Abstract
Historically, mitochondrial reactive oxygen species (mROS) were thought to exclusively cause cellular damage and lack a physiological function. Accumulation of ROS and oxidative damage have been linked to multiple pathologies, including neurodegenerative diseases, diabetes, cancer, and premature aging. Thus, mROS were originally envisioned as a necessary evil of oxidative metabolism, a product of an imperfect system. Yet few biological systems possess such flagrant imperfections, thanks to the persistent optimization of evolution, and it appears that oxidative metabolism is no different. More and more evidence suggests that mROS are critical for healthy cell function. In this review, we discuss this evidence following some background on the generation and regulation of mROS.
Introduction
Reactive oxygen species (ROS) are now appreciated to function as signaling molecules to regulate a wide variety of physiology. This is not a new idea, as it was actually first proposed in the 1990s when hydrogen peroxide was shown to be required for cytokine, insulin, growth factor, AP-1, and NF-κB signaling (Finkel, 1998). Soon thereafter, multiple reports indicated that H2O2 could promote phosphatase inactivation by cysteine oxidation and provided a plausible biochemical mechanism by which ROS can impinge on signaling pathways (Rhee et al., 2000). Concurrent with these initial discoveries that ROS can signal, the popular notion of the mitochondrion as simply the ‘powerhouse of the cell’ was challenged with the discovery that release of cytochrome c mediates apoptosis (Liu et al., 1996). The idea that a mitochondrial protein that participates in oxidative phosphorylation also plays a key role in a signaling pathway opened up the possibility that metabolism is not an isolated system. Although it was clear that metabolism is highly regulated by cellular signaling pathways, it was not widely appreciated that metabolism could feedback to regulate signaling. The dual function of cytochrome c suggested there is cross talk between mitochondrial metabolism and signaling pathways. Perhaps release of mitochondrial ROS (mROS) was another form of this cross talk.
Initial studies to test this possibility showed that mitochondria release H2O2 under physiological hypoxia to activate the transcription factor hypoxia inducible factor 1 (HIF-1), which is required for metabolic adaptation under low oxygen (Chandel et al., 1998). Subsequently, mitochondrial release of H2O2 was shown to activate c-Jun N-terminal kinase 1 (JNK1), p53, and NF-κB (Chandel et al., 2000b; Chandel et al., 2000c; Nemoto et al., 2000). Throughout the past decade, there have been numerous reports highlighting the importance of mROS-dependent signaling in a variety of systems (Collins et al., 2012). Collectively, these data suggest that release of mROS has evolved as a method of communication between mitochondrial function and other cellular processes to maintain homeostasis and promote adaptation to stress.
Mitochondria generate ROS
ROS are molecules derived from oxygen (O2) that can readily oxidize other molecules. Most intracellular ROS are derived from superoxide (O2−•), which is generated by the one electron reduction of O2. Superoxide is converted to hydrogen peroxide (H2O2) by superoxide dismutases (SODs).
There are eight sites in mitochondria that are known to possess the ability to produce O2−• (Brand, 2010), however their contribution to cellular ROS levels in vivo is unclear. Interestingly, while seven of these sites can deposit O2−• into the mitochondrial matrix, only site IIIQo (on complex III) and glycerol 3-phosphate dehydrogenase can release O2−• into the intermembrane space. Intermembrane space ROS theoretically has easier access to the cytosol, as they only need to cross the outer mitochondrial membrane, while matrix ROS need to cross both the inner and the outer mitochondrial membranes (Muller et al., 2004). Thus, O2−• derived from site IIIQo and glycerol 3-phosphate dehydrogenase may possess an advantage with regard to signaling capacity in the cytosol, however further studies are required to definitively determine which site(s) of mROS production is physiologically most important.
Mitochondrial ROS are tightly regulated
Given that quantities of mROS can determine specificity and function, tight regulation of mROS is critical to their ability to participate in physiological cell signaling (Figure 1). Thus, the signaling capacity of mROS is regulated on several levels. First, antioxidant enzymes can eliminate ROS. As mentioned above, SODs convert O2−• to H2O2. SOD1 is located in the mitochondrial intermembrane space and cytosol, SOD2 is located in the mitochondrial matrix, and SOD3 is tethered to the extracellular matrix. There are multiple enzymes that remove H2O2, including peroxiredoxins (PRXs), glutathione peroxidases (GPXs), and catalase. Mammalian cells express 6 PRX isoforms, including PRX3 and PRX5 in the mitochondria. PRXs function by undergoing oxidation by H2O2 at an active site cysteine and then subsequent reduction by thioredoxin, thioredoxin reductase, and NADPH. There are 8 GPXs, which are oxidized by H2O2 and reduced by glutathione (GSH). Catalase is found in peroxisomes. Function of these antioxidant enzymes is dependent on how fast they react with H2O2 (rate constant, k) and the concentration of H2O2 and enzyme in vivo. Importantly, in vivo concentrations of H2O2 are not well defined. Nevertheless, peroxiredoxins have a high rate constant and high abundance and therefore are thought to be responsible for scavenging nanomolar levels of H2O2 associated with signaling. GPXs have similar rate constants, but are less abundant and therefore are likely only important at higher intracellular concentrations of H2O2 when GPXs can begin to compete with PRXs for substrate (Winterbourn and Hampton, 2008). Therefore it is possible that PRXs are critical for turning ROS signaling off, while GPXs are critical for buffering high levels of ROS to bring them to a level at which the cell evades damage and can initiate signaling stress responses. Catalase has an even lower affinity for hydrogen peroxide and is restricted to peroxisomes. Regulation of activity and expression levels of these antioxidants occurs by a variety of mechanisms and functions in part to manage ROS levels.
