ABSTRACT
Intracellular concentration of cyclic diguanylate monophosphate (c-di-GMP), a second messenger molecule, is regulated in bacteria by diguanylate cyclases (DGCs) (synthesizing c-di-GMP) and phosphodiesterases (PDEs) (degrading c-di-GMP). c-di-GMP concentration ([c-di-GMP]) affects motility and sessility in a reciprocal fashion; high [c-di-GMP] typically inhibits motility and promotes sessility. A c-di-GMP sensor domain, PilZ, also regulates motility and sessility. Uropathogenic Escherichia coli regulates these processes during infection; motility is necessary for ascending the urinary tract, while sessility is essential for colonization of anatomical sites. Here, we constructed and screened 32 mutants containing deletions of genes encoding each PDE (n = 11), DGC (n = 13), PilZ (n = 2), and both PDE and DGC (n = 6) domains for defects in motility, biofilm formation, and adherence for the prototypical pyelonephritis isolate E. coli CFT073. Three of 32 mutations affected motility, all of which were in genes encoding enzymatically inactive PDEs. Four PDEs, eight DGCs, four PDE/DGCs, and one PilZ regulated biofilm formation in a medium-specific manner. Adherence to bladder epithelial cells was regulated by [c-di-GMP]. Four PDEs, one DGC, and three PDE/DGCs repress adherence and four DGCs and one PDE/DGC stimulate adherence. Thus, specific effectors of [c-di-GMP] and catalytically inactive DGCs and PDEs regulate adherence and motility in uropathogenic E. coli.
IMPORTANCE
Uropathogenic Escherichia coli (UPEC) contains several genes annotated as encoding enzymes that increase or decrease the abundance of the second messenger molecule, c-di-GMP. While this class of enzymes has been studied in an E. coli K-12 lab strain, these proteins have not been comprehensively examined in UPEC. UPEC utilizes both swimming motility and adherence to colonize and ascend the urinary tract; both of these processes are hypothesized to be regulated by the concentration of c-di-GMP. Here, for the first time, in a uropathogenic strain, E. coli CFT073, we have characterized mutants lacking each protein and demonstrated that the uropathogen has diverged from E. coli K-12 to utilize these enzymes to regulate adherence and motility by distinct mechanisms.
Introduction
Uropathogenic Escherichia coli (UPEC), the most common etiological agent of urinary tract infection (UTI), causes over 80% of uncomplicated UTIs (1). The ability to transition between the planktonic, motile lifestyle and the adherent, sessile lifestyle is necessary for UPEC to ascend the urethra and colonize the bladder and to ascend the ureters to colonize the kidneys. One mechanism by which bacteria regulate the transition between motile and sessile states is by modulating and monitoring the intracellular levels of the second messenger cyclic di-guanylate monophosphate (c-di-GMP) (2, 3). This cyclic nucleotide, synthesized by diguanylate cyclases (DGCs) that contain the characteristic GGDEF domain, is degraded by phosphodiesterases (PDEs) that contain the conserved EAL domain (4). In response to extracellular signals, the DGCs and PDEs modulate the levels of c-di-GMP mediating the conversion from motile to sessile states (5, 6). For example, when functional PDEs are mutated, the local increase in c-di-GMP pools within the cell usually coincides with an increase in biofilm formation, thus encouraging a sessile state, and a decrease in motility. The pyelonephritis isolate E. coli CFT073 (7) carries 30 genes that encode DGC, PDE, or both domains and two genes that encode the c-di-GMP sensor domain, PilZ (8). Several of these domains, however, are degenerate and therefore may not function as direct regulators of c-di-GMP levels in the cell. Degenerate PDEs and DGCs, while not actively affecting c-di-GMP turnover, can nevertheless act as receptors of c-di-GMP (9) or have evolved to function in c-di-GMP-independent mechanisms such as LapD, a protein with both degenerate PDE and DGC domains that affects adherence in Pseudomonas fluorescens (10).
While much work has been conducted on the role of c-di-GMP signaling in environmental and commensal bacteria such as Pseudomonas and E. coli K-12, very little is known about the role of c-di-GMP signaling in pathogenic bacteria. Indeed, pathogens may have evolved to highly regulate c-di-GMP turnover, as c-di-GMP is recognized by the innate immune system protein STING (stimulator of interferon genes) (11, 12). Thus, we endeavored to determine the involvement of each PDE, DGC, and PilZ domain in motility and sessility in E. coli CFT073.
