inhibition by 10 μM metaflumizone was significantly reduced in Nav1.4/V787A channels and completely abolished in Nav1.4/V787K channels. The effects of Val787 mutations on metaflumizone were correlated with the hydrophobicity of the substituted amino acid rather than the extent of slow inactivation. None of the mutations at Val787 significantly affected the sensitivity to inhibition by DCJW or RH3421. These results demonstrate that the impact of mutations at Val787 on sodium channel inhibition by SCI insecticides depends on the specific insecticide examined and is independent of mutation-induced changes in slow inactivation gating. We propose that Val787 may be a unique determinant of metaflumizone binding.
Keywords: Sodium channel, inhibition, insecticide, slow inactivation, indoxacarb, metaflumizone
1. Introduction
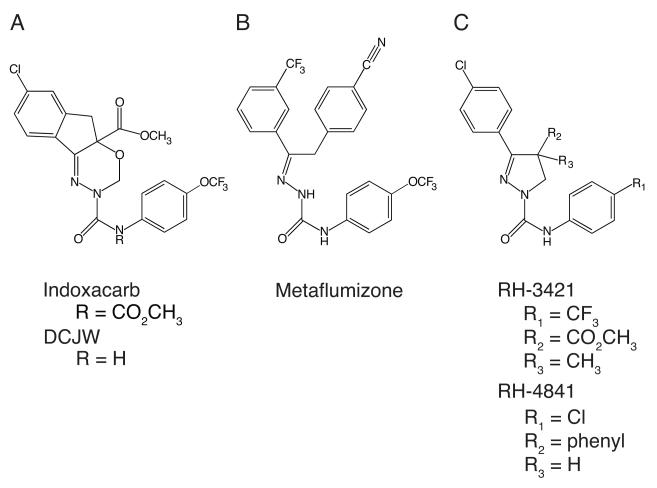
Sodium channel inhibitor (SCI) insecticides are an emerging class of neurotoxic insect control agents that cause ataxia, convulsions, paralysis, and mortality in arthropods (Salgado, 1990). SCI insecticides were first discovered in the early 1970s, though compounds in this insecticide class have only become commercially available within the last decade. The oxadiazine indoxacarb (Fig. 1A), the first SCI insecticide to be commercially registered, is a proinsecticide that is efficiently converted in insects but not mammals to its more potent N-decarbomethoxylated metabolite, DCJW (Fig. 1A) (Wing et al., 2005). The novel semicarbazone metaflumizone (Fig. 1B), the second SCI insecticide to be commercialized, is the first member of this insecticide class introduced into the animal health market (Salgado and Hayashi, 2007). Dihydropyrazoles (e.g. RH-3421 and RH-4841; Fig 1C) exemplify earlier members of the SCI family that exhibited high insecticidal activity but were not developed commercially because of their high subchronic neurotoxicity to mammals (Payne et al., 1998; Silver and Soderlund, 2005a). The continued efforts of the agrochemical industry to develop new, commercially viable SCI compounds reinforces both the value of this type of biological activity and the need to better understand the actions of these compounds that underlie their toxicological properties.
Figure 1. Structures of SCI insecticides.
(A) Indoxacarb and DCJW. (B) Metaflumizone. (C) RH-3421 and RH-4841.
The insecticidal activity of SCI insecticides results from their disruption of electrical signaling in the nervous system by inhibiting voltage-gated sodium channels (Salgado, 1992). Voltage-gated sodium channels are also considered to be the primary targets for the central neurotoxic effects of SCI insecticides in mammals, but there is limited information on the mammalian toxicity of these compounds available in the public literature. Acute oral administration of RH-3421 causes ataxia, tremors, salivation, spasms, and a hunched posture in rats (Salgado, 1992; Silver and Soderlund, 2005a), and similar symptoms were reported in rats administered a high oral dose of indoxacarb (Zhao et al., 1999). A direct linkage between effects of SCIs on sodium channel function and the neurotoxic actions of these compounds in mammals remains to be established.
Voltage-gated sodium channels are integral membrane proteins composed of a large (~260 kDa) α subunit that confers all of the major structural, functional, and pharmacological properties of native channels and either one or two small auxiliary β subunits that modulate channel gating and regulate expression in the cell membrane (Catterall, 2000; Isom, 2001). The α subunit comprises 24 transmembrane α-helical segments that are organized into four homologous domains (DI-DIV) around a central ion-conducting pore. Each domain contains six transmembrane segments, designated S1-S6.
Voltage-gated sodium channels undergo changes in conformation and functional state in response to transient or persistent changes in the voltage difference across the plasma membrane. Transient depolarization from a negative resting potential initiates a cycle of channel activation and fast inactivation that underlies the initiation and termination of a nerve action potential. By contrast, persistent or prolonged depolarizations cause channels to enter non-conducting slow-inactivated states (Goldin, 2003). The transitioning of sodium channels between conformational states not only alters sodium ion permeability but also determines the sensitivity of channels to many neurotoxic and therapeutic agents (McDonough and Bean, 2006).
SCI insecticides are state-dependent inhibitors of sodium channels. They have no effect on channels in the resting state but disrupt channel function through a selective interaction with channels in the non-conducting slow-inactivated state (Salgado, 1992; Lapied et al., 2001; Zhao et al., 2003; Silver and Soderlund, 2005b; von Stein and Soderlund, 2012). However, previous studies provide indirect evidence that some SCIs may also interact with other sodium channel states. Experiments with crayfish axons showed that inhibition of sodium channels by an experimental SCI insecticide (RH-1211) was not disrupted by progressive proteolytic removal of slow and fast inactivation by intracellular perfusion of trypsin and N-bromoacetamide, respectively (Salgado, 1992). Furthermore, metaflumizone differed from other SCI insecticides in its ability to antagonize the inhibition of Nav1.4 sodium channels by the local anesthetic lidocaine under experimental conditions that promoted lidocaine binding to fast-inactivated channels but did not cause insecticide inhibition (von Stein and Soderlund, 2012). These results not only suggest that metaflumizone may bind to fast-inactivated channels without inhibiting them but also imply a fundamental difference in the state-dependent action of metaflumizone compared to other SCI insecticides.
SCI drugs (local anesthetics, class I anticonvulsants and antiarrhythmics), which bind inside the inner pore at the local anesthetic (LA) receptor, preferentially target open or fast-inactivated channels (Ragsdale et al., 1994, 1996). Studies employing site-directed mutagenesis have identified putative drug binding determinants in the S6 transmembrane segments in all domains except domain II (Ragsdale et al., 1994; Yarov-Yarovoy et al., 2001, 2002). Residues in domain IV-S6, particularly Phe1579 and Tyr1586 (rat Nav1.4 numbering) appear to be essential for state-dependent inhibition by SCI drugs. Inhibition of slow-inactivated Nav1.4 channels by SCI insecticides is also modulated by mutations at Phe1579 and Tyr1586 (Silver and Soderlund, 2007; von Stein and Soderlund, 2012). These findings are consistent with the hypothesis that SCI insecticides bind selectively to slow-inactivated sodium channels at or near the LA receptor.