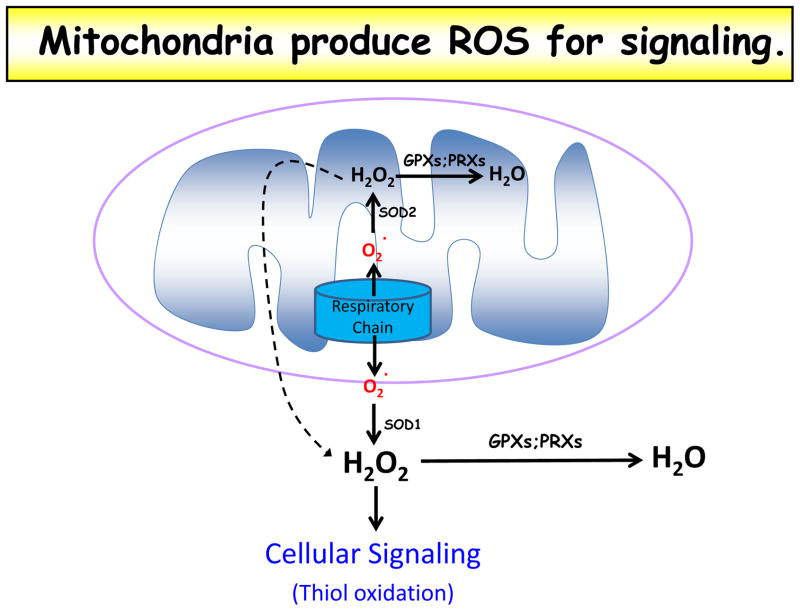
Figure 1. Mitochondria produce ROS for signaling.
Superoxide (O2−) is made at the mitochondrial respiratory chain and emitted into both the matrix and intermembrane space. Matrix superoxide is converted to hydrogen peroxide (H2O2) by superoxide dismutase 2 (SOD2); this hydrogen peroxide can diffuse through both inner and outer mitochondrial membranes to access the cytosol or be converted to water (H2O) by glutathione peroxidases (GPX) or peroxiredoxins (PRX). Intermembrane space superoxide can exit the mitochondria through voltage-dependent anion channels (VDAC) and be converted to hydrogen peroxide in the cytosol by superoxide dismutase 1 (SOD1). The cytosol also possesses glutathione peroxidases and peroxiredoxins that can reduce hydrogen peroxide to water. Cytosolic hydrogen peroxide is believed to be the primary form of signaling ROS in the cell, as it can oxidize protein thiol residues.
In addition to regulation of mROS scavenging, regulation of mROS production can also alter the signaling capacity of mROS. The factors that control ETC generation of ROS in vivo are not fully understood. Much of our current understanding stems from studies with isolated mitochondria and cells (see detailed review (Murphy, 2009)). It has been found that the major determinant of mROS production is the redox state of the ETC. For example, inhibition of ETC electron carriers typically causes them to be more reduced, which increases their propensity to generate superoxide. Another major determinant of mROS production is the proton motive force (pmf), composed of an electrical gradient (ψ, mitochondrial membrane potential) and chemical gradient (pH) across the inner mitochondrial membrane. The pmf is generated as protons are extruded out of the matrix into the intermembrane space by complexes I, III and IV as electrons are transferred through the ETC. An increase in mROS production is observed when pmf increases (Echtay et al., 2002).
Lastly, the signaling capacity of mROS may be altered by mitochondrial localization. ROS are generally short-lived molecules; therefore colocalization of their site of production and their site of signaling function likely enhances their efficiency. Theoretically, the quantity of mitochondria in the cell could also alter quantity of ROS produced and thus their intracellular function, however the mitochondrial biogenesis factor PGC1α also increases expression of antioxidant enzymes to maintain redox balance (St-Pierre et al., 2006). Thus quantity of mitochondria does not necessarily correlate with quantity of mROS.
We will discuss through examples in the latter part of this review how multiple inputs to mitochondria, including Sirt3, FOXOs, immunoreceptors, PI3K, hypoxia, cytokine stimulation, calcium influx, mitophagy, and UCPs, alter these direct regulators of mROS to produce changes in mROS levels necessary for cellular signaling (Figure 2).
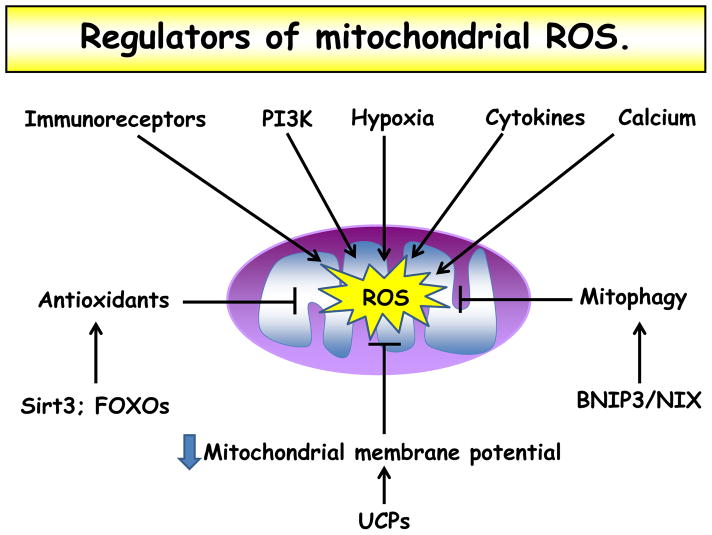
Figure 2. Regulators of mitochondrial ROS.
Levels of mitochondrial ROS are carefully regulated in the cell. Various cell stimuli, including immunoreceptor ligation, cytokine stimulation, and hypoxia increase mROS levels. Increased cytosolic calcium concentration and activation of PI3K also lead to heightened mROS. Antioxidant protein expression, for example through Sirt3 and FOXO activation, decrease mROS. Similarly, mitophagy through activation of BNIP3 or NIX decrease mROS by decreasing overall quantity of mitochondria and quantity of damaged mitochondria that produce more ROS. Finally, mitochondrial membrane potential appears to directly correlate with mROS production; therefore increased expression of the UCPs decreases mitochondrial membrane potential and decreases mROS levels.