Here, we conducted comprehensive screens to identify effects on motility, biofilm formation, adherence, curli synthesis, and cellulose production by construction of individual deletions of each DGC, PDE, and PilZ domain-encoding gene in uropathogenic E. coli CFT073. The effect of chemically complementing the deletion mutants with demonstrable phenotypes was assessed by expression of exogenous PDE or DGC in each mutant background. Thus, the phenotypes conferred by both enzymatically active and inactive proteins were determined.
RESULTS
Motility of c-di-GMP phosphodiesterase, diguanylate cyclase, and PilZ mutants of E. coli CFT073.
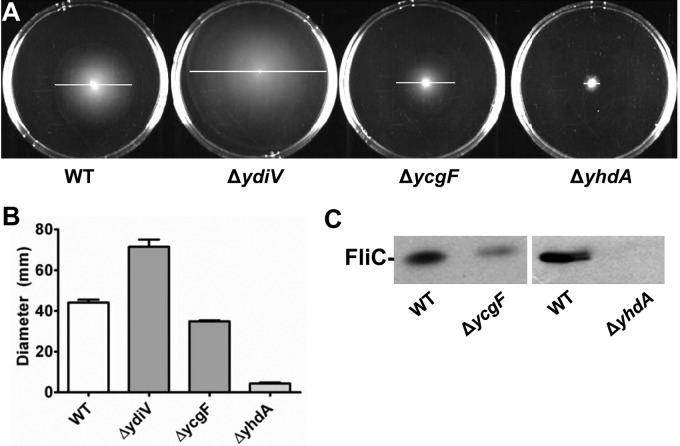
Since the putative phosphodiesterase YdiV partially restores motility in a type 1 fimbria locked-on mutant (Fim L-ON) and, in a wild-type background, increases motility almost 2-fold (13), we hypothesized that related proteins that modulate the levels of c-di-GMP would also affect motility in E. coli CFT073. We therefore systematically constructed single deletions of the 10 other genes encoding putative phosphodiesterases (PDEs) (ycgF, yahA, ylaB, yliE, c1246, c1610, yoaD, rtn, yhjH, and yjcC), as well as 13 putative diguanylate cyclase (DGC)-encoding genes (ydeH, yeaI, yeaJ, yeaP, yedQ, yfiN, yaiC, yliF, ycdT, ydaM, c1918, c1919, and yneF), six genes encoding both domains (yciR, yegE, yfeA, yfgF, yhdA, and yhjK) (Table 1), and two genes that encode the c-di-GMP binding domain PilZ (ycgR and bcsA-yhjO). Three of the deletion constructs in E. coli CFT073 significantly affected motility when swimming diameter was measured in soft agar plates: ydiV, ycgF, and yhdA (Fig. 1A and B; Table 2). As demonstrated previously, ΔydiV displayed increased motility (71.5- ± 6.2-mm swim diameter; P < 0.0001) compared to wild-type E. coli CFT073 (42.4 ± 7.2 mm), whereas ΔycgF (34.9 ± 0.8 mm; P = 0.0145) and ΔyhdA (4.3 ± 0.8 mm; P < 0.0001) showed decreased motility. All three genes, ydiV, ycgF, and yhdA, encode degenerate PDE domains, and yhdA also encodes a degenerate DGC domain.
TABLE 1 .