The present study was initiated to test the hypothesis that the extent of sodium channel inhibition by SCI insecticides depends directly on the availability of slow-inactivated channel states. We employed experimental mutations at the highly conserved Val787 residue in sodium channel DII-S6 that either increase or decrease the propensity of channels to enter slow inactivated states (O’Reilly et al., 2001). Replacement of Val787 by lysine (V787K) profoundly enhances slow inactivation, whereas replacement by either alanine or cysteine (V787A, V787C) inhibits slow inactivation compared to wildtype channels. We anticipated that mutations at Val787 would selectively modify slow inactivation without affecting binding of SCI insecticides to the channel because this residue has not been identified previously as part of the LA receptor (Wang et al., 2001; Kondratiev and Tomaselli, 2003). We expressed wildtype Nav1.4 channels and Nav1.4 channels possessing the V787A, V787C, or V787K mutation in combination with the rat β1 subunit in Xenopus laevis oocytes and assessed the sensitivity of channels to inhibition by SCI insecticides. Surprisingly, the impact of mutations at Val787 on insecticide inhibition depended on the properties of the amino acid introduced at this position and the specific insecticide examined independent of any mutation-induced changes in slow inactivation gating. These results suggest that the Val787 residue in DII-S6 may be part of or in close proximity to the SCI insecticide binding site, the molecular determinants of which may differ between members of this chemically diverse insecticide class.
2. Materials and Methods
2.1. Choice of sodium channel isoform
The rat skeletal muscle sodium channel (Nav1.4) was chosen for the present study because it is a well-defined model system for studying the actions of SCI insecticides on cloned mammalian sodium channels (Silver and Soderlund, 2005b, 2007; von Stein and Soderlund, 2012). The use of Nav1.4 also ensured that mutations at Val787 yielded the expected residue-specific effects on slow inactivation that have been reported elsewhere (O’Reilly et al., 2001). Furthermore, oocytes expressing Nav1.4 channels consistently gave robust sodium currents that facilitated the reliable recording of currents under experimental conditions that promoted partial channel inactivation and insecticide inhibition. We coexpressed the rat Nav1.4 α subunit with the rat β1 subunit because Nav1.4 α subunits yield channels that exhibit aberrant inactivation gating when expressed alone in Xenopus laevis oocytes (Balser et al., 1996). Previous studies (Silver and Soderlund, 2006) demonstrated that the effects of SCI insecticides on rat Nav1.4 channels were qualitatively consistent with those on other rat sodium channel isoforms that are the presumptive molecular targets for the central neurotoxic effects of SCI insecticides.
2.2. Molecular biology
The cDNA encoding the wildtype rat Nav1.4 skeletal muscle α subunit (Kallen et al., 1990) was obtained from R.G. Kallen (University of Pennsylvania, Philadelphia, PA) and the cloned rat brain β1 cDNA was obtained from W.A. Catterall (University of Washington, Seattle, WA). Sodium channel mutations were introduced by polymerase chain reaction using a commercial site-directed mutagenesis kit (QuikChange XL, Stratagene, La Jolla, CA) and oligonucleotide primers (Sigma Genosys, The Woodlands, TX) encoding the mutation of interest at Val787 in the rat Nav1.4 cDNA. DNA sequencing of the region surrounding the mutation confirmed the structures of all mutated cDNAs. The cDNA plasmids were linearized with restriction enzymes to provide templates for in vitro cRNA synthesis using a commercial kit (mMessage mMachine, Ambion, Austin, TX). The integrity of all cRNAs was assessed by electrophoresis in 1% agarose-formaldehyde gels.
2.3. Oocyte preparation and sodium channel expression
Ovaries were either surgically removed from anesthetized female Xenopus laevis frogs (Nasco, Ft. Atkinson, WI) or purchased as freshly-dissected whole tissues (Nasco). Oocytes from both of these sources gave identical results in expression assays. The surgical procedure was performed in accordance with National Institutes of Health guidelines and followed a protocol that was approved by the Cornell University Animal Care and Use Committee. Ovaries were first washed with Ca2+-free saline (containing in mM: 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, adjusted pH to 7.6 with NaOH) before being digested in 1 mg/mL collagenase (type 1A, Sigma-Aldrich, St. Louis, MO) for about 1 hr to degrade the ovarian tissue. Mature (stage V and VI) oocytes were selected and the outer follicle cells were manually removed with surgical forceps. Defolliculated oocytes were incubated overnight at 16° C in ND-96 (containing in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, adjusted to pH 7.6 with NaOH) solution supplemented with 6% horse serum (Sigma-Aldrich), 0.5% penicillin/streptomycin and 2.5 mM sodium pyruvate (Goldin, 1992). Healthy oocytes were pressure-injected with a 1:2 (mass ratio) mixture of wildtype or mutated rat Nav1.4 channel α subunit and β1 cRNAs, respectively. The concentration of the α subunit was between 0.5 and 12 ng/μL, which was sufficient to generate sodium currents with amplitudes between -10 and -30 μA. Oocytes were incubated at 16° C in supplemented ND-96 media for 2-5 days prior to use.
2.4. Electrophysiology
The two-microelectrode voltage-clamp technique was used to record macroscopic sodium currents from injected oocytes using a Geneclamp 500B amplifier (Axon Instruments, Foster City, CA) and pClamp 10.2 software (Axon Instruments, Burlingame, CA. All electrophysiological experiments were performed at room temperature (~23° C) in disposable recording chambers manufactured from 0.25 in. Plexiglass and a glass coverslip sealed together with ethyl cyanoacrylate as described elsewhere (Smith and Soderlund, 1998). Oocytes were continuously perfused with ND-96 saline at ~0.45 ml/min using a custom-designed passive capillary perfusion system similar to that described previously (Tatebayashi and Narahashi, 1994; Silver and Soderlund, 2005b). The entire bath perfusate could be exchanged in approximately 1 min. Recording microelectrodes were pulled from borosilicate glass capillaries (World Precision Instruments, Inc.) and filled with a filtered 3 M KCl solution. Filled electrodes had 0.5- to 0.7-MΩ tip resistances when submerged in ND-96 saline. All perfusion capillaries, recording chambers, and recording electrodes were discarded after single use to prevent potential cross-contamination between experiments. Sodium currents were filtered at 5 kHz with a low-pass 4-pole Bessel filter and digitized at 50 kHz with a Digidata 1320A (Axon Instruments). Capacitive transient currents were subtracted using the P/4 subtraction method (Bezanilla and Armstrong, 1977). Experiments with oocytes that expressed Nav1.4, Nav1.4/V787A, or Nav1.4/V787C channels were performed >5 min after the membrane was clamped at a hyperpolarized potential of −120 mV to allow sodium channels to recover from slow inactivation following hyperpolarization from a typical oocyte resting potential of about −55 mV (Catterall, 1976). The Nav1.4/V787K channels typically required >10 min at a holding potential of −140 mV to stabilize the sodium current, reflecting the enhanced slow inactivation of this mutant.
