Abstract
Introduction
The true post-operative arteriovenous malformation (AVM) recurrence incidence in the pediatric population remains largely unreported. Some literature suggests that delayed imaging six months to one year after a negative post-operative angiogram should be obtained. The aim of this study was to describe the timing of AVM recurrences after resection and the modalities on which the recurrences were detected.
Methods
This study was a retrospective cohort of all pediatric patients treated surgically for AVM resection by a single neurosurgeon from 2005 to 2010. Patients were followed radiographically after resection with magnetic resonance angiography (MRA) or cerebral angiography, when possible, at various time points. A visual scale of initial AVM nidus compactness was used and correlated with probability of recurrence after surgery.
Results
A total of 28 patients (13 female, 15 male) underwent an AVM resection. Eighteen patients (64.3%) received an intra-operative angiogram. In four cases, the intra-operative angiogram revealed residual AVM and the AVMs were re-resected immediately. Recurrent AVMs were found in 4 children (14.3%) at 50, 51, 56, and 60 weeks from the initial resection. Recurrence risk was 0.08 per person-year. No patient with a normal angiogram at 1 year developed a recurrence on either a 5-year angiogram or an angiogram at 18 years of age. All patients with recurrence had a compactness score of 1 (diffuse AVM). Lower compactness score was associated with recurrence, p=0.0003.
Conclusions
All recurrences in our cohort occurred less than 15 months from the initial resection. We recommend intraoperative angiography to help ensure complete resection at the time of the surgery. Follow-up vascular imaging is crucial for detecting recurrent AVMs, and conventional angiogram is preferred since MRA can miss smaller AVMs. One year follow-up imaging detected these recurrences and no one who had a negative angiogram at 1 year had a late recurrence. However, not all the subjects have been followed for 5 years or to age 18, so longer follow is required for these patients. Lower compactness score predicted recurrent AVM in this cohort.
Keywords: arteriovenous malformation, AVM, surgery, angiogram, recurrence, pediatric, compactness
Introduction
Cerebral arteriovenous malformations (AVMs) are congenital lesions that form due to an embryological failure of capillary and venous development that occurs around the third week of gestation (2, 19). Despite their congenital origin, AVMs typically do not become symptomatic in patients until the second to fourth decade of life, with hemorrhage and seizure as the most common primary presentations (26). However, the natural history of pediatric AVMs shows a high risk of hemorrhage, demonstrating the need for curative therapy. Previous pediatric studies show the risk of hemorrhage to be 2–4% per year, with a resulting 5–10% mortality rate and a 50% risk of serious neurological morbidity associated with each hemorrhagic event (1, 5). Rebleeding is also of concern, with reports occurring in up to 30% of patients (7).
The goal of treatment is complete obliteration of the AVM. Management of AVMs in the pediatric population includes resection, endovascular embolization, radiosurgery, or a combination of these modalities. Surgical resection is traditionally the first choice because it can immediately eliminate the risk of hemorrhage; however, a multidisciplinary approach is often used to minimize the treatment-associated morbidity of these lesions (11, 20). Even if the AVM is surgically resected, it can recur. In adults, a negative post-operative angiogram almost always ensures that the AVM will not recur. In children, however, recurrences are much more common (1, 15). Pediatric patients must be followed much more closely to detect recurrences as soon as possible. Since complications can occur from angiography, especially in children it is important to limit the radiation exposure of angiograms while balancing the goal of detecting recurrences. There are very few literature reports regarding the true post-operative residual AVM incidence (1, 2, 10, 13). In the adult population, some surgeons describe use of an immediate post-operative angiogram and immediate surgical re-excision if residual AVM is seen (10). In the pediatric population, it is postulated that immature vessels may undergo active angiogenesis thus contributing to the higher rate of recurrence of AVMs (13, 16, 17). Some authors suggest obtaining delayed imaging at least six months to one year after a negative post-operative angiogram to exclude recurrent AVM (13, 20).
The aim of this study is to report our experience with the follow-up 28 cases of pediatric intracranial AVMs treated at the Children’s Hospital of Philadelphia (CHOP) by a single surgeon between 2005 and 2010. By examining the post-operative follow-up of these patients, the goal was to determine the best imaging modality and optimal time frame for follow-up of pediatric AVM patients after surgical resection.
Methods
Study design and subjects
A neurosurgery database of vascular malformations was cross-referenced with the neurology stroke database and queried to identify 48 pediatric patients presenting with an AVM at the Children’s Hospital of Philadelphia (CHOP) and followed by a neurologist or neurosurgeon between 2005 and 2010. Of these patients, 10 were treated with radiosurgery (4 with proton beam therapy; 6 with gamma knife radiosurgery), 5 underwent a resection by another neurosurgeon, and 2 patients were embolized as the sole treatment. Three patients had AVMs that were deemed inoperable and unable to be treated without significant morbidity and mortality by embolization or radiation. The known follow-up and outcomes for the 20 patients excluded from the current cohort are presented in Figure 1. Our cohort included 28 patients who underwent surgical resection of an AVM by a single neurosurgeon at CHOP. With institutional review board approval, retrospective analysis of these 28 patients was performed.
Figure 1.
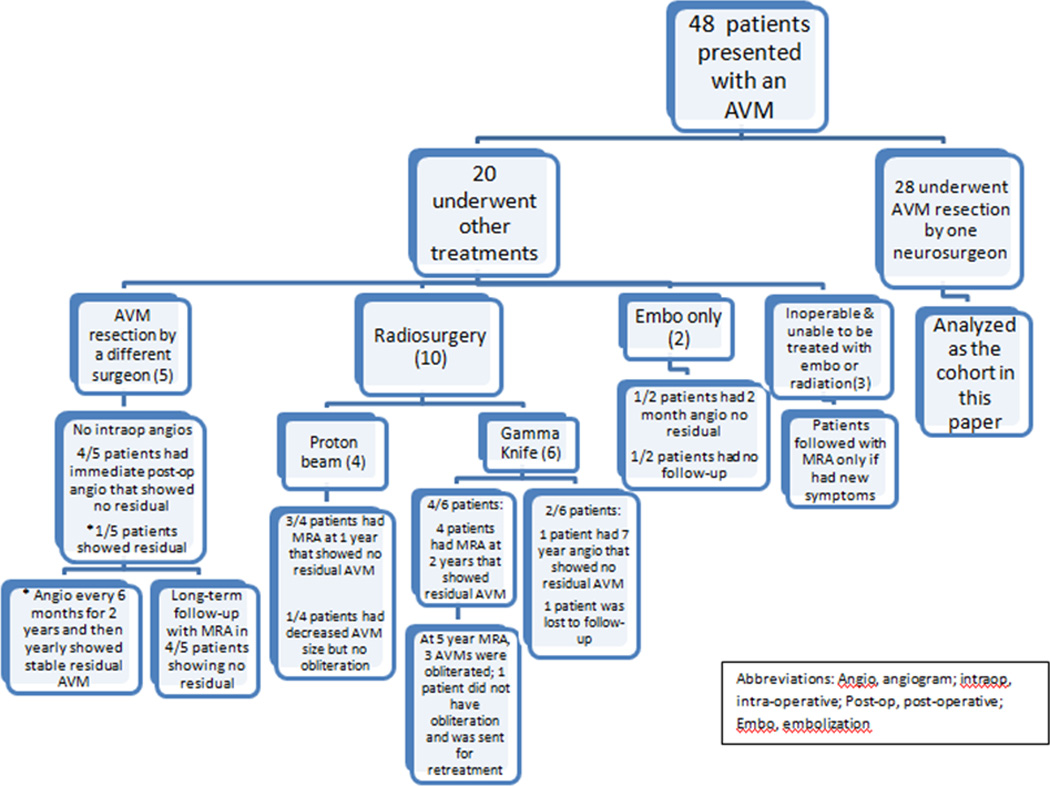
Flow-diagram of 48 subjects presenting to our institution from 2005–2010.