Physiological targets of ROS
More and more evidence suggests that ROS can cause reversible post-translational protein modifications to regulate signaling pathways. Specifically, H2O2 can oxidize thiol groups (—SH) on cysteine residues to form sulphenic acid (—SOH), which can react with GSH to become glutathionylated (—SSG), with adjacent thiols to form a disulfide bond (—SS—), or with amides to form a sulfenyl amide (—SN—) (Finkel, 2012). Each of these modifications can change the activity of the target protein, thereby altering its function in a signaling pathway. Phosphatases appear to be susceptible to regulation by ROS in this manner, as they possess a reactive cysteine their catalytic domain that can be reversibly oxidized, which inhibits their dephosphorylation activity (Rhee et al., 2000). Specific examples of phosphatases known to be regulated in this manner are PTP1b, PTEN, and MAPK phosphatases (Tonks, 2005).
A working model
Mitochondrial ROS are now known to be biologically important in a variety of physiological systems, including adaptation to hypoxia, regulation of autophagy, regulation of immunity, regulation of differentiation, and regulation of longevity (Figure 3), and we will review the evidence for each of these now. In these examples, ROS appear to be induced in response to a stress and function as a signaling intermediate to facilitate cellular adaptation to this stress. It remains to be determined whether ROS are important to maintain homeostasis in the absence of cellular stress. A working hypothesis is that at baseline, there is a tonal level of ROS that have a wide range of permissive effects to maintain homeostasis, while following stress, this level of ROS may fluctuate to alter signaling pathways. Importantly, in many of the following examples, the specific molecular target of ROS is still unknown. This will be an important area of investigation in the future.
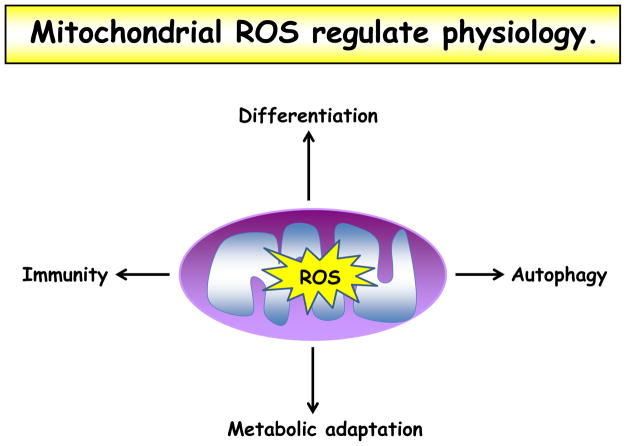
Figure 3. Mitochondrial ROS regulate physiology.
Mitochondrial ROS have been demonstrated to play a role in varied cellular processes, including differentiation, autophagy, metabolic adaptation, and immune cell activation.
Mitochondrial ROS regulate adaptation to hypoxia
Oxygen is critical for the survival of aerobic organisms due to its role in the production of ATP by mitochondria. When mammalian cells encounter lower oxygen levels (hypoxia; 0.3–3% O2), they invoke transcriptional and non-transcriptional responses to increase oxygen supply while simultaneously decreasing oxygen usage. Ironically, many of these adaptations to low oxygen conditions are invoked or enhanced by mROS. Although the mechanism by which hypoxia increases mROS is unknown, we will discuss the varied functions and long-term regulation of hypoxic mROS.
The family of hypoxia-inducible factors (HIFs), HIF-1 and HIF-2, orchestrate the transcriptional response to hypoxia, promoting expression of erythropoietin (EPO) to enhance red blood cell production, vascular endothelial growth factor (VEGF) to promote new blood vessel formation, and glycolytic enzymes to maintain ATP levels. HIF-1 and HIF-2 are heterodimers consisting of two basic helix-loop-helix/PAS proteins, HIFα and aryl hydrocarbon nuclear trans-locator (ARNT or HIF-1β). Under normal oxygen conditions, prolyl hydroxylase domain protein 2 (PHD2) hydroxylates HIFα at two proline residues, which target the protein for pVHL-dependent proteasomal degradation (Kaelin and Ratcliffe, 2008). When oxygen levels decrease below 5%, PHD2 is inhibited and HIFα is stabilized. HIFα is then free to dimerize with HIF-1β and bind HIF-response elements (HRE) to initiate gene transcription.
Early evidence suggesting that mitochondria regulate HIFs during hypoxia came from the observation that cells depleted of mitochondrial DNA (ρ° cells) do not stabilize HIFα under hypoxia (Chandel et al., 1998). These ρ° cells lack a functional electron transport chain and could not increase mROS under hypoxia. Wild-type cells treated with mitochondrial electron transport chain inhibitors phenocopied the ρ° cells (Chandel et al., 2000a). Later came genetic evidence to support these early data. Loss of the complex III subunit Rieske iron sulfur protein (RISP) or cytochrome c prevented hypoxic increase in ROS and stabilization of HIF-α (Brunelle et al., 2005; Guzy et al., 2005; Mansfield et al., 2005). Yet all of these early perturbations in mitochondrial function under hypoxia inhibited both mitochondrial oxygen consumption and mROS production. The critical experiment that showed that mROS, not oxidative phoshorylation, are needed for HIF stabilization under hypoxia utilized cells lacking the complex III subunit cytochrome b (Bell et al., 2007b). These cytochrome b-null cells cannot perform oxidative phosphorylation, but can produce complex III ROS and stabilize HIF under hypoxia. Pharmacologic or genetic reduction of ROS in these cells prevents HIF stabilization under hypoxia. In fact, an unbiased chemical screen to find inhibitors of hypoxic activation of HIF revealed strong inhibition by small molecules that inhibit the mitochondrial electron transport chain (Lin et al., 2008). It is now known that FOXO3a and SIRT3 function as negative regulators of mROS generation and subsequent HIF activation under hypoxia (Bakker et al., 2007; Bell et al., 2011; Emerling et al., 2008; Ferber et al., 2012). Recent reports indicate that non-hypoxic stimuli known to activate HIFs also require mROS (Patten et al., 2010). An important question for future investigation is: how do mROS inhibit HIFα hydroxylation resulting in HIF activation?