2.5. Pulse protocols
Protocols used to characterize the voltage dependence of channel activation and inactivation were carried out at a holding potential (Vh) of −120 mV except for Nav1.4/V787K channels, which were assayed at a Vh of −140 mV due to the altered voltage dependence of slow inactivation observed for this mutant channel. The voltage dependence of activation was determined by measuring sodium currents during a 20-ms depolarization to potentials ranging from −80 to 30 mV in 10-mV increments. To determine the voltage dependence of steady-state fast inactivation, sodium currents were measured during a 20-ms test pulse to −10 mV (Nav1.4, Nav1.4/V787C, and Nav1.4/V787K channels) or 0 mV (Nav1.4/V787A channels) after a 200-ms conditioning pulse to potentials ranging from −100 mV to 0 mV in 10-mV increments. To determine the voltage dependence of slow inactivation, a 100-s conditioning pulse to potentials ranging from Vh to 0 mV in 10-mV steps was followed by a 50-ms hyperpolarization to Vh (to fully recover fast-inactivated channels) and a 20-ms test pulse to −10 mV (Nav1.4, Nav1.4/V787C, and Nav1.4/V787K channels) or 0 mV (Nav1.4/V787A channels). With Nav1.4/V787K channels there was no difference in the extent of slow inactivation after a 10-s conditioning pulse compared to a 100-s conditioning pulse (data not shown) so we employed a 10-s conditioning pulse to characterize the voltage-dependence of slow inactivation in this mutant (O’Reilly et al., 2001). Oocytes were held at Vh for >2 min between pulses in the slow inactivation protocol to allow recovery from slow inactivation caused by previous pulses. To assess the time course of inhibition by SCI insecticides, oocytes were held at a depolarized Vh of −30 mV and sodium currents were measured once every minute during a 20-ms test pulse preceded by a 2-s hyperpolarization to −120 mV to partially recover channels to the resting state except with Nav1.4/V787K channels. In assays with Nav1.4/V787K channels, test pulses were preceded by a 2-s step to −140 mV from either a Vh of −140 mV or −110 mV because of enhanced slow inactivation caused by this mutation. Stable sodium currents were recorded for 15 min prior to insecticide perfusion. Control sodium currents were typically stable for >60 min (data not shown). Further details of voltage protocols are provided in the figure legends.
2.6. Insecticides
Metaflumizone and indoxacarb were purchased from Sigma-Aldrich and ChemService (West Chester, PA), respectively. RH-3421 and RH-4841 were provided by G. Carlson (Rohm and Haas Company, Spring House, PA) and DCJW was a gift from K. Wing (DuPont Agricultural Product, Newark, DE). Stock solutions of insecticides (10 mM) were prepared in dimethyl sulfoxide (DMSO) and were diluted in ND-96 just prior to use to a final concentration of 10 μM, the highest concentration attainable in ND-96 medium, to maximize insecticide effects. The DMSO concentration of the bath perfusate never exceeded 0.1% v/v, a concentration that had no effect on sodium currents.
2.7. Data and Statistical analysis
Data were analyzed using Origin 8.1 (OriginLab Corp., Northampton, MA). The voltage dependence of sodium conductance and sodium channel inactivation plots were fitted using the Boltzmann equation [y = (A1-A2)/{1 + exp[(V-V1/2)/k]} + A2] where V1/2 is the midpoint of the curve, k is the slope factor, and A1 and A2 are the maximum and minimum values in the fit, respectively. We calculated time constants for sodium current inhibition by fitting the time course of inhibition data with a single-exponential decay equation [y = A1*exp(-x/τ1) + y0], where x is time, τ1 is the time constant, and y0 is the non-inactivating component. All fitted data are given as the mean ± S.E. All statistical analyses were completed using Prism 5.0 (GraphPad Software, La Jolla, CA). The statistical significance of two or more mean values from a single control data set was determined by one-way analysis of variance (ANOVA) and Dunnett’s post hoc analysis. Differences were considered statistically significant when P < 0.05.
3. Results
3.1. Effects of mutations at Val787 on voltage-dependent gating of Nav1.4 sodium channels
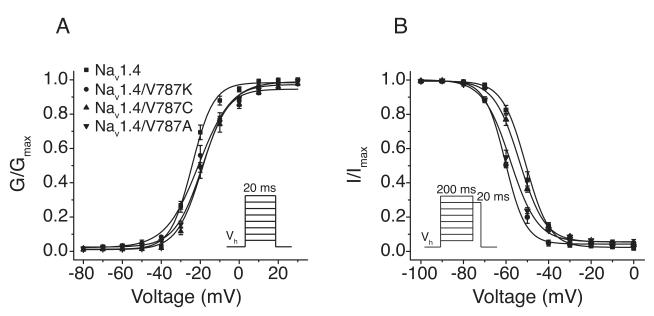
We expressed the wildtype and mutated Nav1.4 sodium channel α subunits in combination with the rat β1 subunit to give channels that reflect the putative native heteromeric structure and exhibit native-like gating properties in Xenopus oocytes (Isom et al., 2001). The activation (conductance-voltage) curve was significantly shifted in the direction of depolarization and less steep for Nav1.4/V787A and Nav1.4/V787C channels than for wildtype channels (Fig. 2A; Table 1). Although the V787K mutation did not significantly affect the midpoint potential (V1/2) for activation, the slope of the voltage response was less steep compared to wildtype. The V1/2 for steady-state fast inactivation was significantly more hyperpolarized for Nav1.4/V787A and Nav1.4/V787K channels but not for Nav1.4/V787C channels compared to wildtype channels (Fig. 2B; Table 1).
Figure 2. Voltage-dependent activation and steady-state fast inactivation of Nav1.4 and V787-mutated sodium channels.