Clinical data
All patients presented to CHOP or were referred by other institutions. All available inpatient/outpatient charts, pathology reports, operative reports, endovascular reports, and radiologic films were retrospectively reviewed. The size of the AVM was determined by the maximal diameter of the nidus as measured on cerebral angiography, by the volume on magnetic resonance imaging angiography (MRI/MRA), or by computed tomography angiography (CTA). Spetzler-Martin grade was assigned to each AVM on the basis of size, venous drainage, and eloquence of location as previously described by a board-certified pediatric neuroradiologist who was blinded to the presence of recurrent AVM (22). A visual AVM nidus compactness score of 1 to 3 was also assigned by the neuroradiologist, similar to the concept previously described by Du et al., in a modified, non-computerized fashion (6). Of the 28 patients, 23 had a pre-operative conventional angiogram on which compactness could be measured. A compactness score of 1 is the most diffuse, and a score of 3 is the most compact (Figure 2). Patients were followed radiographically with MRI/MRA or cerebral angiography, when possible, at varying time-points. Twelve patients received their first follow-up angiogram approximately 3 months after surgery, six patients received their first post-operative angiogram at approximately 6 months post resection, nine patients had their first angiogram around 1 year post resection, and the remaining patient received an angiogram 2 years post-operatively. For long-term follow-up, patients received an angiogram at 5 years post-resection or when they reached 18 years of age depending on which was longer. Recurrent AVM was defined as an AVM evident on follow-up imaging after complete surgical resection and in the same location as the primary AVM. One patient did not have complete resection during the initial surgery at the time of incision closure and was excluded from all recurrence analyses.
Figure 2.
Visual AVM nidus Compactness score
A. AVM with compactness score of 1 showing a diffuse nidus.
B. AVM with compactness score of 2.
C. Highly compact AVM nidus with compactness score of 3.
Statistical analysis
STATA version 11.1 (Stata Corporation, College Station, TX) was used for all analyses. Descriptive statistics were performed using frequency distributions and proportions for categorical variables and medians with interquartile ranges for continuous variables. Wilcoxon rank-sum test was used to explore whether recurrent AVM was associated with age at first resection, Spetzler-Martin grade, compactness score, or nidus size. Fisher’s exact test was used to explore whether the absence of pre-operative embolization was associated with recurrence. Recurrent AVM-free survival was evaluated by survival analysis with the time origin defined as surgical treatment of the AVM and failure defined as the time of diagnosis of recurrent AVM. The child without complete initial resection was excluded from all analyses of risk factors for recurrence. Children with missing information on a variable of interest were excluded from that particular analysis. A two-sided p-value of <0.05 was considered statistically significant.
Results
Patient characteristics and presentation
From 2005 to 2010, 28 patients underwent an AVM resection. Table 1 shows the patient demographics. Fifteen were male (53.6%) and thirteen were female (46.4%). Median age at the time of AVM resection was 12.3 years (range 1.2 to 16.4 years). The most common presentation was hemorrhage in 18 (64.3%). Hemorrhage was primarily intraparenchymal in 16 and was isolated intraventricular in 2. The most common presenting symptom of hemorrhage was a headache, often accompanied by altered mental status. Of the 10 children without hemorrhage, headache was also a common presenting symptom.
Table 1.
Patient characteristics
| No. of patients | 28 | ||
| Sex | 15 male (53.6%) | ||
| 13 female (46.4%) | |||
| Age | |||
| Mean | 11.3 yr | ||
| Median | 12.3 yr | ||
| Range | 1.2 yr – 16.4 yr | ||
|
Presenting signs and symptoms |
Number of patients | Percent of total | |
| Hemorrhage | 18 | 64.3% | |
| Headache Workup | 4 | 14.3% | |
| Incidental Finding after trauma |
1 | 3.6% | |
| Syncope | 1 | 3.6% | |
| Papilledema workup | 1 | 3.6% | |
| Seizures | 1 | 3.6% | |
| Failure to thrive | 1 | 3.6% | |
| Bulging Fontanelle | 1 | 3.6% | |
Pre-treatment imaging
Table 2 shows the location of the AVMs. AVMs were left-sided in 15 (53.6%) and right-sided in 13 (46.4%); one right-sided AVM also affected the midline via the corpus collosum. One patient with hereditary hemorrhagic telangiectasia (HHT) had two AVMs that were resected during the same operation, but a third distinctly separate AVM was found at 3 months post-operatively and was not treated surgically.
Table 2.
AVM location characteristics
| Location | Number of Patients | Percent of total |
|---|---|---|
| Frontal | 8 | 28.6% |
| Temporal | 6 | 21.4% |
| Parietal | 4 | 14.3% |
| Occipital | 4 | 14.3% |
| Frontal-Parietal | 3 | 10.7% |
| Cerebellar | 2 | 7.1% |
| Parieto-Occipital | 1 | 3.6% |
Spetzler-Martin grades (SM) were available in 27 of 28 subjects; 6 (22.2%) were SM grade 1, 10 (37.1%) were SM grade 2, 6 (22.2%) were SM grade 3, and 5 (18.5%) were SM grade 4. Pre-surgical nidus size was available in 27 of 28 and children, and the median size was 29 mm with a range of 6–63mm. The most common location of the AVM was frontal. The majority of patients had an AVM nidus size less than 30mm, superficial venous drainage, and an AVM in non-eloquent cortex. Compactness score was available in 24 of 28 patients. Compactness score was 1 in 6 subjects (25.0%), 2 in 16 (66.7%), and 3 in 2 (8.3%). Table 3 shows the Spetzler-Martin grade, size, eloquence, venous drainage, and compactness score of the AVMs.
Table 3.
Spetzler-Martin grade characteristics
| Spetzler-Martin grade | Number of Patients (*) | Percent of total |
|---|---|---|
| I | 6 | 22.2% |
| II | 10 | 37.0% |
| III | 6 | 22.2% |
| IV | 5 | 18.5% |
| Size | Number of patients | Percent of total |
| <3cm | 14 | 51.9% |
| 3–6cm | 12 | 44.4% |
| >6cm | 1 | 3.7% |
| Pattern of venous drainage | Number of patients | Percent of total |
| Deep | 2 | 7.4% |
| Superficial | 16 | 59.3% |
| Both | 9 | 33.3% |
| Location | Number of patients | Percent of total |
| Eloquent site | 9 | 33.3% |
| Non-Eloquent site | 18 | 66.7% |
| Compactness Score | Number of patients ** | Percent of total |
| 1 | 6 | 25.0% |
| 2 | 16 | 66.7% |
| 3 | 2 | 8.3% |
±, total = total number for which the data was available
cm, centimeter
In 1 patient, S-M grade could not be determined
In 4 patients, Compactness Score could not be determined
Treatment
Of the children who presented with hemorrhage, the median time to resection was 9 days after the hemorrhage; 3 children had resection on hemorrhage day 0. However, in two patients, the AVM was not evident at the time of hemorrhage, and these AVMs were resected at 17.7 and 25 months, respectively, when they were first evident on neuroimaging. Twenty patients (71.4%) had the AVM embolized prior to surgery, most within 48 hours prior to the resection. Eighteen patients (64.3%) received an intra-operative angiogram. This procedure enabled the surgeon to make certain that the entire AVM was resected before closing the incision. In four cases, the intra-operative angiogram revealed residual AVM, which was then resected immediately before closing the incision. Two of these patients went on to have a recurrence at 50 weeks and 56 weeks despite re-resection and no residual AVM seen on the second intra-operative angiogram (Patients 2 and 4, Table 4). In one case, the surgeon visualized residual AVM at the time of closure and the immediate post-operative angiogram confirmed a small residual AVM. The patient was treated with gamma knife radiation 4 months later because of the unacceptable morbidity of further surgery. This patient was excluded from analyses for recurrence risk because the persistent AVM was the result of residual, not recurrence. Patients received a diagnostic angiogram during their post-operative hospital course if an intra-operative angiogram was not performed.
Table 4.