Although an increase in mROS is important to activate HIFs, a sustained increase in ROS production can be detrimental. Interestingly, cells lacking HIF-1 maintain elevated ROS levels under chronic hypoxia and eventually succumb to ROS-induced cell death (Kim et al., 2006). This suggests that HIF-1 feeds back and dampens mROS levels as an adaptive mechanism for cell survival under chronic hypoxia. Independent of HIF-1, cells appear to switch cytochrome c oxidase (COX) subunit 4 isoforms from COX4-2 to COX4-1 during chronic hypoxia, which also appears to limit ROS production (Fukuda et al., 2007). Recently a study implicated HIF-1 induction of the mitochondrial complex I NDUFA4L2 protein as another mechanism to prevent overproduction of ROS during hypoxia (Tello et al., 2011). Thus, cells utilize an acute increase in mROS to stabilize HIF under hypoxia and subsequently restrain ROS production under chronic hypoxia to avoid cellular damage.
Though it is clear that HIFs are regulated by mROS on the protein level, a recent report indicated that VEGF, a quintessential HIF target, is regulated by mROS on the transcription level. The authors show that mitochondria display perinuclear clustering during hypoxia, which increases nuclear ROS and was required for oxidative modifications in the VEGF HRE (hypoxia response element) and VEGF transcription (Al-Mehdi et al., 2012).
In mammals, the non-transcriptional response to hypoxia includes contraction of pulmonary vasculature to divert blood from hypoxic lung regions, release of neurotransmitters by the carotid body to increase respiration rate, and reduction of ATP usage. Hypoxia triggers contraction of pulmonary vasculature by inducing influx of calcium into pulmonary arterial smooth muscle cells (PASMCs), which activates calmodulin- and actin/myosin-mediated cellular contraction (Mark Evans and Ward, 2009). Several studies suggest that contraction of PASMCs under hypoxia requires mROS. The primary evidence for this is that mitochondrial inhibitors and mitochondrial-targeted antioxidants block hypoxic pulmonary vasoconstriction in isolated rat lungs and contraction of PASMCs under hypoxia (Waypa et al., 2001). Treatment with exogenous H2O2 was sufficient to invoke contraction in PASMCs and increase calcium during normoxia, while transfection of PASMCs with catalase prevented calcium influx under hypoxia. Several studies suggest that modulating the mitochondrial ETC alters carotid body activation under hypoxia, however it is unclear whether this is through mROS (Wyatt and Buckler, 2004).
In addition to increasing oxygen supply, hypoxia diminishes oxygen demand by reversibly decreasing cellular ATP consumption (Chandel et al., 1997). Hypoxia has been shown to induce endocytosis of the α subunit of the Na/K ATPase, reducing its activity and consumption of ATP (Dada et al., 2003). This occurs through AMPK activation, which directly phosphorylates PKCζ at Thr410 to promote Na/K-ATPase endocytosis (Gusarova et al., 2009). Interestingly, mROS have been shown to be required for both AMPK activation and endocytosis of the α subunit of the Na/K ATPase under hypoxia (Gusarova et al., 2011). Though AMPK is classically activated by an increase in the intracellular AMP/ATP ratio, acute hypoxia does not alter this ratio (Emerling et al., 2009). Instead, hypoxia regulates AMPK activity through mROS regulation of Ca2+/calmodulin-dependent kinase kinase (CaMKK) (Mungai et al., 2011).
Although we know that hypoxia induces increased mROS production, how this occurs is unclear. One possibility is that rearrangement of electron transport chain complexes into supercomplexes, which has been shown to occur under hypoxia, alters ROS production (Diaz et al., 2012). Alternatively, hypoxia may alter mROS emission from matrix to intermembrane space (Waypa et al., 2010). Further studies should address this question.
Mitochondrial ROS regulate autophagy
Autophagy is the process by which cells engulf and breakdown intracellular proteins and organelles in the lysosome and repurpose the constituents for new biosynthesis. It occurs continuously under normal conditions to remove and recycle damaged proteins and organelles as a method of quality control. For example, cells that lack the ability to undergo autophagy, such as Atg5−/−, accumulate dysfunctional mitochondria that would normally be removed and recycled through autophagy of mitochondria, termed ‘mitophagy’ (Pua et al., 2009). Accordingly, these cells accumulate mROS as a result of both increased numbers of total mitochondria and increased numbers of damaged mitochondria that secrete higher levels of ROS (Tal et al., 2009). In fact, mitophagy-deficient cells have been used specifically to study the effects of increased mROS in a variety of contexts. Thus, mitophagy is a mechanism to limit ROS levels.
In addition to its role in maintenance of homeostasis, autophagy is also an important response to cellular stress, including starvation, ischemia/reperfusion, and pathogen infection (Kroemer et al., 2010; Levine et al., 2011). Under starvation, it is thought that autophagy functions to recycle intracellular molecules when external nutrients are limiting. Mitochondrial ROS are required for induction of autophagy under starvation (Scherz-Shouval et al., 2007). The authors of this study found that starvation induced PI3K activation, which induced mROS, which subsequently oxidized and inactivated the cysteine protease Atg4 to promote autophagy. Further, a recent paper indicates that BNIP3 is regulated by HIF-2, which requires mROS for stabilization (Qi et al., 2012). As such, mROS and mitophagy can form a feedback loop, whereby mROS induce mitophagy, which limits further production of ROS by reducing mitochondria quantity.