Characteristics and conserved residues in the domains of the phosphodiesterases and diguanylate cyclases of E. coli CFT073
| Gene | TM domaina |
N-terminal signal sequenceb |
Other domainc |
GGDEF domain (consensus) |
EAL domain (consensus) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metal binding site (D[D/E]) |
I site (DR) |
A site (KNDHDRGG[D/E]EF) |
EALxR (EALR) |
c-di-GMP binding (QRDD) |
Catalysis (TE) |
Mg2+ binding (ENEEDKEQ) |
||||
| yahA | LuxR-C-like DNA binding |
EVLR | QRDD | TE | ENEEDKEQ | |||||
| ydiV | ELIH | FHND | ND | E-LEGMGQ | ||||||
| ylaB | ✓ (2) | ✓ | CSS motif | EALR | QRDD | TE | ENEEDKEQ | |||
| yliE | ✓ (2) | ✓ | EALR | QRDD | TE | ENEEDKEQ | ||||
| ycgF | BLUF | EAIQ | NQHS | TA | ENEEDKMQ | |||||
| c1610 | EVLR | QRDD | TE | ENEEDKEQ | ||||||
| yoaD | ✓ (1) | ✓ | CSS motif | EILR | QRDD | TE | ENEEDKEQ | |||
| rtn | ✓ (11) | ✓ | CSS motif | EVLR | QRDD | TE | ENEEDKEQ | |||
| yhjH | ELLV | QVDA | VE | ENEEDKEQ | ||||||
| yjcC | ✓ (11) | ✓ | CSS motif | EALR | QRDD | TE | ENEEDKEQ | |||
| c1246 | EMLD | EDDS | SE | ENEEDKEQ | ||||||
| yaiC | ✓ (36) | ✓ | MASE2 | DD | DR | KNDHDRGGDEF | ||||
| yliF | ✓ (11) | ✓ | DD | GE | KNDHDRGGDEF | |||||
| ydeH | DE | ER | KNDHDRGGEEF | |||||||
| yeaI | ✓ (2) | DE | DR | KNAHDREGEVF | ||||||
| yeaJ | ✓ (11) | DD | DR | KNDHDRGGDEF | ||||||
| yeaP | GAF | DD | ER | KNDHDRGGDEF | ||||||
| yedQ | ✓ (11) | ✓ | DE | DR | KNDHDRGGEEF | |||||
| yfiN | ✓ (11) | ✓ | Inner membrane | DD | HA | KNDHDRGGDEF | ||||
| ycdT | ✓ (24) | ✓ | DE | DR | KNDHDRGGEEF | |||||
| ydaM | PAS, sensory box | DE | DT | KNDHDRGGEEF | ||||||
| c1918 | AtoS, PAS (2) | G- | − | GQIDYD------ | ||||||
| c1919 | − | − | KHYADIDEALY | |||||||
| yneF | ✓ (2) | ✓ | DE | GK | KNDHDRGGEEF | |||||
| yciR | PAS, RNase II stability modulator |
DD | QR | KNDHDRGGDEF | EALR | QRDD | TE | ENEEDKEQ | ||
| yegE | ✓ (18) | ✓ | MASE1, PAS, PAC-PAS | DD | DR | KNDHDRGGDEF | EIIE | SLQD | AD | EPIESLGY |
| yfeA | ✓ (2) | ✓ | MASE1 | RS | EN | ESRLVQPGSEL | EILR | QRDD | TE | ENEEDKEQ |
| yfgF | ✓ (24) | ✓ | MASE1 | SGNDL | EILR | QRDD | TE | ENEEDKEQ | ||
| yhdA | ✓ (11) | ✓ | HAMP | RS | AQ | NSDHERHRSDF | ELM R | KRQH | AR | EVEENKTQ |
| yhjK | ✓ (11) | ✓ | HAMP | TY | MQ | RDQSGYDF | EVLR | QRDD | TE | ENEEDKEQ |
Presence of transmembrane domains (✓) as predicted by the TMHMM server. Numbers of transmembrane domains are in parentheses.
Presence of an N-terminal sequence (✓) as predicted by the TMHMM server.
Other domains listed in NCBI gene database.
FIG 1 .
Motility and flagellin expression of the strains with the single deletions ΔydiV, ΔyhdA, and ΔycgF. (A) Motility plates inoculated with wild-type E. coli CFT073 and single-deletion ΔydiV, ΔyhdA, and ΔycgF strains. White bars indicate swimming diameter. (B) Swimming motility diameter of wild-type E. coli CFT073 and single-deletion ΔydiV, ΔyhdA, and ΔycgF strains cultured at 30°C in soft LB agar plates for 16 h. Data are averages from three independent experiments performed in triplicate. Error bars indicate standard errors of the means. For all data, P was <0.05 as assessed by Student’s t test. (C) Western blot of flagellin protein FliC from wild-type E. coli CFT073 (WT) and the mutant ΔyhdA and ΔycgF strains. All cultures were diluted to an OD of 0.3 before the samples were boiled to ensure equal loading of protein.