Patient characteristics of recurrent intracranial AVM after surgical resection
| Pt | Age (years) |
Presentation | Location | Size (mm) |
Eloquent Location |
Drainage | S-M grade |
Compact - ness score |
Pre- op embo |
Intra- op angio |
Recurrence (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | Hemorrhage/ Hemiparesis |
Left Frontal |
11 | Yes | S | 2 | 1 | No | Yes | 60 |
| 2 | 8 | Papilledema | Left Frontal |
63 | No | S | 3 | 1 | Yes | Yes | 56 |
| 3 | 5 | Hemorrhage/ Headache |
Left Frontal |
12 | No | D | 2 | 1 | No | No | 51 |
| 4 | 15 | Syncope | Left Frontal |
39 | No | B | 3 | 1 | Yes | Yes | 50 |
Pt, patient; mm, millimeter; S, Superficial; D, Deep; B, Both; S-M grade, Spetzler-Martin grade; Pre-op, preoperative; Intra-op, intraoperative; Embo, embolization; Angio, angiogram
Out of the four recurrences, Patient 1 and 2 (Table 4) had intra-operative angiograms showing complete resections and post-operative angiograms at 3 and 6 months respectively that showed no residual AVM. Patient 3 had an immediate post-operative day 2 routine angiogram that showed no residual AVM. Patient 4 was taken to the angiography suite intra-operatively, and an angiogram was performed with the same equipment and technique used during routine angiograms that showed no residual AVM. This data suggests that the intra-operative angiogram was sensitive enough to find residual AVM after resection and that our 4 recurrences in his cohort were true recurrences.
Post-operative imaging & follow-up treatment
The median time to last follow-up with vascular imaging for the cohort was 60 weeks (13.7 months) from the initial resection [interquartile range 51– 125 weeks ( 11.7–28.8 months)], for a total of 48.1 person-years. Patients were followed with angiograms and/or MRI/MRA at varying intervals post-surgery. Attempts were made to follow all patients with neuroimaging at about 1 year from surgery; however, some patients’ “one-year” angiograms occurred up to 3 months later. Twelve patients received their first follow-up angiogram approximately 3 months after surgery. One patient with a negative 3-month angiogram went on to have a recurrence at 1 year, suggesting that a negative 3 month angiogram does not rule out later recurrence. Four patients received their first post-operative angiogram at approximately 6 months post AVM resection, and one of these patients was later discovered to have a recurrence on a one-year angiogram. Five patients had their first angiogram at 1 year after surgery, and the remaining patients received angiograms later than 1 year post-operatively.Six patients had an MRA before or at their 1 year follow-up imaging and the other 22 patients had a diagnostic angiogram at their 1 year follow-up. Of the 6 patients followed by MRA, all had either an intraoperative angiogram, immediate post-operative, or a diagnostic angiogram at 3 or 6 months showing no residual AVM.
Recurrent AVMs were found in 4 children (14.3%) at 50, 51, 56, and 60 weeks from the initial resection. Even though 2 of the children had recurrences found on angiograms later than one year after surgery, the angiogram performed at this “later-than-one-year” date was supposed to be their 1-year postoperative follow-up angiogram. Because of scheduling conflicts, the 1-year angiogram may have been delayed by a maximum of 3 months. Incidence of AVM recurrence after resection was 0.08 per person-year. One-year and 5-year recurrence free survival were 91% (95% CI 68%-98%) and 79% (95% CI 52%-92%), respectively. Three recurrences were found on diagnostic angiography performed at time points described above, i.e. 3 months, 6 months or 1 year post-operation and not due to irregularities or suspicious areas on CT (computed tomography) or MRI/MRA. One recurrence (Patient 3, Table 4) had an MRA at 10 months post-resection because of a clinical seizure even though she was scheduled for a diagnostic angiography at 1 year post-resection. The MRA showed no recurrent AVM;, however, the diagnostic angiogram performed 2 months later showed a Spetzler-Martin grade 2 residual AVM. Importantly, no recurrences were found secondary to recurrent hemorrhage. Figure 3 shows the AVM-free recurrence for the cohort. Figure 4 shows the pre-operative, post-operative, and recurrence angiogram for one of the five patients with recurrence. Of the 4 recurrent AVMs, all four were treated with surgery. The AVM characteristics for patients with recurrent AVMs are shown in Table 4.
Figure 3.
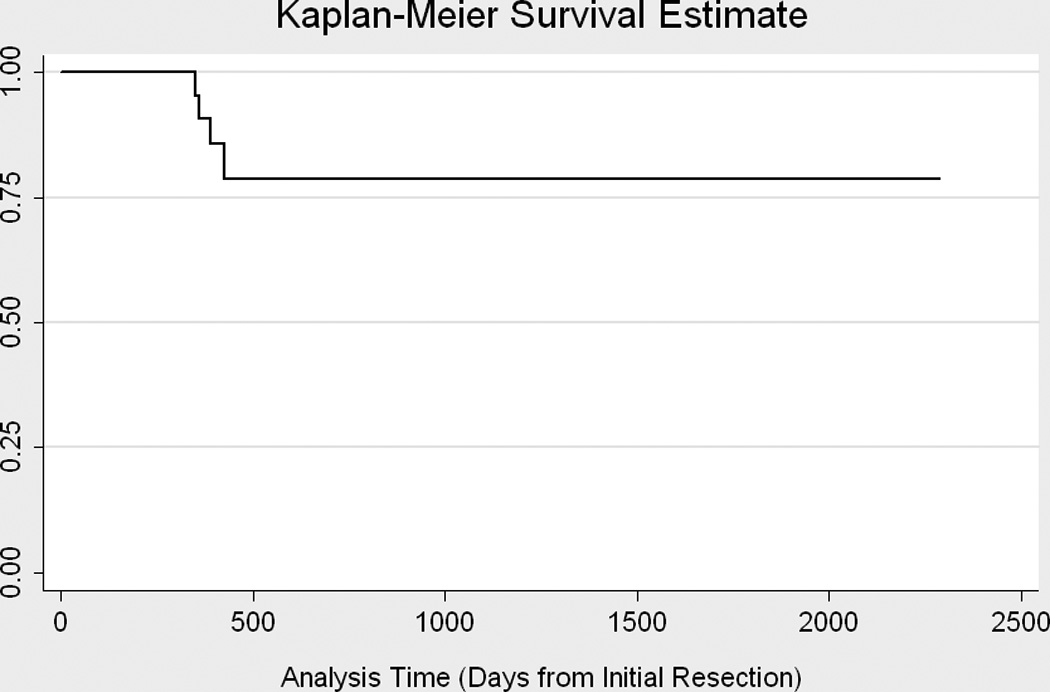
Time to Recurrence. Kaplain-Meyer Survival Curve.
Figure 4.