(A) Conductance-voltage plots for sodium channel activation; peak sodium currents measured on depolarization from −120 mV to test potentials ranging from − 80 mV to 30 mV were transformed to conductances (G) using the equation G = I/(Vt-Vrev), where I is the peak current, Vt is the voltage of the test potential, and Vrev is the reversal potential; conductances were normalized to the maximal conductance (Gmax) for that oocyte; values are means ± S.E. of 15 (Nav1.4), 9 (Nav1.4/V787A), 7 (Nav1.4/V787C), or 11 (Nav1.4/V787K) separate experiments with different oocytes. (B) Voltage dependence of steady-state fast inactivation; conditioning pulses (200 ms) from −120 mV to potentials ranging from −100 mV to 0 mV (Vp) were followed immediately by 20-ms test pulses to either −10 mV (Nav1.4, Nav1.4/V787C, Nav1.4/V787K) or 0 mV (Nav1.4/V787A); peak currents were normalized to the maximal current measured during the inactivation protocol for that oocyte; values are means ± S.E. of 11 (Nav1.4), 9 (Nav1.4/V787A), 5 (Nav1.4/V787C), or 4 (Nav1.4/V787K) separate experiments with different oocytes; curves were fitted to the mean values using the Boltzmann equation.
Table 1.
Voltage-dependent gating parameters for wildtype and Val787 mutated Nav1.4 sodium channels a.
| Activation |
Fast inactivation |
Slow inactivation |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel | V1/2 (mV) | k | [n] | V1/2 (mV) | k | [n] | V1/2 (mV) | k | [n] |
| Nav1.4 | −25.8 ± 0.4 | 5.0 ± 0.4 | [15] | −52.2 ± 0.3 | 5.5 ± 0.2 | [11] | −45.8 ± 0.8 | 9.4 ± 0.7 | [7] |
| Nav1.4/V787A | −18.8 ± 0.6* | 7.0 ± 0.5* | [9] | −59.2 ± 0.6* | 5.9 ± 0.5 | [9] | −39.8 ± 1.8 | 10.8 ± 1.6 | [4] |
| Nav1.4/V787C | −18.3 ± 0.6* | 6.6 ± 0.5† | [7] | −53.8 ± 0.4 | 5.6 ± 0.4 | [5] | −41.4 ± 3.4 | 10.4 ± 2.9 | [5] |
| Nav1.4/V787K | −23.9 ± 0.7 | 6.6 ± 0.6§ | [11] | −60.1 ± 0.5* | 5.1 ± 0.5 | [4] | −92.6 ± 1.2* | 11.4 ± 1.0 | [6] |
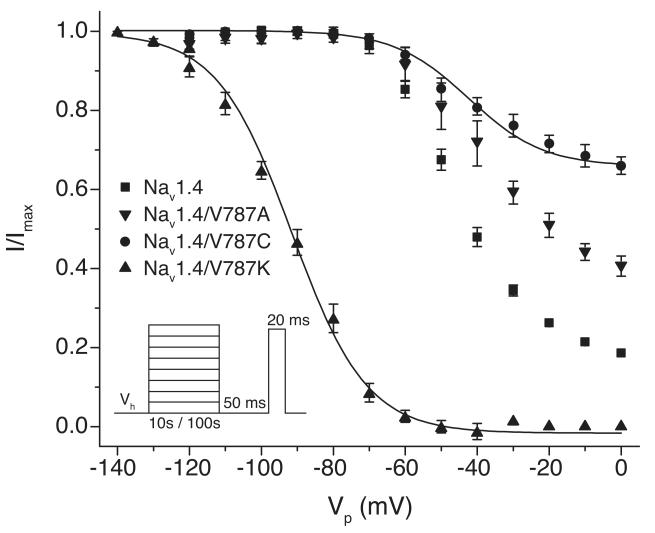
Amino acid substitutions at Val787 strongly affected the propensity of sodium channels to enter the slow-inactivated state in response to depolarization. With Nav1.4/V787K channels, slow inactivation developed at more hyperpolarized potentials (~95% slow inactivation at −70 mV) than with wildtype channels (~2% slow inactivation at −70 mV) and the V1/2 of the apparent voltage dependence of steady-state slow inactivation curve was shifted ~50 mV in the direction of hyperpolarization compared to wildtype (Fig. 3; Table 1). By contrast, the steady-state probability of slow inactivation was significantly lower for Nav1.4/V787A (~60% slow inactivation at 0 mV) and Nav1.4/V787C (~34% slow inactivation at 0 mV) channels than for wildtype (~82% slow inactivation at 0 mV), though the midpoint potentials for steady-state slow inactivation in Nav1.4/V787A (-39.8 ± 1.8 mV) and Nav1.4/V787C (-41.4 ± 3.4 mV) did not differ significantly from that of wildtype (-45.8 ± 0.8 mV; Fig. 3; Table 1).
Figure 3. Voltage dependence of slow inactivation of Nav1.4 and Val787 mutated sodium channels.
Oocytes were clamped at a holding potential (Vh) of either −120 mV (Nav1.4, Nav1.4/V787A, Nav1.4/V787C channels) or −140 mV (Nav1.4/V787K channels) and stimulated with either a 100-s (Nav1.4, Nav1.4/V787A, Nav1.4/V787C) or 10-s (Nav1.4/V787K) conditioning pulse (Vp) to potentials ranging from Vh to 0 mV followed by a 50-ms hyperpolarization to Vh and a test pulse to either −10 mV (Nav1.4, Nav1.4/V787C, Nav1.4/V787K) or 0 mV (Nav1.4/V787A); peak currents were normalized to the maximal current measured during the inactivation protocol for that oocyte; values are means ± S.E. of 7 (Nav1.4, 4 (Nav1.4/V787A), 5 (Nav1.4/V787C), or 6 (Nav1.4/V787K) separate experiments with different oocytes; curves were fitted to the mean values using the Boltzmann equation.