Although autophagy has been widely shown to be a survival pathway, under certain circumstances that are not completely understood at this time, autophagy can guide cell death in a process called ‘autophagic cell death.’ This method of controlled cell death is distinct from apoptosis. Some evidence suggests that mROS guide the cell fate decision to induce autophagic cell death. Autophagic cell death is induced by selective degradation of catalase, which leads to vast accumulation of H2O2 and subsequent cell death presumably by nonspecific oxidative damage (Yu et al., 2006). The source of H2O2 appears to be mitochondria, as inhibitors of complex I and complex II can directly induce autophagic cell death by increasing mROS (Chen et al., 2007). Therefore mROS may play dual functions in the context of autophagy – low levels may promote survival by inducing autophagy to allocate intracellular resources under starvation, while high levels may promote controlled autophagic cell death when survival is not possible.
Mitochondrial ROS regulate immunity
ROS in immune cells are best known for their role in directly killing pathogens through the oxidative burst mediated by NADPH oxidases (NOXs) in phagosomes. However, more subtle changes in intracellular redox state mediated by mROS appear to be essential for a wide range of innate immune function, including antiviral, antibacterial, and antiparasitic responses. Over a decade ago, it was observed that Uncoupling Protein 2 (UCP2) knockout mice possessed increased immunity to Toxoplasma gondii and Salmonella typhimurium (Arsenijevic et al., 2000). Remarkably, while all control mice succumbed to T. gondii infection, the UCP2−/− mice were protected. UCP2 expression negatively correlates with mROS production (Mailloux and Harper, 2011). Indeed, isolated UCP2−/− macrophages generated more ROS and more efficiently eliminated T. gondii tachyzoites in vitro, an effect that was eliminated by antioxidant treatment (Arsenijevic et al., 2000). It was later delineated that LPS downregulates UCP2 in wild-type macrophages specifically to increase mROS for MAPK activation and oxidative burst augmentation for pathogen elimination (Emre et al., 2007; Kizaki et al., 2002).
More recent studies have shown that mROS are essential for multiple Toll-like receptor (TLR)-initiated pathways, not just the LPS/TLR4 pathway. West et al. reported that stimulation of cell surface TLRs (TLR1, TLR2, and TLR4), but not endosomal TLRs (TLR3, TLR7, TLR8, and TLR9), leads to an increase in mROS production through TRAF6 and ECSIT signaling (West et al., 2011). When ECSIT was depleted or when catalase was overexpressed in the mitochondria, macrophages displayed reduced mROS induction and impaired clearance of Salmonella typimurium. Regulation of the TLR/mROS pathway appears to be critical in human disease, as cells from patients with tumor necrosis factor receptor-associated periodic syndrome (TRAPS) have enhanced responsiveness to LPS due to increased mROS production (Bulua et al., 2011). This hyperactivity of innate immune cells in TRAPS promotes autoinflammation characterized by recurrent fevers, peritonitis, rash, myalgia and arthralgia.
Like the cell surface TLRs, the cytosolic RIG-I-like receptors (RLRs) also signal through mROS. This was discovered with the use of cells lacking Atg5, which are deficient in autophagy and thus accumulate mitochondria and possess higher mROS levels (Tal et al., 2009). Atg5−/− cells exhibited enhanced RLR signaling, increased IFN secretion, and resistance to infection by vesicular stomatitis virus, and these traits could be reduced by treatment with an antioxidant. That mROS modulate RLR signaling perhaps should not be surprising, as the outer mitochondrial membrane serves as a platform for formation of the RLR molecular complex, placing it right in the line of fire of mROS generation (Moore and Ting, 2008). Mitochondrial ROS are the simply newest addition to the substantial list of mitochondrial molecules that participate in RLR signaling, including MAVS, TOM70, HSP90, and STING.
Inflammatory cytokines also appear to signal through mitoROS in several cell types. More than a decade ago, mROS were found to be induced by TNFR signaling through TRAF2 to promote NF-κB signaling (Chandel et al., 2001). In endothelial cells, TNFα induces a calcium-dependent increase in mROS that causes shedding of TNFR1 and reduction of microvascular inflammation (Rowlands et al., 2011). More recently, IFNγ was shown to induce mitochondrial oxidative phosphorylation and mROS production through PGC1β/ERRα in macrophages, which was required for clearance of listeria monocytogenes (Sonoda et al., 2007).
The inflammasome recognizes a wide variety of microbial pathogen-associated molecular patterns (PAMPs) and endogenous damage-associated molecular patterns (DAMPs) to induce proteolytic processing of the pro-inflammatory cytokines interleukin 1β (IL-1β) and interleukin-18 (IL-18). The NLRP3 inflammasome requires ROS for activation (Allen et al., 2009; Cruz et al., 2007; Tschopp and Schroder, 2010). Early reports of the finding that ROS are needed for NLRP3 inflammasome activation propose a role for NOX proteins (Cruz et al., 2007; Dostert et al., 2008), yet interpretation of these data may be skewed by the assumption that DPI is a specific inhibitor of NOX proteins. DPI is also a potent inhibitor of mitochondrial complex I (Majander et al., 1994), and defects of NOX subunits did not affect NLRP3 inflammasome activation (Meissner et al., 2010). Zhou et al. showed that inhibitors of electron transport that increase mitochondrial ROS, as well as inhibition of mitophagy, are sufficient to activate the NLRP3 inflammasome (Zhou et al., 2011). Most recently, it was shown that inhibition of autophagy by deletion of LC3B increased mROS levels and NLRP3 activation by LPS and ATP (Nakahira et al., 2011). NLRP3 activation in both WT and LC3B KO macrophages was inhibited by treatment with the mitochondrial-targeted antioxidant, Mito-TEMPO, or by treating the cells with ethidium bromide to make them ρ°. Thus, it appears ROS needed for NLRP3 inflammasome activation are derived from mitochondria.