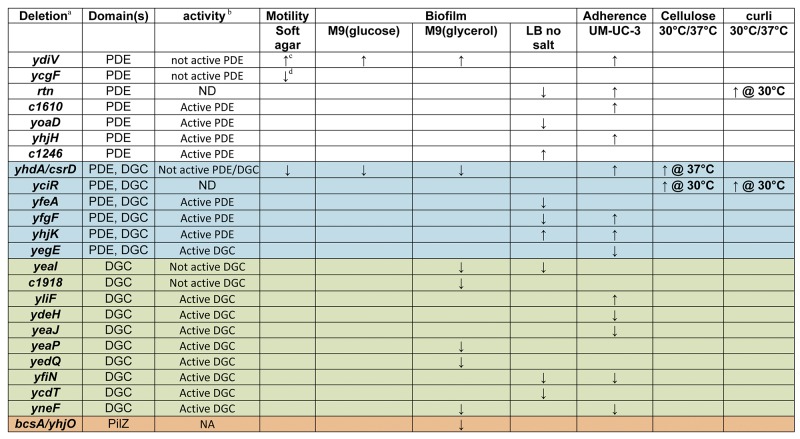
TABLE 2 .
Phenotypes of Deletions in E. coli CFT073
Deletions for which no phenotype was determined are not listed.
Activity as determined by complementation by VC1086 (known active PDE) or WspR (known active DGC).
↑, statistically significant increase in the phenotype compared to wild-type E. coli CFT073.
↓, statistically significant decrease in the phenotype compared to wild-type E. coli CFT073.
Both ΔyhdA and ΔycgF were tested for their effect on motility in the Fim L-ON background to determine if the motility phenotypes of these mutants were affected by type 1 fimbrial expression. As observed previously, the Fim L-ON construct has reduced motility, with an average diameter of 10.0 mm, compared to the wild-type parental strain (average diameter of 42.4 mm). Both mutants further decreased motility in the Fim L-ON background (diameter for the ΔyhdA mutant = 1.9 ± 0.7 mm [P < 0.0001]; diameter for the ΔycgF mutant = 7.0 ± 0.3 mm [P = 0.0112]), demonstrating that deletion of either yhdA or ycgF suppresses motility regardless of the fimbriation state.
Since deletion of ydiV (average diameter of 18.5 mm) partially restores motility in E. coli CFT073 Fim L-ON, mutation of yhdA and ycgF was assessed in a ΔydiV Fim L-ON background to determine if either the low- or high-motility phenotype was dominant. Deletion of yhdA significantly decreased motility in the ΔydiV Fim L-ON background (3.7 mm; P = 0.0013) but compared to ΔyhdA Fim L-ON, ΔyhdA ΔydiV Fim L-ON was significantly more motile (P = 0.034), suggesting that ΔyhdA and ΔydiV likely act through different regulatory pathways and thus have an additive effect. When placed in the ΔydiV Fim L-ON background, ΔycgF no longer affected motility; thus, the ydiV deletion is dominant over ycgF.
Motility phenotypes correlate with flagellin production.
Since deletion of yhdA, ycgF, and ydiV affected the swimming motility of E. coli CFT073, we estimated the production of flagellin by Western blotting to determine whether the effects on motility were due to a change in the amount of flagellin synthesized. Consistent with their reduced-motility phenotypes, both the ΔyhdA and ΔycgF mutants yielded reduced levels of flagellin protein compared to the wild-type strain (Fig. 1C), while the ΔydiV mutant demonstrated increased FliC expression (as previously shown [13]), commensurate with its increased motility.
Exogenous phosphodiesterase or diguanylate cyclase activity and motility in mutants.
While c-di-GMP affects motility in many bacterial species (5, 6), in E. coli CFT073 all three deletions affecting motility were of degenerate PDEs. Therefore, we endeavored to determine whether E. coli CFT073 produces this cyclic dinucleotide. However, under all conditions tested (culture in LB and in minimal medium, at both exponential and stationary growth phases), the level of c-di-GMP was below the 7.8 nM limit of detection by liquid chromatography-tandem mass spectrometry. We next induced expression of WspR, a known active diguanylate cyclase from Pseudomonas aeruginosa, from an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter (14) to determine if increasing [c-di-GMP] in E. coli CFT073 affects motility. Likewise, a known active phosphodiesterase from Vibrio cholerae, VC1086, under the control of an IPTG-inducible promoter (15) was utilized to determine if decreasing [c-di-GMP] affects motility. However, induction of neither WspR nor VC1086 in E. coli CFT073 significantly affected motility. Therefore, either [c-di-GMP] does not regulate motility in E. coli CFT073 or [c-di-GMP] is so tightly regulated in E. coli CFT073 that the addition of an exogenous PDE or DGC does not sufficiently alter [c-di-GMP] to produce a visible change in swimming diameter.
Biofilm formation by PDE, DGC, and PilZ mutants of E. coli CFT073.