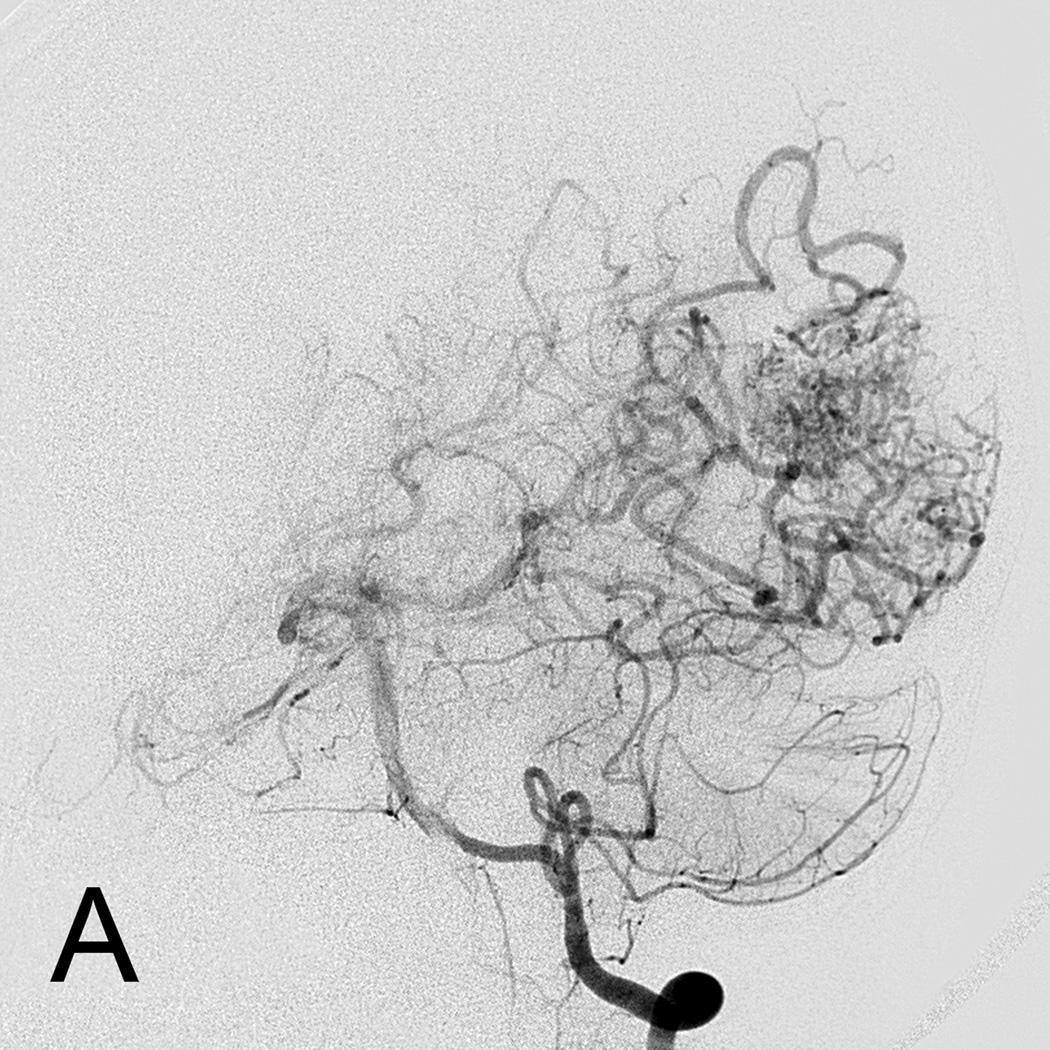
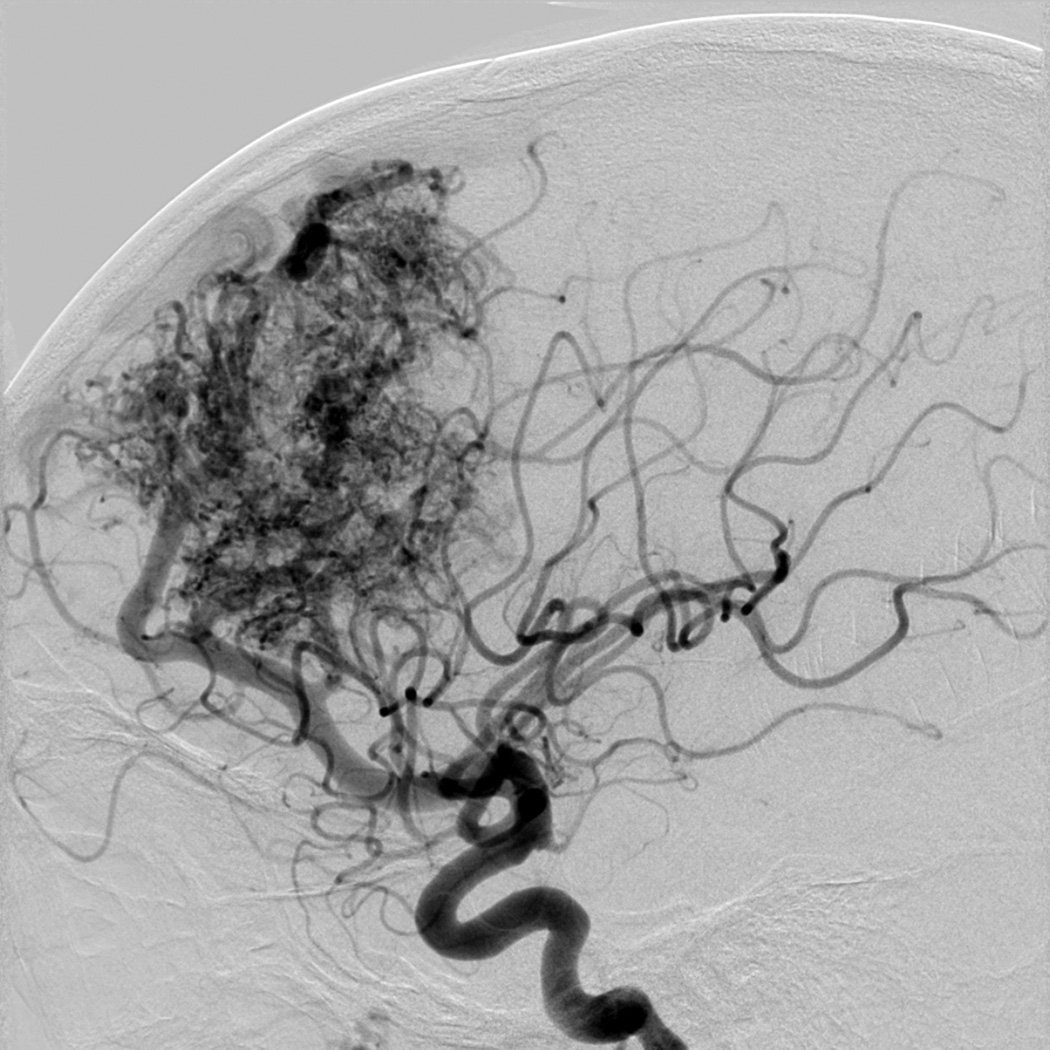
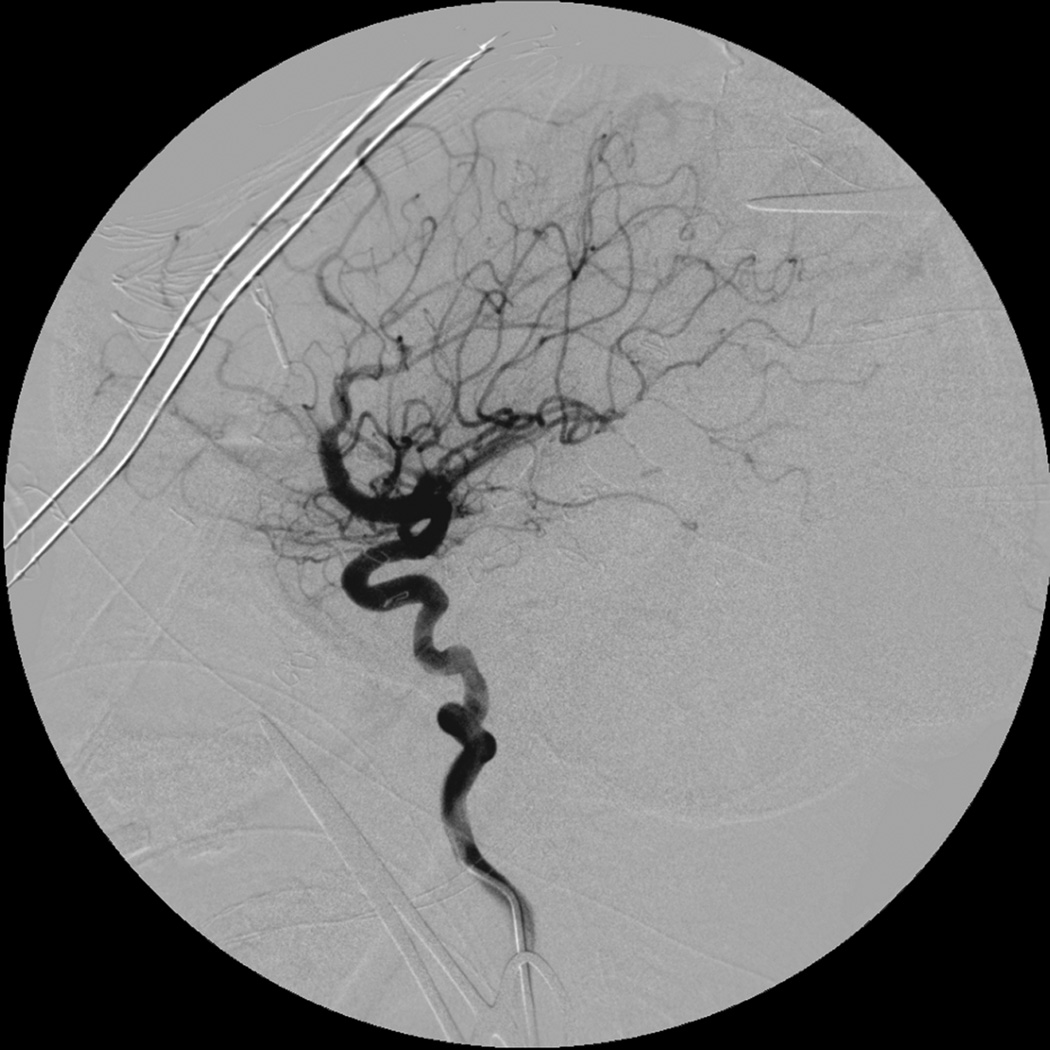
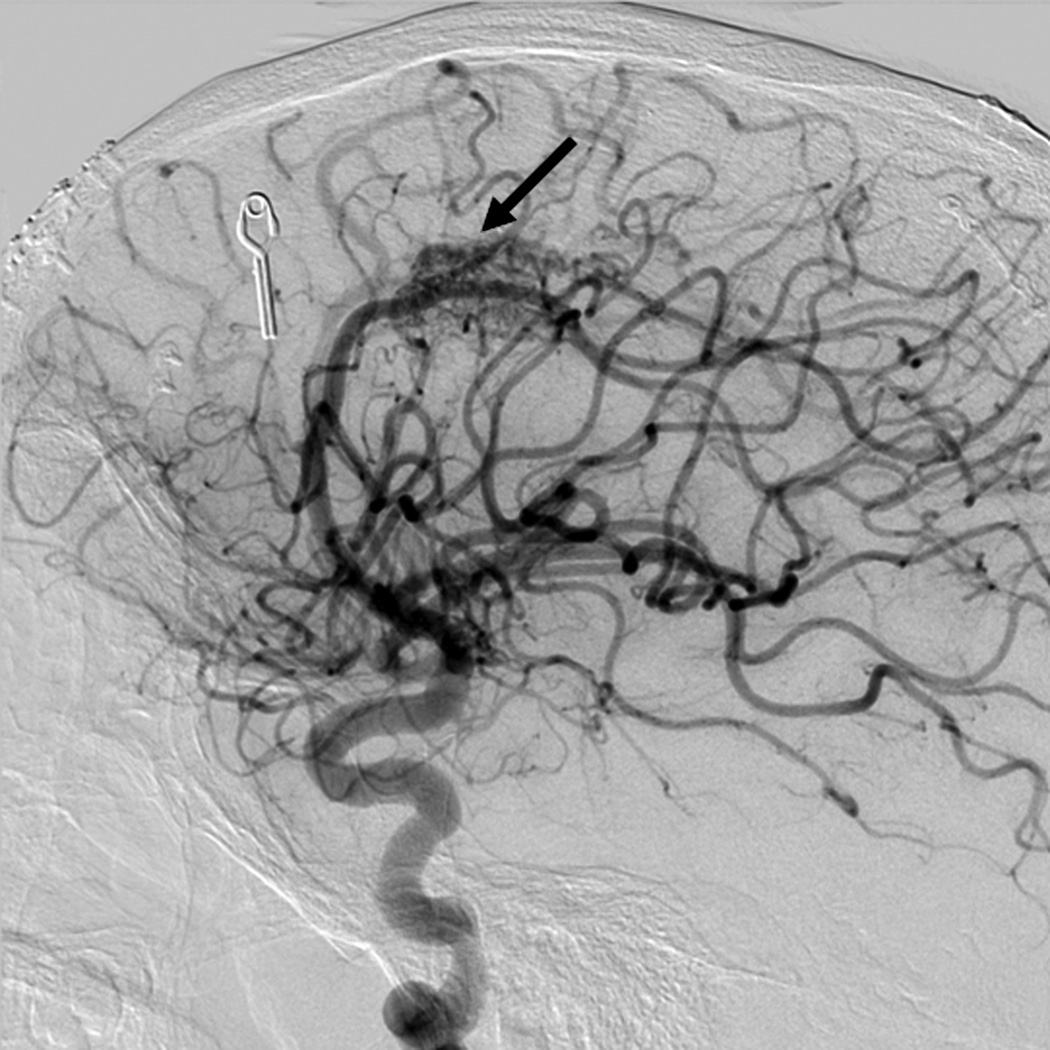
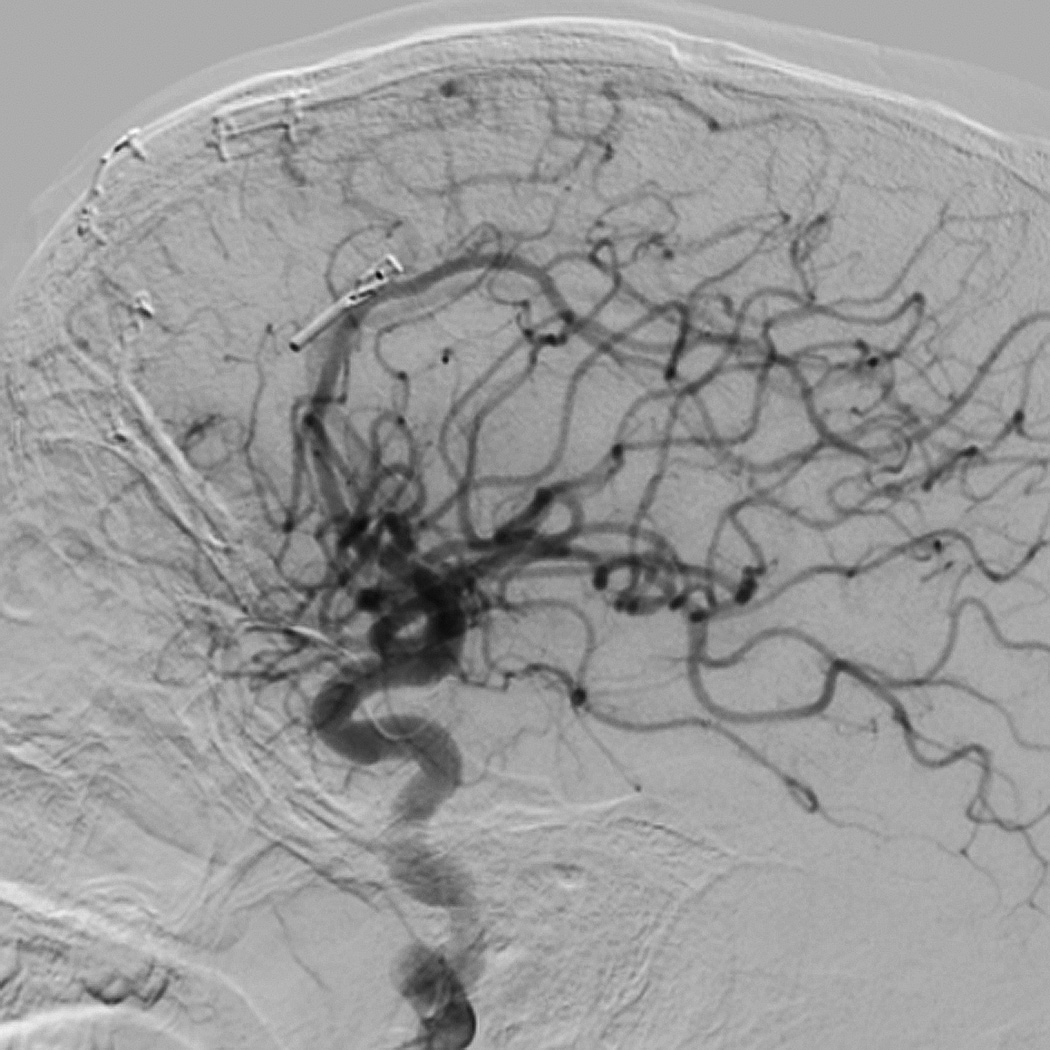
Eight year old male who presented with