Several lines of evidence point to a role for mROS in adaptive immune cell function as well as innate immune cell function. Treatment of primary T cells with pharmacologic antioxidants inhibits proliferation and production of IL-2 following TCR/CD28 stimulation, indicating that ROS are required during early stages of T cell activation (Chaudhri et al., 1986). Additionally, treatment of mice with antioxidants administered orally reduced the expansion of T cells following viral infection (Laniewski and Grayson, 2004), suggesting that ROS are also required for T cell function in vivo. The source of activation-induced ROS is likely the mitochondrion, although this has not been shown genetically or in vivo. While T cells do express low levels of the phagocyte-type NADPH oxidase NOX2, T cells deficient in components of this protein still produce ROS after TCR stimulation (Jackson et al., 2004). In contrast, rotenone, a mitochondrial complex I inhibitor, completely ablated activation-induced ROS and production of IL-2 and IL-4 in the Jurkat T cell line (Kaminski et al., 2010). Furthermore, CD8+ T cells treated with rotenone are unable to be activated (Yi et al., 2006). Interestingly, mitochondria have recently been shown to be localized to the immunological synapse during T cell activation, where they appear to regulate local Ca2+ influx (Quintana et al., 2006; Schwindling et al., 2010). Since mitochondrial TCA enzymes are activated by Ca2+ (McCormack et al., 1990), this may support the idea that Ca2+ and mROS constitute a feedback loop during T cell activation. To date, the requirement of mROS for T cell activation and proliferation in vivo has not been deciphered.
Mitochondrial ROS regulate differentiation
Embryonic stem cells give rise to all cells of the multicellular organism and adult stem cells replenish cells throughout the life of the organism and following injury. Stem cells are characterized by their ability to both self-renew to maintain the stem cell pool and also differentiate to give rise to fresh specialized tissue. Molecular mechanisms that guide this cell fate decision to renew or differentiate are not completely understood, but recent studies indicate that mROS play a significant role.
Stem cells are described to have minimal mitochondrial structures with few cristae. As bone marrow-derived human mesenchymal stem cells (MSC) differentiate to osteoblasts, mitochondrial content increases driven by PGC-1α (Chen et al., 2008). Mitochondrial mass and oxygen consumption were also shown to increase during differentiation of human embryonic stem cells (Cho et al., 2006) as well as during differentiation of induced pluripotent stem cells (Prigione and Adjaye, 2010). These descriptions might indicate that increased mitochondrial function is important to differentiation, but they do not address whether it is a cause or effect.
A few recent studies addressed the question whether mROS are required for differentiation of stem cells. Owusu-Ansah et al. discovered that scavenging ROS by overexpression of GTPx-1 retards differentiation of Drosophila multipotent hematopoietic progenitors, while increasing ROS by depletion of the mitochondrial complex I protein ND75 or deletion of SOD2 increased differentiation (Owusu-Ansah and Banerjee, 2009). Recently, we found that reduction of mROS by knockdown of the complex III protein Rieske Iron Sulfur Protein or mitochondrial-targeted antioxidants prohibited differentiation of human MSCs to adipocytes (Tormos et al., 2011), suggesting that mROS are required for differentiation in the mammalian system as well. Mitochondrial ROS are likely important for human cell differentiation as well, as human pluripotent stem cells (hPSCs) were shown to repress UCP2 during differentiation, a process that generally increases production of mROS (Zhang et al., 2011). Future studies should address whether mROS are a common requirement across multiple systems of differentiation and whether they are required for in vivo differentiation. An interesting question is whether division of mitochondria is asymmetrical during stem cell division and whether asymmetrical mitochondrial segregation dictates ROS levels in daughter cells and ultimately cell fate.
Mitochondrial ROS regulate aging
Aging is an inevitable, universal phenomenon observed in all living organisms, yet its cause is open to debate. Some argue aging is preprogrammed, while others suggest it is merely the effect of accumulation of damage to proteins, lipids, and DNA. In the 1950s, Denham Harman proposed the ‘free radical theory of aging’ as a molecular explanation for why aging occurs (Harman, 1956). The theory is that free radicals, as byproducts of oxidative metabolism, cause cumulative cellular damage resulting in overall loss of organismal fitness over time. If this theory is correct, then increasing antioxidant capacity should increase organismal lifespan. Yet over the past two decades, there has not been a consensus whether or not this occurs, and there are contradictory reports and reviews suggesting that ROS are both causal and mere bystanders of the aging process (Hekimi et al., 2011). From our perspective, since mROS are required to maintain normal physiology (see above), increasing antioxidant capacity would not only limit oxidative damage, but it would also limit normal adaptation to stress. Indeed, recent data suggest that low levels of ROS activate stress responses that are beneficial to the organism and extend lifespan. We highlight a few of these studies that provide compelling evidence to support this idea across different organisms.
In yeast, inhibition of TOR (target of rapamycin) extends chronological lifespan by increasing mROS (Pan et al., 2011). Furthermore, caloric restriction (CR) extends chronological lifespan in yeast by inducing H2O2 (Mesquita et al., 2010; Zuin et al., 2010). Both diminishing TOR and CR have been shown to extend lifespan in mammals, though a connection to mROS has not been established. In Drosophila, mROS levels were shown to increase with age, but did not alter with interventions that increase lifespan (Cocheme et al., 2011). In C. elegans, glucose restriction increases mROS to increase lifespan (Schulz et al., 2007). In fact, several different mutations in the mitochondrial electron transport chain increases lifespan by increasing mROS levels in C. elegans. Diminishing the insulin-growth factor (IGF) pathway also extends life span of C. elegans in part by increasing mROS through proline dehydrogenase (Zarse et al., 2012). In mice, there is a correlation between increased mROS and prolonged lifespan—mice that are heterozygous for Mclk1, an enzyme in involved in ubiquinone synthesis, exhibit both increased mitochondrial oxidative stress and lifespan (Liu et al., 2005).