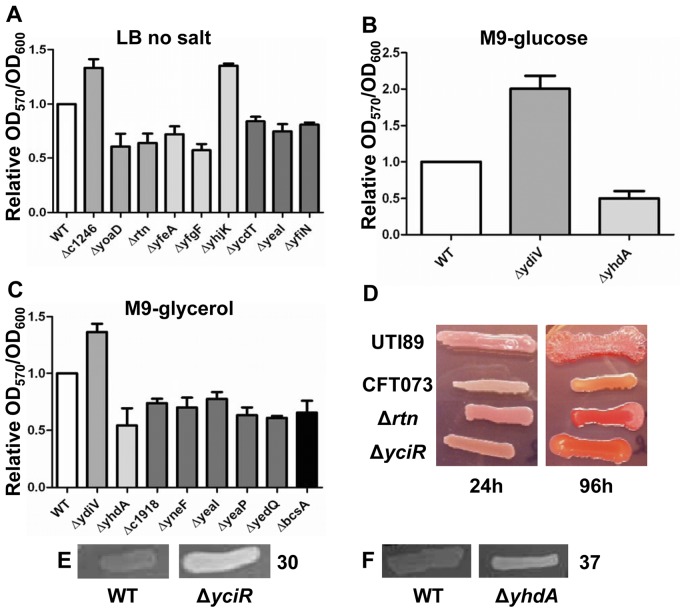
Biofilm formation, a sessile phenotype regulated by c-di-GMP, is increased as levels of c-di-GMP increase in some bacteria (3). Therefore, all 32 mutants were tested using the crystal violet assay to determine the contribution of individual PDE, DGC, and PilZ domain-encoding genes to biofilm formation when bacteria were cultured statically for 24 h at 37°C in LB without salt or M9 minimal salts medium with either 0.2% glucose or 0.4% glycerol as carbon source. In LB medium without salt, wild-type E. coli CFT073 forms a robust biofilm ring on the side of the well at the air-liquid interface. When corrected for the optical density at 600 nm (OD600) to account for any growth differences in the mutants and normalized to wild-type, the ΔycdT, ΔyeaI, and ΔyfiN mutants (DGCs), the Δc1246, ΔyoaD, and Δrtn mutants (PDEs), and the ΔyhjK, ΔyfgF, and ΔyfeA mutants (PDE/DGCs) produced amounts of biofilm significantly different from that of the wild-type strain (P < 0.044) (Fig. 2A; Table 2). Deletion of yhjK or c1246 increased biofilm biomass, whereas ΔycdT, ΔyeaI, ΔyfiN, ΔyoaD, Δrtn, ΔyfgF, and ΔyfeA reduced biofilm formation.
FIG 2 .
Biofilm formation of PDE, DGC, PDE/DGC, and PilZ mutants relative to the wild-type strain. Biofilm formation was measured following growth in three media. (A) LB, no salt; (B) M9 minimal salts medium with 0.2% glucose; (C) M9 minimal salts medium with 0.4% glycerol. All data are the averages from four independent experiments performed in duplicate. Error bars indicate standard errors of the means. For all data, P was <0.05 as assessed by Student’s t test. (D) E. coli UTI89, E. coli CFT073 wild-type, E. coli CFT073 Δrtn, and E. coli CFT073 ΔyciR grown on YESCA medium containing Congo red demonstrate that E. coli UTI89, E. coli CFT073 Δrtn, and E. coli CFT073 ΔyciR produce curli, whereas wild-type E. coli CFT073 does not at 30°C. (E) Calcofluor white staining of cellulose production at 30°C in wild-type E. coli CFT073 and E. coli CFT073 ΔyciR. (F) Calcofluor white staining of cellulose production at 37°C in wild-type E. coli CFT073 and E. coli CFT073 ΔyhdA.
In M9 minimal salts medium, with either glucose or glycerol as the carbon source, the ΔydiV mutant produced significantly more biofilm (P = 0.0048 and P = 0.0073, respectively) while the ΔyhdA mutant formed significantly less biofilm (P = 0.0078, and P = 0.0398, respectively) than the wild-type strain (Fig. 2B and C; Table 2). None of the other deletions affected biofilm formation when glucose served as the carbon source. However, with glycerol as the sole carbon source, the Δc1918, ΔyneF, ΔyeaP, ΔyedQ, and ΔyeaI mutants (DGCs) and the ΔbscA (PilZ) mutant also significantly decreased biofilm formation compared to the wild-type strain (P < 0.03) (Fig. 2C and Table 2).