papilledema during routine eye examination. (Patient 2, Table 4
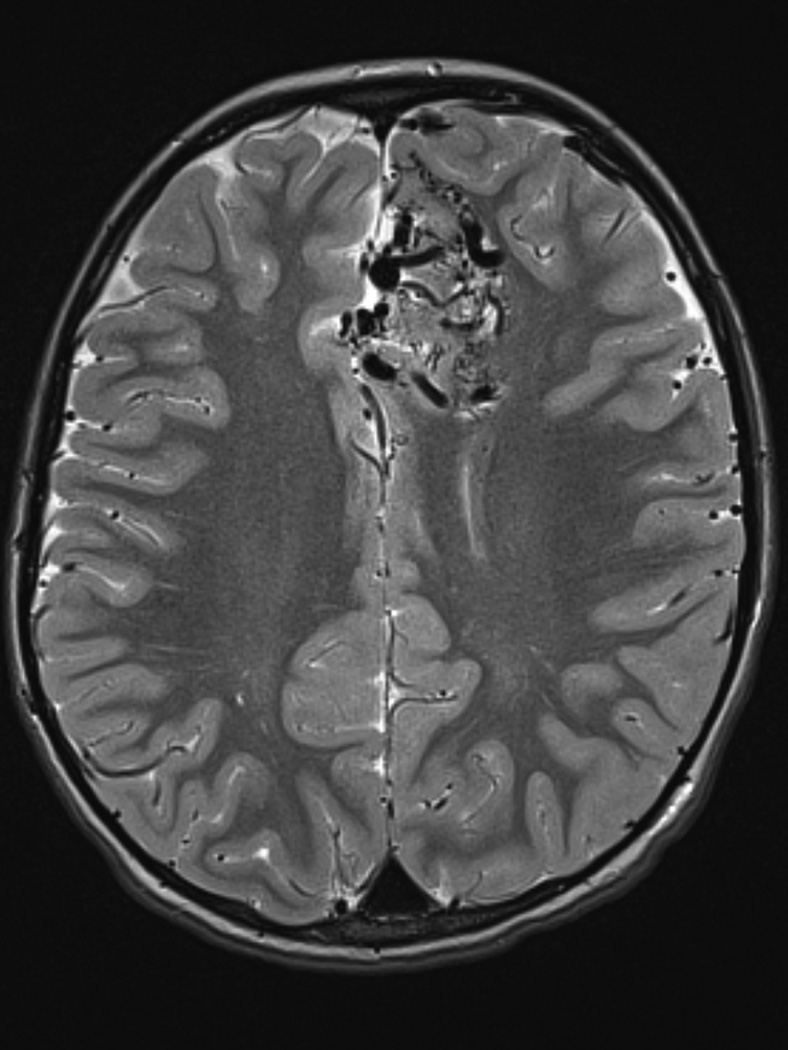
A. T2-weighted axial MRI demonstrates serpiginous flow voids of various sizes in the left frontal lobe suggestive of AVM.
B. Lateral left internal carotid artery injection digital subtraction angiogram demonstrates a 6 cm left frontal AVM fed predominantly by both anterior cerebral arteries (ACAs), but predominately by the left ACA with superficial drainage. A compactness score of 1 was assigned.