The data regarding regulation of lifespan of humans is scarce. Replicative senescence of human cells in vitro has been studied as a surrogate for studying human lifespan. Interestingly, an early pillar of the free radical theory comes from the observation that human diploid fibroblasts undergo an increase in replicative lifespan under hypoxia (Packer and Fuehr, 1977). It was surmised that decreased oxygen decreases ROS and the accumulation of oxidative damage, thereby increasing replicative lifespan. However, as noted above, hypoxia paradoxically increases ROS. Furthermore, these ROS were shown to be required to increase replicative lifespan of human fibroblasts under hypoxia (Bell et al., 2007a). It is unclear whether factors thought to increase human lifespan such as mutations in IGF1R (Suh et al., 2008) do so through increasing mROS. Nevertheless, an emerging theory from multiple studies in lower organisms is that low levels of mROS positively affect aging.
Currently, the downstream targets of mROS to enhance organismal fitness are not fully understood, but there is some evidence that the activation of HIF-1 by mROS is important. Long-lived C. elegan mitochondrial mutants rely on ROS-dependent activation of HIF-1 for increased longevity, and long lived Mclk1+/− mice have heightened activation of HIF-1 (Lee et al., 2010; Wang et al., 2010). Further, mammalian cells increase replicative lifespan under hypoxia through HIF-1 activation (Bell et al., 2007a). In the future, it will be important to decipher the downstream targets of mROS that increase lifespan because they might serve as better pharmacological targets given that mROS are likely only beneficial in a tightly controlled quantity.
Conclusion
Following review of the divergent biological processes that are regulated by mROS, we can make some generalizations regarding the induction and function of mROS. Importantly, mROS are generally induced by cell stress – hypoxia, starvation, pathogen infection (or molecular harbingers of pathogen infection), and growth factor stimulation. Thus, mROS are poised to serve as an alarm to notify the cell that the extracellular environment is changing. This mROS alarm system is titrated such that the gravity of the stressor correlates with the quantity of mROS induced – a stressor that is incompatible with cell viability induces larger quantities of mROS, which nonspecifically produce cell damage and subsequent cell death, while a moderate stressor induces smaller quantities of mROS (Figure 4). As we have described, these small inductions of mROS promote adaptation to the stressor – HIF activation under hypoxia, autophagy activation under starvation, inflammatory cytokine production under pathogen infection, differentiation under receptor dependent stimulation – and consequently promote cell survival.
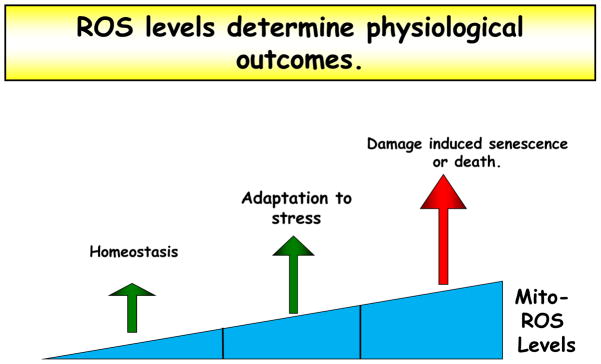
Figure 4. H2O2 levels determine physiological outcomes.
The level of mitochondria-derived hydrogen peroxide determines its function and the physiological outcome. While very high quantities of mROS directly damage proteins, lipids, and nucleic acids, lower levels of mROS function as signaling molecules to adapt to the stress. Even lower levels of mROS are required for normal cell homeostasis. Therefore mROS are not categorically harmful, and therapeutics must consider the essential role of low levels of mROS.
As mentioned earlier, mROS are implicated in a variety of diseases including diabetes, cancer, inflammatory disorders, and neurodegeneration. These diseases exhibit alterations in the physiological cellular redox system. For example, neurodegeneration is associated with overproduction of mROS that induces cellular damage and subsequent neuronal deficits (Schon and Przedborski, 2011). In contrast, cancer cells utilize mROS to constitutively activate proliferation pathways to promote tumor growth (Cairns et al., 2011). Yet a large number of clinical trials have failed to demonstrate beneficial effects of antioxidants on these pathologies, and a handful of trials suggested antioxidants increased mortality (Bjelakovic et al., 2007).
We hypothesize that the dual function of mROS to both promote cell damage and promote cell adaptation makes it a potentially difficult therapeutic target. In other words, inhibition of mROS by antioxidants does not have a predictable outcome on cell function since the role of mROS changes under differing environmental conditions. Going forward, it will be important to identify specific molecular targets of mROS under different environmental conditions with the goal of modulating pathways downstream of mROS that increase adaptation to stress.