3.2. Inhibition of wildtype and mutated Nav1.4 sodium channels by SCI insecticides
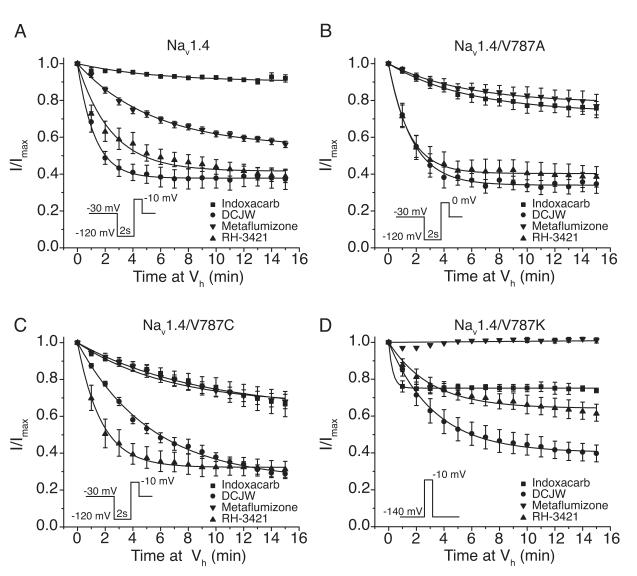
Consistent with the results of previous studies (Silver and Soderlund, 2005b; von Stein and Soderlund, 2012), SCI insecticides had no effect on sodium currents through wildtype channels at a hyperpolarized holding potential (-120 mV) where channels predominantly occupied the resting state (data not shown). Holding oocytes at a depolarized potential of −30 mV facilitated slow inactivation of wildtype channels and inhibition by SCI insecticides. Fig 4A shows the time course of inhibition of Nav1.4 sodium channels by 10 μM indoxacarb, DCJW, metaflumizone, and RH-3421 at a holding potential of −30 mV. After 15 min of exposure, peak transient sodium currents were inhibited 8.3 ± 1.4%, 62.8 ± 5.5%, 43.9 ± 1.7%, and 61.2 ± 3.3% by indoxacarb, DCJW, metaflumizone, and RH-3421, respectively when compared to currents measured in the same oocytes prior to insecticide exposure. The time courses of inhibition were fitted best by a single-exponential decay equation that yielded first-order decay constants (τ) of 6.1 ± 1.1 min, 1.3 ± 0.1 min, 5.9 ± 0.6 min, and 1.4 ± 0.3 min for indoxacarb, DCJW, metaflumizone, and RH-3421, respectively.
Figure 4. Time course of inhibition of (A) Nav1.4, (B) Nav1.4/V787A, (C) Nav1.4/V787C, or (D) Nav1.4/V787K sodium channels by SCI insecticides.
Oocytes were clamped at a holding potential (Vh) of either −30 mV (Nav1.4, Nav1.4/V787A, Nav1.4/V787C) or −140 mV (Nav1.4/V787K); sodium currents were measured once every minute with a test pulse (20 ms) preceded by a hyperpolarization (2 s) to either −120 mV (Nav1.4, Nav1.4/V787A, Nav1.4/V787C) or −140 mV (Nav1.4/V787K); currents measured in the presence of an insecticide were normalized to the peak current recorded in that oocyte prior to insecticide perfusion; values are means ± S.E. of 3-5 experiments (Nav1.4), 3-7 (Nav1.4/V787A, Nav1.4/V787C), or 4-5 (Nav1.4/V787K) separate experiments with different oocytes; curves were fitted to the mean values using a single-exponential decay equation.
Preliminary experiments verified that Nav1.4 channels bearing either the V787A or V787C mutation were insensitive to inhibition by SCI insecticides at a holding potential of −120 mV (data not shown). The time course of inhibition of sodium currents through Nav1.4/V787A (Fig. 4B) and Nav1.4/V787C (Fig. 4C) channels by SCI insecticides was assessed at a holding potential of −30 mV. At this potential, inhibition by indoxacarb after 15 min of perfusion was significantly greater for Nav1.4/V787A (24.2 ± 3.6%) and Nav1.4/V787C (33.3 ± 6.8%) channels compared to wildtype channels. By contrast, the extent of inhibition by metaflumizone was significantly lower for Nav1.4/V787A channels (20.4 ± 5.5%) after 15 min, whereas inhibition by metaflumizone of Nav1.4/V787C channels did not differ significantly from inhibition of wildtype channels. Inhibition of either Nav1.4/V787A or Nav1.4/V787C channels by DCJW or RH-3421 also did not differ significantly from inhibition of wildtype channels by these compounds. However, the V787C mutation significantly retarded the rate of onset of inhibition by indoxacarb (τ = 11.9 ± 3.5 min) and DCJW (τ = 4.5 ± 0.3 min) compared to wildtype. Inhibition of Nav1.4/V787C channels by indoxacarb and DCJW did not reach a steady-state plateau after 15 min of perfusion, so our results may underestimate the extent to which this mutation enhanced inhibition by the these compounds.
We first examined the time course of inhibition of Nav1.4/V787K channels by SCI insecticides at a hyperpolarized holding potential of −140 mV (Fig. 4D) because this channel exhibited complete slow inactivation at −30 mV, the holding potential employed for other channel variants (Fig. 3). Limitations of the experimental system prohibited us from achieving a stable voltage clamp of the membrane at potentials more hyperpolarized than −140 mV for the time (> 25 min) required for measuring the slow inhibition of sodium channels by SCI insecticides. Sodium currents through Nav1.4/V787K channels were significantly inhibited by 10 μM indoxacarb (26.3 ± 1.5%), DCJW (60.3 ± 4.6%), and RH-3421 (38.6 ± 5.1%) after 15 min of perfusion, but were completely resistant to inhibition by 10 μM metaflumizone when compared to currents measured in the same oocytes prior to insecticide exposure . Inhibition by indoxacarb reached a steady-state level by 1 min of perfusion, whereas DCJW and RH-3421 required ~15 min of perfusion to approach an apparent steady-state inhibition. First order exponential decay fits of the time course of sodium current inhibition by DCJW and RH-3421 yielded time constants of 3.7 ± 0.2 min and 3.7 ± 0.4 min, respectively. The rapid onset of inhibition by indoxacarb prevented an accurate first-order exponential decay fit of the time course of inhibition at a stimulation frequency of once per minute.
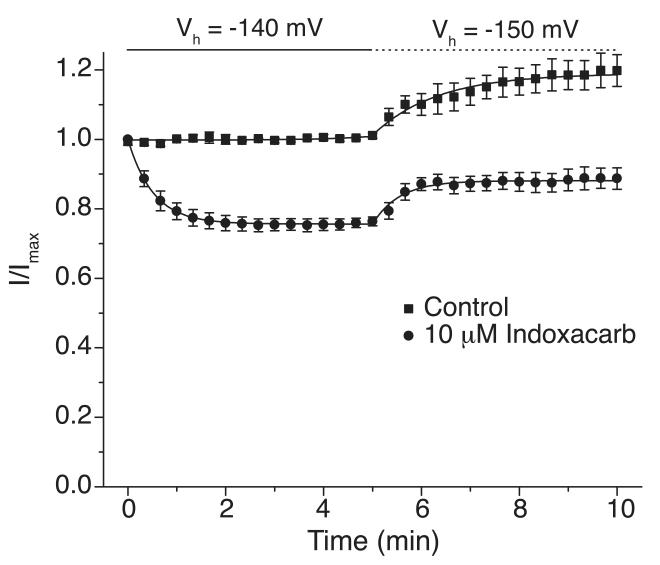
To determine if insecticide inhibition of Nav1.4/V787K channels at −140 mV reflected insecticide binding to channels in the slow-inactivated state, we examined the effect of hyperpolarization to −150 mV on the amplitude of the peak sodium current in both the absence and presence of 10 μM indoxacarb (Fig. 5). Sodium current amplitudes increased in both the absence and presence of indoxacarb in response to hyperpolarization of the membrane to −150 mV. Oocytes were stimulated at a frequency of 0.05 Hz, which permitted us to fit the onset of inhibition by indoxacarb to a first order exponential decay function that yielded a time constant of 0.7 ± 0.1 min.