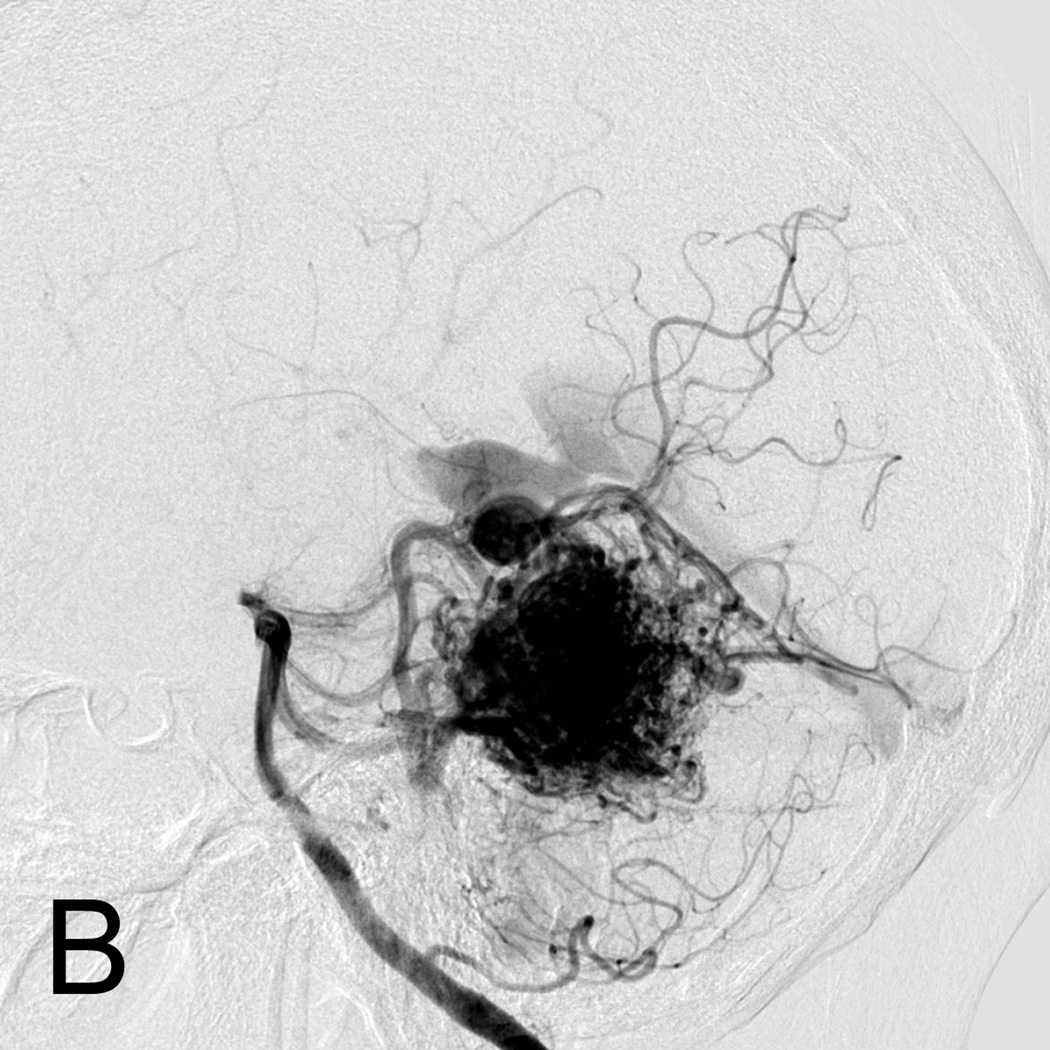
C. Intra-operative angiography following resection was performed showing complete resection of the AVM on lateral view.
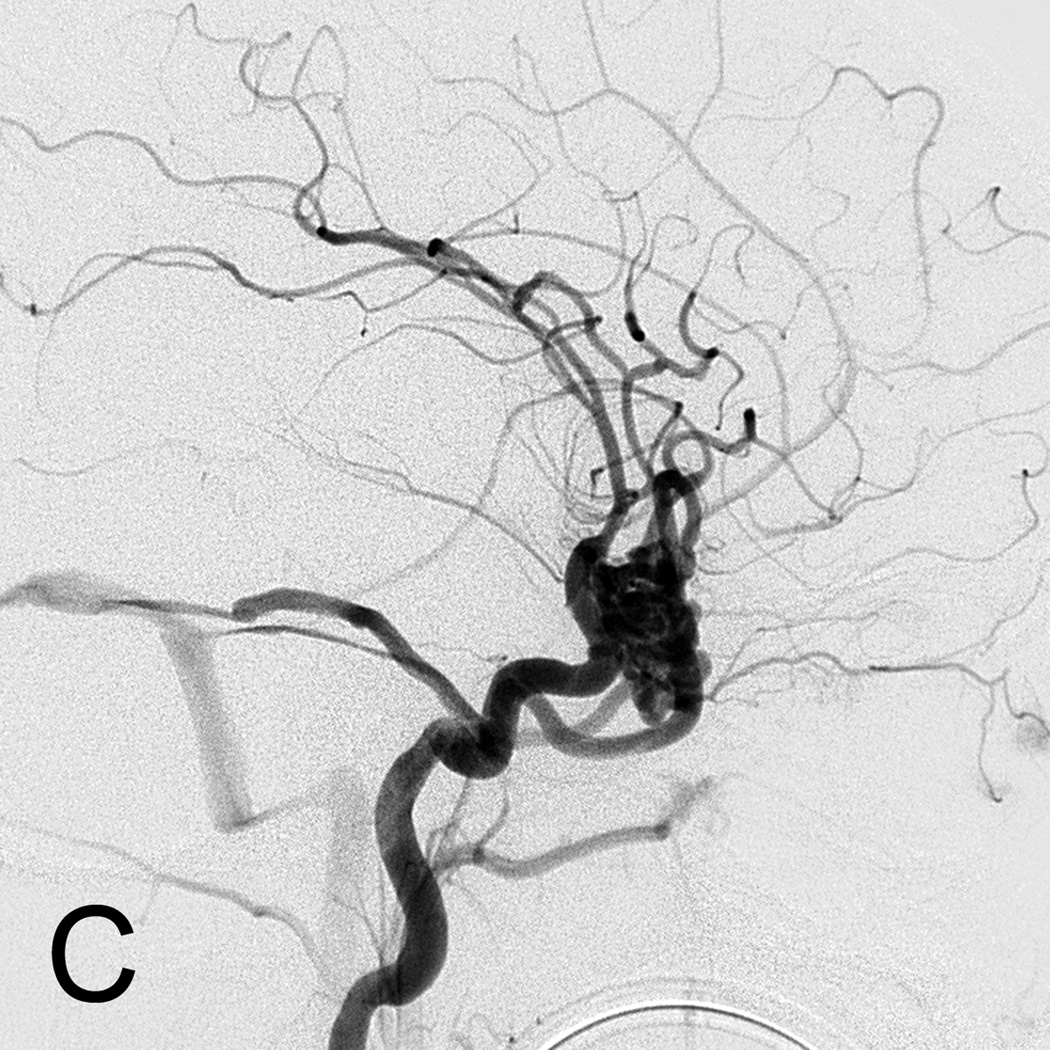
D. Lateral left internal carotid artery injection digital subtraction angiogram 1 year post-resection demonstrating recurrence of the AVM (arrow). Patient underwent craniotomy for recurrent AVM resection.
E. Lateral left internal carotid artery injection digital subtraction angiogram 1 year post-operatively after second resection demonstrating no recurrent AVM.
All 4 patients with AVM recurrence had a compactness score of 1, and only 1 patient with a compactness score of 1 did not have recurrence. The patient with residual AVM at the time of closure also had a compactness score of 1. Lower compactness score was associated with AVM recurrence (p=0.0003, Wilcoxon rank-sum). The median SM grade for the children with recurrent AVM was 2.5 (IQR 2–3), not statistically different from the median SM grade of 2 (IQR 1–3) for the children without recurrence (p=0.55 Wilcoxon rank-sum). There was also no significant difference between the median nidus size of 25.5mm (interquartile range 11.5–51mm) for recurrence and 29mm (interquartile range 16–38mm) without recurrence (p=0.89 Wilcoxon rank-sum). Median age for children with recurrence, 11.5 years (interquartile range 7.0–15.1 years), and that for children without recurrence, 12 years (interquartile range 9.1–14.5 years), was also not statistically significant (p=1.00 Wilcoxon rank-sum). Of the 4 patients with recurrent AVM, 2 had pre-operative embolization and 2 did not. Of the 23 patients without recurrent AVM, 17 had pre-operative embolization and 6 did not. The patient who did not undergo a complete resection did have a pre-operative embolization. Lack of pre-operative embolization was not associated with recurrence (risk ratio 2.4, 95% confidence interval 0.4 to 14.0, p=0.56, Fisher’s exact.).
Discussion
The incidence of incomplete surgical resection or recurrence after complete resection of AVMs is not well-reported in the literature, especially in the pediatric population (1, 2, 10). Literature suggests that children tend to be more prone to hemorrhage than adults and may have higher recurrence rates after treatment (3, 9, 21). In this modern cohort, the incidence of recurrent AVM was 0.08 per person-year. It has been the recent trend to treat AVMs with a multidisciplinary approach (4, 11, 12, 18) and with intra-operative angiograms to document complete AVM resection (8, 10). Partial resection of an AVM is particularly dangerous since it does not offer any protection from hemorrhage (10). In certain cases, the risk of hemorrhage of a partially resected AVM may be even higher than an untreated AVM (13, 14). Previous studies have indicated that a negative post-operative angiogram within the first week post resection in the pediatric population does not necessarily indicate a cure and that the risk of future hemorrhage may not be reduced without follow-up studies (13, 16, 20).
The natural history of the tendency of AVMs to increase in size has been documented in the literature, but the pathogenesis behind this phenomenon is unclear (13, 17). Some authors suggest that the shunt created by abnormal vasculature itself may stimulate proliferation of new abnormal blood vessels (17), and others describe shifts in hemodynamics causing increased flow into draining veins and recruitment of collateral vessels (23, 25). Even newer reports have shown evidence of aberrant signal transduction pathways in recurrent AVMs by immunohistochemical analysis (24).
Our data show that intra-operative angiogram can detect residual AVM which can then be resected immediately. The fact that the intra-operative angiogram detected residual that could not otherwise be seen during surgery does strongly suggest that intra-operative angiogram is important and should be used for all patients when possible. A cerebral angiogram is likely more sensitive than other imaging modalities in detecting recurring AVMs. Patient 3 (Table 4) had MRI/MRA 10 months from her original resection that showed no residual, but a conventional angiogram 2 months later showed a Spetzler-Martin grade 2 recurrent AVM. However, the other 3 patients with recurrent AVM did not have MRI and MRA performed prior to the angiogram diagnostic of the recurrence, making it difficult to assess fully the comparative sensitivity of MRA compared to angiogram for detecting recurrences. It is important to weigh the risks and potential benefits of repeat angiography, especially in pediatric patients. Ruling out a recurrence of the AVM is a priority, but unnecessary angiograms should be avoided whenever possible to minimize radiation exposure and other risks of angiography and anesthesia. Although most patients with negative 3-month or 6-month post-operative angiograms were negative at all subsequent angiograms, one patient did recur after a negative 3 month post-operative angiogram and one patient recurred after a negative 6 month post-operative angiogram. This study shows that the time point of about 1 year is crucial. Less than 1 year was too short of an interval to rule out a recurrence, and no patient with a negative cerebral angiogram at about 1 year (note two patients with recurrent AVMs had their “one year” angiogram at 56 and 60 weeks) has had a recurrence at our latter time point, although this cohort needs to be followed for several more years to make recommendations about long term risk of recurrence. Factors that predict recurrent AVM in the pediatric population are poorly understood, but our study suggests that lower compactness score is a strong predictor of recurrence, identifying a subgroup that may warrant more frequent vascular imaging. Additional study in a larger cohort is necessary to adequately evaluate whether other factors like Spetzler-Martin grade, nidus size, or lack of pre-operative embolization are also predictors of recurrent AVM. Since only 4 recurrent events occurred in our cohort, this limited our power to analyze multiple risk factors. Importantly, no patient with recurrent AVM in this cohort had recurrent hemorrhage, emphasizing that timely detection and treatment of recurrent AVMs may prevent additional morbidity and mortality from recurrent hemorrhage.