Acknowledgments
This work was supported by NIH grants F30ES019815 (L.A.S.), T32-HL76139 (L.A.S.), 5P01HL071643 (N.S.C.), RO1CA123067 (N.S.C.). We apologize to those investigators whose important work we did not discuss for the sake of conciseness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF, Gillespie MN. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal. 2012;5:ra47. doi: 10.1126/scisignal.2002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 4.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Bell E, Klimova T, Eisenbart J, Schumacker P, Chandel N. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007a;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–2996. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007b;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 9.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 13.Chandel N, Budinger G, Choe S, Schumacker P. Cellular respiration during hypoxia. Role of cytochrome oxidase as the oxygen sensor in hepatocytes. J Biol Chem. 1997;272:18808–18816. doi: 10.1074/jbc.272.30.18808. [DOI] [PubMed] [Google Scholar]
- 14.Chandel N, Maltepe E, Goldwasser E, Mathieu C, Simon M, Schumacker P. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandel N, McClintock D, Feliciano C, Wood T, Melendez J, Rodriguez A, Schumacker P. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000a;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 16.Chandel N, Schumacker P, Arch R. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem. 2001;276:42728–42736. doi: 10.1074/jbc.M103074200. [DOI] [PubMed] [Google Scholar]
- 17.Chandel N, Trzyna W, McClintock D, Schumacker P. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000b;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 18.Chandel N, Vander Heiden M, Thompson C, Schumacker P. Redox regulation of p53 during hypoxia. Oncogene. 2000c;19:3840–3848. doi: 10.1038/sj.onc.1203727. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhri G, Clark IA, Hunt NH, Cowden WB, Ceredig R. Effect of antioxidants on primary alloantigen-induced T cell activation and proliferation. J Immunol. 1986;137:2646–2652. [PubMed] [Google Scholar]
- 20.Chen C, Shih Y, Kuo T, Lee O, Wei Y. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport- chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 22.Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park do J, Park KS, Lee HK. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472–1478. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins Y, Chouchani ET, James AM, Menger KE, Cocheme HM, Murphy MP. Mitochondrial redox signalling at a glance. J Cell Sci. 2012;125:801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 25.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz F, Enriquez JA, Moraes CT. Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV. Mol Cell Biol. 2012;32:415–429. doi: 10.1128/MCB.06051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 30.Emerling B, Weinberg F, Liu J, Mak T, Chandel N. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a) Proc Natl Acad Sci U S A. 2008;105:2622–2627. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emerling B, Weinberg F, Snyder C, Burgess Z, Mutlu G, Viollet B, Budinger G, Chandel N. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012;19:968–979. doi: 10.1038/cdd.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 35.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5:pe10. doi: 10.1126/scisignal.2002943. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 37.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI. Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol Cell Biol. 2011;31:3546–3556. doi: 10.1128/MCB.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guzy R, Hoyos B, Robin E, Chen H, Liu L, Mansfield K, Simon M, Hammerling U, Schumacker P. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 41.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 43.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Kaminski MM, Sauer SW, Klemke CD, Suss D, Okun JG, Krammer PH, Gulow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 45.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Kizaki T, Suzuki K, Hitomi Y, Taniguchi N, Saitoh D, Watanabe K, Onoe K, Day NK, Good RA, Ohno H. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci U S A. 2002;99:9392–9397. doi: 10.1073/pnas.142206299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laniewski NG, Grayson JM. Antioxidant treatment reduces expansion and contraction of antigen-specific CD8+ T cells during primary but not secondary viral infection. J Virol. 2004;78:11246–11257. doi: 10.1128/JVI.78.20.11246-11257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin X, David C, Donnelly J, Michaelides M, Chandel N, Huang X, Warrior U, Weinberg F, Tormos K, Fesik S, et al. A chemical genomics screen highlights the essential role of mitochondria in HIF-1 regulation. Proc Natl Acad Sci U S A. 2008;105:174–179. doi: 10.1073/pnas.0706585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 54.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 55.Majander A, Finel M, Wikstrom M. Diphenyleneiodonium inhibits reduction of iron-sulfur clusters in the mitochondrial NADH-ubiquinone oxidoreductase (Complex I) J Biol Chem. 1994;269:21037–21042. [PubMed] [Google Scholar]
- 56.Mansfield K, Guzy R, Pan Y, Young R, Cash T, Schumacker P, Simon M. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mark Evans A, Ward J. Hypoxic pulmonary vasoconstriction--invited article. Adv Exp Med Biol. 2009;648:351–360. doi: 10.1007/978-90-481-2259-2_40. [DOI] [PubMed] [Google Scholar]
- 58.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 59.Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leao C, Costa V, Rodrigues F, Burhans WC, Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci U S A. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore CB, Ting JP. Regulation of mitochondrial antiviral signaling pathways. Immunity. 2008;28:735–739. doi: 10.1016/j.immuni.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 63.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol. 2000;20:7311–7318. doi: 10.1128/mcb.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–425. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- 69.Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of Yeast Chronological Life Span by TORC1 via Adaptive Mitochondrial ROS Signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell. 2010;21:3247–3257. doi: 10.1091/mbc.E10-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prigione A, Adjaye J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol. 2010;54:1729–1741. doi: 10.1387/ijdb.103198ap. [DOI] [PubMed] [Google Scholar]
- 72.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 73.Qi Y, Tian X, Liu J, Han Y, Graham AM, Simon MC, Penninger JM, Carmeliet P, Li S. Bnip3 and AIF cooperate to induce apoptosis and cavitation during epithelial morphogenesis. J Cell Biol. 2012;198:103–114. doi: 10.1083/jcb.201111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 75.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 76.Rowlands DJ, Islam MN, Das SR, Huertas A, Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, et al. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest. 2011;121:1986–1999. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Schwindling C, Quintana A, Krause E, Hoth M. Mitochondria positioning controls local calcium influx in T cells. J Immunol. 2010;184:184–190. doi: 10.4049/jimmunol.0902872. [DOI] [PubMed] [Google Scholar]
- 81.Sonoda J, Laganiere J, Mehl IR, Barish GD, Chong LW, Li X, Scheffler IE, Mock DC, Bataille AR, Robert F, et al. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007;21:1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 83.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordonez A, Corral-Escariz M, Soro I, Lopez-Bernardo E, Perales-Clemente E, et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metab. 2011;14:768–779. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 89.Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/− mouse mutants. J Immunol. 2010;184:582–590. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]
- 90.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 91.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 94.Wyatt CN, Buckler KJ. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J Physiol. 2004;556:175–191. doi: 10.1113/jphysiol.2003.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yi JS, Holbrook BC, Michalek RD, Laniewski NG, Grayson JM. Electron transport complex I is required for CD8+ T cell function. J Immunol. 2006;177:852–862. doi: 10.4049/jimmunol.177.2.852. [DOI] [PubMed] [Google Scholar]
- 96.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci U S A. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. Impaired Insulin/IGF1 Signaling Extends Life Span by Promoting Mitochondrial L-Proline Catabolism to Induce a Transient ROS Signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 100.Zuin A, Carmona M, Morales-Ivorra I, Gabrielli N, Vivancos AP, Ayte J, Hidalgo E. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J. 2010;29:981–991. doi: 10.1038/emboj.2009.407. [DOI] [PMC free article] [PubMed] [Google Scholar]