Figure 5. Effect of hyperpolarization on Nav1.4/V787K currents and their inhibition by indoxacarb.
Oocytes were clamped at a holding potential (Vh) of −140 mV for 5 min followed by 5 min at a Vh of −150 mV while stimulated at a rate of 0.05 Hz with a test pulse (20 ms) to −10 mV; sodium currents were normalized to the maximum current recorded in the same oocyte at − 140 mV prior to indoxacarb perfusion; values are means ± S.E. of 7 separate experiments in different oocytes; curves were fitted to the mean values using a single-exponential decay equation.
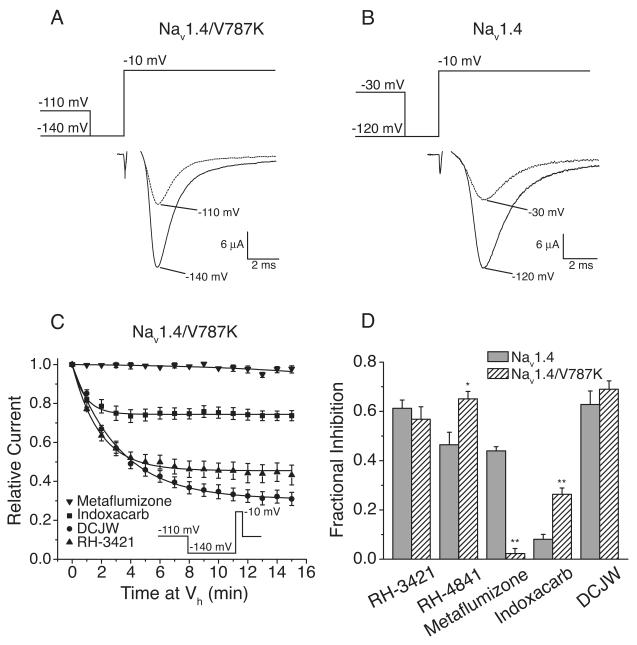
We recently reported (von Stein and Soderlund, 2012) that the extent of Nav1.4 sodium channel inhibition by metaflumizone was strongly dependent on the relative fraction of channels in the slow-inactivated state. To determine if the resistance of Nav1.4/V787K channels to inhibition by metaflumizone at −140 mV (Fig. 4D) reflected a relatively low steady-state probability of slow inactivation, we examined the time course of inhibition of currents through Nav1.4/V787K channels at a more depolarized holding potential of −110 mV (Fig. 6). Holding oocytes at this potential in the absence of insecticides reduced the peak sodium current amplitude over several minutes to ~50% of the maximum current elicited from a holding potential of −140 mV (Fig. 6A), an extent of apparent slow inactivation that was comparable to the partial slow inactivation produced in wildtype channels at −30 mV (Fig. 6B). However, it is likely there was greater fractional slow inactivation in Nav1.4/V787K at −110 mV compared to wildtype channels at −30 mV because some slow inactivation in Nav1.4/V787K was evident at −140 mV (Fig. 5). The time course of the onset of Nav1.4/V787K inhibition by SCI insecticides at −110 mV (Fig. 6C) showed there was no difference in the extent of metaflumizone inhibition compared to −140 mV (Fig. 4D). The statistical analyses of these data in comparison to inhibition of currents through wildtype channels after 15 min of perfusion at −30 mV are summarized in Fig. 6D. At a holding potential of −110 mV, the V787K mutation paradoxically increased sensitivity to 10 μM indoxacarb and conferred resistance to 10 μM metaflumizone, whereas the extent of inhibition by 10 μM DCJW and RH-3421 was not significantly different from that measured with wildtype channels. We also examined the impact of the V787K mutation on inhibition by RH-4841, a SCI insecticide that resembles a heterocylic ring-closed analog of metaflumizone. Sodium currents through Nav1.4/V787K channels were significantly more sensitive to 10 μM RH-4841 than wildtype after 15 min of perfusion (Fig. 6D).
Figure 6. Comparative inhibition of Nav1.4 and Nav1.4/V787K sodium channels by SCI insecticides.
(A) Representative sodium current traces from an oocyte expressing Nav1.4/V787K at a holding potential (Vh) of −140 mV and −110 mV in the absence of insecticides. (B) Representative sodium current traces from an oocyte expressing Nav1.4 sodium channels at a Vh of −120 mV and −30 mV in the absence of SCI insecticides. (C) Time course of inhibition of Nav1.4/V787K by SCI insecticides; oocytes were clamped at a Vh of −110 mV and stimulated once every minute with a test pulse (20 ms) to −10 mV preceded by a hyperpolarization (2 s) to − 140 mV; currents measured in the presence of an insecticide were normalized to the peak sodium current recorded in that oocyte prior to insecticide perfusion; values are means ± S.E. of 5-7 separate experiments with different oocytes; curves were fitted to the mean values using a single-exponential decay equation. (D) Comparison of the fractional inhibition of Nav1.4 (Fig. 4A) and Nav1.4/V787K channels by SCI insecticides after 15 min of insecticide perfusion at a Vh of either −30 (Nav1.4) or −110 mV (Nav1.4/V787K).
4. Discussion
SCI insecticides are a class of neurotoxic agents whose commercial development was impeded for decades by adverse neurotoxic effects in mammals. In this context, information on the mode of action of SCI insecticides on mammalian sodium channel targets is relevant to understanding the risks posed by the use of current and future members of this insecticide class. The current hypothesis for the action of SCI insecticides on voltage-gated sodium channels proposes that inhibition involves a selective interaction with channels in the slow-inactivated state. The primary objective of this study was to determine if insecticide inhibition of Nav1.4 sodium channels was modulated by specific mutations at Val787 in DII-S6 that exhibited altered slow inactivation. The effects of mutations at residue Val787 on slow inactivation reported here are consistent with previous studies of these mutations expressed in mammalian cells without the β1 subunit (O’Reilly et al., 2001). The V787C and V787A mutation inhibited slow inactivation, whereas the V787K mutation significantly enhanced slow inactivation compared to wildtype. Consistent with previous results (O’Reilly et al., 2001), the V787K mutation shifted the voltage dependence of slow inactivation ~50 mV in the direction of hyperpolarization compared to wildtype. However, this previous study also reported much larger depolarizing shifts (~24 mV) in the voltage dependence of steady-state slow inactivation of Nav1.4/V787A and Nav1.4/V787C channels compared to wildtype channels than we observed in the present study. These discrepancies may reflect differences in experimental conditions, such as the choice of expression system, presence of the β1 subunit, or differences in the duration of conditioning prepulses used to assay slow inactivation.