Conclusion
Pediatric patients with AVMs have a substantial risk of recurrence and need follow up imaging with a cerebral angiogram at 1 year. More patients and longer follow up is required to determine the long term recurrence rate several years after a negative angiogram at 1 year. Based on our cohort results, we recommend the use of intra-operative angiogram as they are useful for detecting residual AVM before craniotomy closure. The residual AVM can be resected immediately, decreasing the need for re-operations in the future.
The utility of the visual AVM nidus compactness score in predicting AVM recurrence should be validated in larger future studies
Acknowledgments
Funding: Dr. Beslow has been funded by NIH T32-NS007413, The L. Morton Morley Funds of The Philadelphia Foundation, and NINDS K12-NS049453. Dr. Vossough receives funding from NIH 1R15CA115464-01A2, 5R01HL090615-02, 1R21EY020662-01A1, and 1R01NS072338-01A1.
Footnotes
None of the authors has any disclosures or conflicts of interest.
References
- 1.Ali MJ, Bendok BR, Rosenblatt S, Rose JE, Getch CC, Batjer HH. Recurrence of pediatric cerebral arteriovenous malformations after angiographically documented resection. Pediatr Neurosurg. 2003;39:32–38. doi: 10.1159/000070878. [DOI] [PubMed] [Google Scholar]
- 2.Andaluz N, Myseros JS, Sathi S, Crone KR, Tew JM., Jr Recurrence of cerebral arteriovenous malformations in children: report of two cases and review of the literature. Surg Neurol. 2004;62:324–330. doi: 10.1016/j.surneu.2003.11.030. discussion 330-321. [DOI] [PubMed] [Google Scholar]
- 3.Bristol RE, Albuquerque FC, Spetzler RF, Rekate HL, McDougall CG, Zabramski JM. Surgical management of arteriovenous malformations in children. J Neurosurg. 2006;105:88–93. doi: 10.3171/ped.2006.105.2.88. [DOI] [PubMed] [Google Scholar]
- 4.Deruty R, Pelissou-Guyotat I, Amat D, Mottolese C, Bascoulergue Y, Turjman F, Gerard JP. Multidisciplinary treatment of cerebral arteriovenous malformations. Neurol Res. 1995;17:169–177. doi: 10.1080/01616412.1995.11740307. [DOI] [PubMed] [Google Scholar]
- 5.Di Rocco C, Tamburrini G, Rollo M. Cerebral arteriovenous malformations in children. Acta Neurochir (Wien) 2000;142:145–156. doi: 10.1007/s007010050017. discussion 156-148. [DOI] [PubMed] [Google Scholar]
- 6.Du R, Keyoung HM, Dowd CF, Young WL, Lawton MT. The effects of diffuseness and deep perforating artery supply on outcomes after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2007;60:638–646. doi: 10.1227/01.NEU.0000255401.46151.8A. discussion 646-638. [DOI] [PubMed] [Google Scholar]
- 7.Gerosa MA, Cappellotto P, Licata C, Iraci G, Pardatscher K, Fiore DL. Cerebral arteriovenous malformations in children (56 cases) Childs Brain. 1981;8:356–371. doi: 10.1159/000120000. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, Levy ML, Stanley P, Nelson M, Giannotta SL, McComb JG. Intraoperative angiography in the management of pediatric vascular disorders. Pediatr Neurosurg. 1999;30:16–22. doi: 10.1159/000028754. [DOI] [PubMed] [Google Scholar]
- 9.Hladky JP, Lejeune JP, Blond S, Pruvo JP, Dhellemmes P. Cerebral arteriovenous malformations in children: report on 62 cases. Childs Nerv Syst. 1994;10:328–333. doi: 10.1007/BF00335172. [DOI] [PubMed] [Google Scholar]
- 10.Hoh BL, Carter BS, Ogilvy CS. Incidence of residual intracranial AVMs after surgical resection and efficacy of immediate surgical re-exploration. Acta Neurochir (Wien) 2004;146:1–7. doi: 10.1007/s00701-003-0164-5. discussion 7. [DOI] [PubMed] [Google Scholar]
- 11.Hoh BL, Ogilvy CS, Butler WE, Loeffler JS, Putman CM, Chapman PH. Multimodality treatment of nongalenic arteriovenous malformations in pediatric patients. Neurosurgery. 2000;47:346–357. doi: 10.1097/00006123-200008000-00015. discussion 357-348. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys RP, Hoffman HJ, Drake JM, Rutka JT. Choices in the 1990s for the management of pediatric cerebral arteriovenous malformations. Pediatr Neurosurg. 1996;25:277–285. doi: 10.1159/000121140. [DOI] [PubMed] [Google Scholar]
- 13.Kader A, Goodrich JT, Sonstein WJ, Stein BM, Carmel PW, Michelsen WJ. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg. 1996;85:14–18. doi: 10.3171/jns.1996.85.1.0014. [DOI] [PubMed] [Google Scholar]
- 14.Kiris T, Sencer A, Sahinbas M, Sencer S, Imer M, Izgi N. Surgical results in pediatric Spetzler-Martin grades I–III intracranial arteriovenous malformations. Childs Nerv Syst. 2005;21:69–74. doi: 10.1007/s00381-004-1025-0. discussion 75-66. [DOI] [PubMed] [Google Scholar]
- 15.Klimo P, Jr, Rao G, Brockmeyer D. Pediatric arteriovenous malformations: a 15-year experience with an emphasis on residual and recurrent lesions. Childs Nerv Syst. 2007;23:31–37. doi: 10.1007/s00381-006-0245-x. [DOI] [PubMed] [Google Scholar]
- 16.Kondziolka D, Humphreys RP, Hoffman HJ, Hendrick EB, Drake JM. Arteriovenous malformations of the brain in children: a forty year experience. Can J Neurol Sci. 1992;19:40–45. [PubMed] [Google Scholar]
- 17.Krayenbuhl HA. Angiographic contribution to the problem of enlargement of cerebral arteriovenous malformations. Acta Neurochir (Wien) 1977;36:215–242. doi: 10.1007/BF01405393. [DOI] [PubMed] [Google Scholar]
- 18.Lasjaunias P, Hui F, Zerah M, Garcia-Monaco R, Malherbe V, Rodesch G, Tanaka A, Alvarez H. Cerebral arteriovenous malformations in children. Management of 179 consecutive cases and review of the literature. Childs Nerv Syst. 1995;11:66–79. doi: 10.1007/BF00303807. discussion 79. [DOI] [PubMed] [Google Scholar]
- 19.Mullan S, Mojtahedi S, Johnson DL, Macdonald RL. Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg. 1996;85:1–8. doi: 10.3171/jns.1996.85.1.0001. [DOI] [PubMed] [Google Scholar]
- 20.Niazi TN, Klimo P, Jr, Anderson RC, Raffel C. Diagnosis and management of arteriovenous malformations in children. Neurosurg Clin N Am. 2010;21:443–456. doi: 10.1016/j.nec.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Mejia RO, Chennupati SK, Gupta N, Fullerton H, Young WL, Lawton MT. Superior outcomes in children compared with adults after microsurgical resection of brain arteriovenous malformations. J Neurosurg. 2006;105:82–87. doi: 10.3171/ped.2006.105.2.82. [DOI] [PubMed] [Google Scholar]
- 22.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 23.Spetzler RF, Wilson CB. Enlargement of an arteriovenous malformation documented by angiography. Case report. J Neurosurg. 1975;43:767–769. doi: 10.3171/jns.1975.43.6.0767. [DOI] [PubMed] [Google Scholar]
- 24.Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Early regrowth of juvenile cerebral arteriovenous malformations: report of 3 cases and immunohistochemical analysis. World Neurosurg. 73:100–107. doi: 10.1016/j.surneu.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Waltimo O. The change in size of intracranial arteriovenous malformations. J Neurol Sci. 1973;19:21–27. doi: 10.1016/0022-510x(73)90052-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Wang S, Li J, Qi W, Sui D, Zhao Y. Clinical characteristics and surgical results of patients with cerebral arteriovenous malformations. Surg Neurol. 2005;63:156–161. doi: 10.1016/j.surneu.2004.04.021. discussion 161. [DOI] [PubMed] [Google Scholar]