The interpretation of results from studies using site-directed mutagenesis to map binding determinants of state-dependent SCIs inside the inner pore is often confounded by the fact that mutations in this region of the channel can indirectly affect binding via modification of gating (Mike and Lukacs, 2010). However, our data for the effects of mutations at residue Val787 on insecticide sensitivity cannot be explained by changes in slow inactivation gating. For example, the V787K mutation strongly enhanced slow inactivation relative to wildtype but completely abolished inhibition by 10 μM metaflumizone. Furthermore, the V787A and V787C mutations destabilized slow inactivation but increased the sensitivity to inhibition by indoxacarb compared to wildtype. These results indicate that the changes in insecticide sensitivity caused by mutations at Val787 were not the result of the altered slow inactivation gating of the mutated channels.
SCI insecticides are unified functionally by a common mechanism of action despite their structural diversity. The dihydropyrazoles (e.g. RH-3421 and RH-4841) contain a central pyrazoline ring, whereas indoxacarb contains a fused 3-ring system incorporating an oxadiazine ring and metaflumizone contains a linear semicarbazone motif in place of central heterocyclic elements. Although metaflumizone can be viewed as a ring-opened analog of RH-4841, the greater rotational flexibility conferred by the semicarbazone motif distinguishes it from other compounds in this insecticide class, which are very rigid, nearly planar molecules (Wellinga et al., 1977; Grosscurt et al., 1979; Hasan et al., 1996). We recently reported (von Stein and Soderlund, 2012) that the inhibition of fast-inactivated Nav1.4 sodium channels by lidocaine was selectively attenuated by metaflumizone but not RH-4841 or DCJW. These data were interpreted to suggest that the state dependence of insecticide-channel interactions for metaflumizone may differ from other compounds in this insecticide class and may involve different binding determinants. Here, we show that mutations at residue Val787 affect SCI insecticide sensitivity in a compound-specific manner. All mutations at Val787 that we tested significantly increased the sensitivity to inhibition by indoxacarb, whereas sensitivity to metaflumizone was significantly reduced with Nav1.4/V787A and Nav1.4/V787K channels compared to wildtype channels. Interestingly, the extent of inhibition by RH-4841 was significantly increased with Nav1.4/V787K channels compared to wildtype channels. None of the mutations at residue Val787 significantly affected the extent of inhibition by DCJW or RH-3421. These data suggest that the molecular determinants for the binding of SCI insecticides may not be identical for all members of this insecticide class. Consistent with this possibility, Silver and Soderlund (2005b) showed that co-application of indoxacarb was effective at reducing the sodium channel-inhibiting efficacy of DCJW but not RH-3421.
The orientation of the S6 helices of sodium channel domains I, II, and III in relation to the ion pore is controversial (Mike and Lukacs, 2010). The orientation of DII-S6 is particularly ambiguous because no residues in this segment have been directly implicated in the binding of SCI drugs. Mike and Lukacs (2010) recently proposed a plausible orientation of the four S6 helices in the rat Nav1.2 sodium channel. According to their model, residue Val974 (analogous to Val787 in rNav1.4) in DII-S6 is oriented away from the pore in the protein-protein interface between domains II and III, at least in the open or fast-inactivated channel states that are the high-affinity conformations for the binding of numerous SCI drugs. Consistent with this model, the thiol group of V787C is only available for covalent modification by the sulfhydryl-reactive agent methanethiosulfonate ethylammonium (MTSEA) under experimental conditions that promote slow inactivation (O’Reilly et al., 2001). Thus, slow inactivation in sodium channels is associated with molecular rearrangement(s) at or near Val787. However, it is not clear whether MTSEA accesses the V787C residue via a hydrophilic (i.e. the pore) or hydrophobic (i.e. the membrane) pathway because a significant fraction of MTSEA is un-protonated at physiological pH and can therefore reach the site by either pathway (O’Reilly et al., 2001). Analogous experiments (O’Reilly and Shockett, 2006; Chancey et al., 2007) performed using the human Nav1.5 sodium channel yielded similar results, suggesting that molecular rearrangement at or near residues corresponding to Val787 in DII-S6 plays an important role in conformational changes underlying slow inactivation of other sodium channel isoforms.
Our data do not permit us to discern whether mutations at Val787 affect the binding of individual SCIs directly by changing the structure of the receptor or indirectly via allosteric changes in the binding site. For indoxacarb, the increase in sensitivity caused by all three mutations at Val787 was not well-correlated with the size or physiochemical properties of the substituted amino acid, which suggests that this residue does not contribute directly to the binding site for indoxacarb. We postulate that the increase in affinity for indoxacarb caused by mutations at Val787 may reflect an alleviation of steric hindrance that normally impedes the interaction between the insecticide and its receptor, as suggested for rat Nav1.4 channels bearing the Y1586A mutation in DIV-S6 (Silver and Soderlund, 2007). By contrast, the effects of mutations at Val787 on the affinity for metaflumizone were well-correlated with the hydrophobicity of the substituted amino acid. Replacing the native hydrophobic valine with a hydrophobic cysteine did not significantly affect the affinity for metaflumizone, whereas a less hydrophobic alanine substitution significantly lowered the affinity for metaflumizone and a hydrophilic lysine substitution rendered the channels completely insensitive to inhibition by 10 μM metaflumizone. These data suggest that Val787 may participate directly with metaflumizone binding to slow-inactivated channels through hydrophobic interactions.
Further experiments involving replacement of Val787 amino acids with a broader range of physiochemical properties are needed to assess more definitively the involvement of Val787 in metaflumizone binding. Nonetheless, our data provide provocative evidence that this residue directly interacts with metaflumizone. We conclude from these data that mutations at Val787 modulate SCI insecticide sensitivity in a compound-specific manner that is independent of mutation-induced changes in slow inactivation gating. The data presented here provide not only new evidence for the involvement of residues outside the local anesthetic drug receptor in the binding and action of select SCI insecticides but also insight into the molecular properties that distinguish between neurotoxic and therapeutic SCIs.
Research Highlights.
Mutations at V787 in rat Nav1.4 channels modulate slow inactivation gating.
V787 mutations modulate SCI insecticide inhibition independent of changes in gating.
Effects of V787 mutations on SCI insecticide inhibition are compound specific.
Effects of V787 mutations on metaflumizone correlate with substituent hydrophobicity.
V787 may be a unique determinant of metaflumizone binding.
Acknowledgments
This work was supported in part by a grant (R01-ES014591) from the National Institute of Environmental Health Sciences, National Institutes of Health. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences. We thank P. Adams and S. Kopatz for technical assistance and R. Araújo and S. McCavera for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors declare that they have no conflicts of interest with regard to sources of funding for this research or the design and interpretation of the experiments described herein.
References
- Balser JR, Nuss HB, Orias DW, Johns DC, Marban E, Tomaselli GF. Local anesthetic as effectors of allosteric gating. J Clin Invest. 1996;98:2874–86. doi: 10.1172/JCI119116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel. J Gen Physiol. 1977;70:549–66. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Membrane potential dependent binding of scorpion toxin to action potential Na+ channel ionophore. Proc Natl Acad Sci USA. 1976;73:2682–6. doi: 10.1073/pnas.73.8.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Chancey JH, Shockett PE, O’Reilly JP. Relative resistance to slow inactivation of human cardiac Na+ channel hNav1.5 is reversed by lysine or glutamine substitution at V930 in D2-S6. Am J Physiol Cell Physiol. 2007;293:C1895–C1905. doi: 10.1152/ajpcell.00377.2007. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–97. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol. 2003;13:284–90. doi: 10.1016/s0959-4388(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Grosscurt AC, van Hes R, Wellinga K. 1-Phenylcarbamoyl-2-pyrazolines, a new class of insecticides. 3. Synthesis and insecticidal properties of 3,4-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J Agric Food Chem. 1979;27:406–9. [Google Scholar]
- Hasan R, Nishimura K, Okada M, Akamatsu M, Inoue M, Ueno T. Stereochemical basis for the insecticidal activity of carbamoylated and acylated pyrazoline. Pestic Sci. 1996;46:105–12. [Google Scholar]
- Isom I. Sodium channel β subunits: anything but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- Kallen RG, Sheng ZH, Yang J, Chen L, Rogart RB, Barchi RL. Structure and expression of a sodium channel characteristic of denervated skeletal muscle. Neuron. 1990;4:233–42. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- Kondratiev A, Tomaselli GF. Altered gating and local anesthetic block mediated by residues in the I-S6 and II-S6 transmembrane segments of voltage-dependent Na+ channels. Mol Pharmacol. 2003;64:741–52. doi: 10.1124/mol.64.3.741. [DOI] [PubMed] [Google Scholar]
- Lapied B, Grolleau F, Satelle DB. Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels. Brit J Pharmacol. 2001;132:587–95. doi: 10.1038/sj.bjp.0703853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Bean BP. State-dependent drug interactions with ion channels. In: Triggle D, editor. Voltage-Gated Ion Channels as Drug Targets. Wiley-VCH Verlag GmbH & Co; Weinheim Germany: 2006. pp. 19–36. 2006. [Google Scholar]
- Mike A, Lukacs P. The enigmatic drug binding site for sodium channel inhibitors. Curr Mol Pharmacol. 2010;3:129–44. doi: 10.2174/1874467211003030129. [DOI] [PubMed] [Google Scholar]
- O’Reilly JP, Wang S-Y, Wang GK. Residue-specific effects on slow inactivation at V787 in D2-S6 of Nav1.4 sodium channels. Biophys J. 2001;81:2100–11. doi: 10.1016/S0006-3495(01)75858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JP, Shockett PE. Slow-inactivation induced conformational change in domain 2-segment 6 of cardiac Na+ channel. Biochem Biophys Res Commun. 2006;345:59–66. doi: 10.1016/j.bbrc.2006.04.049. [DOI] [PubMed] [Google Scholar]
- Payne GT, Deecher DC, Soderlund DM. Structure-activity relationships for the action of dihydropyrazole insecticides on mouse brain sodium channels. Pestic Biochem Physiol. 1998;60:177–85. [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–8. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado VL. Mode of action of insecticidal dihydropyrazoles: selective block of impulse generation in sensory nerves. Pestic Sci. 1990;28:389–411. [Google Scholar]
- Salgado VL. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol Pharmacol. 1992;41:120–6. [PubMed] [Google Scholar]
- Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker insecticide. Vet Parasitol. 2007;150:182–9. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Silver KS, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pestic Biochem Physiol. 2005a;81:136–43. [Google Scholar]
- Silver KS, Soderlund DM. State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides. Neurotoxicology. 2005b;26:397–406. doi: 10.1016/j.neuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Silver KS, Soderlund DM. Differential sensitvity of rat voltage-sensitive sodium channel isoforms to pyrazoline-type insecticides. Toxicol Appl Pharmacol. 2006;214:209–17. doi: 10.1016/j.taap.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Silver KS, Soderlund DM. Point mutations at the local anesthetic receptor site modulate the state-dependent block of rat Nav1.4 sodium channels by pyrazoline-type insecticides. Neurotoxicology. 2007;28:655–63. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. Neurotoxicology. 1998;19:823–32. [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin resistant sodium channels. J Pharmacol Exp Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- von Stein RT, Soderlund DM. Role of the local anesthetic receptor in the state-dependent inhibition of voltage-gated sodium channels by the insecticide metaflumizone. Mol Pharmacol. 2012;81:366–74. doi: 10.1124/mol.111.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Barile M, Wang GK. Disparate role of Na(+) channel D2-S6 residues in batrachotoxin and local anesthetic action. Mol Pharmacol. 2001;59:1100–7. doi: 10.1124/mol.59.5.1100. [DOI] [PubMed] [Google Scholar]
- Wellinga K, Grosscurt AC, van Hes R. 1-Phenylcarbamoyl-2-pyrazoline: a new class of insecticides. 1. Synthesis and insecticidal properties of 3-phenyl-1-phenylcarbamoyl-2-pyrazoline. J Agric Food Chem. 1977;25:987–92. [Google Scholar]
- Wing KD, Andaloro JT, McCann SF, Salgado VL. Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology, and biology in insects. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Elsevier; New York: 2005. pp. 30–53. [Google Scholar]
- Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel α subunit. J Bio Chem. 2001;276:20–7. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel α subunit in voltage-dependent gating and drug block. J Bio Chem. 2002;277:35393–401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Nagata K, Marszalec W, Yeh JZ, Narahashi T. Effects of the oxadiazine indoxacarb, DPX-MP062, on neuronal nicotinic acetylcholine receptors in mammalian neurons. Neurotoxicology. 1999;20:561–70. [PubMed] [Google Scholar]
- Zhao X, Ikeda T, Yeh JZ, Narahashi T. Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology. 2003;24:83–96. doi: 10.1016/s0161-813x(02)00112-2. [DOI] [PubMed] [Google Scholar]