Complementation of biofilm defects by exogenous phosphodiesterase or diguanylate cyclase activity.
To determine if the biofilm defects observed were due to a change in [c-di-GMP] because of loss of either PDE or DGC activity, the mutants were complemented with either WspR (IPTG-inducible DGC) or VC1086 (IPTG-inducible PDE). An enzymatically inactive mutant of the PDE (VC1086*) was included as a control. In M9 minimal salts with glucose, induction of VC1086 decreased biofilm formation (52% ± 6% of that of VC1086*; P < 0.0001) (see Fig. S1B in the supplemental material) and induction of WspR increased biofilm formation (144% ± 17% of that of the uninduced wild type; P = 0.0243) (see Fig. S1A in the supplemental material), as expected if c-di-GMP regulates biofilm formation under this condition. However, ΔydiV and ΔyhdA were not complemented by VC1086 (see Fig. S1B) or WspR, in the case of ΔyhdA (see Fig. S1A), suggesting that these deletions affect biofilm formation without modulation of [c-di-GMP].
In M9 minimal medium with glycerol, induction of VC1086 unexpectedly increased biofilm formation in wild-type E. coli CFT073 (144% ± 21% of that of VC1086*); therefore, no conclusions could be drawn about PDE activity under this condition. However, induction of WspR increased biofilm formation in wild-type E. coli CFT073 (160% ± 13% of uninduced wild-type; P = 0.0066) (see Fig. S1C in the supplemental material) as expected since an increase in [c-di-GMP] generally increases biofilm formation. ΔyhdA, ΔyeaP, ΔyneF, and ΔyedQ were all complemented by induction of WspR (see Fig. S1C), suggesting that the biofilm defects in these mutants are due to a loss of DGC function. However, ΔyeaI and Δc1918 could not be complemented by expression of WspR (see Fig. S1C), suggesting that YeaI and C1918 do not actively produce c-di-GMP in this medium and therefore that they affect biofilm formation by another mechanism.
Wild-type E. coli CFT073 biofilm formation increases in no-salt LB medium when WspR is induced (121% ± 6% of that of the uninduced wild type; P = 0.0130) (see Fig. S2A), demonstrating that DGC activity promotes biofilm development and that biofilm formation decreases when VC1086 is induced (67% ± 7% of that of VC1086*; P = 0.0016) (see Fig. S2B), due to PDE activity. Three mutants with single DGC domains, the ΔyfiN, ΔyeaI, and ΔycdT mutants, were complemented by induction of WspR (see Fig. S2A), suggesting that these are active DGCs in no-salt LB medium. All three mutants with single PDE domains, the ΔyoaD, Δc1246, and Δrtn mutants, were complemented by induction of VC1086-encoded PDE (see Fig. S2B). However, Δrtn was also complemented by VC1086*; therefore, no conclusion could be drawn for Rtn, as it may be PDE activity or another regulatory function underlying the phenotype. The cause of the biofilm defects in the ΔyfgF, ΔyfeA, and ΔyhjK (PDE and DGC) mutants were also undetermined, as induction of both VC1086 and the WspR-encoded DGC was able to complement these mutations; therefore, no conclusion could be drawn as to which domain is enzymatically active under this condition (see Fig. S2A and B).
Curli and cellulose biosynthesis.
In environmental E. coli biofilms, the extracellular matrix may be comprised of cellulose and curli, which are both regulated by [c-di-GMP] in E. coli K-12 and Salmonella strains (16, 17). Using E. coli strain UTI89 as a positive control for curli production (18), all 32 mutants, along with wild-type E. coli CFT073, were tested for curli biosynthesis during culture on YESCA agar containing Congo red. Plates were incubated for 24 h at 30° or 37°C. Curli production was observed as red growth due to binding of Congo red by the curli amyloid structure (19). Wild-type E. coli CFT073 remained white under all conditions, while E. coli UTI89 turned red at 30°C, demonstrating that this strain produced curli (Fig. 2D). Two mutants in E. coli CFT073, ΔyciR and Δrtn, produced red colonies at 30°C suggesting that YciR and Rtn suppress curli synthesis (Fig. 2D; Table 2). Furthermore, after incubation for 96 h at 30°C, E. coli UTI89 produced the typical red, dry, and rough (rdar) phenotype, whereas wild-type E. coli CFT073 remained white, mucoid, and smooth. The E. coli CFT073 ΔyciR mutant, however, produced a red, mucoid, and rough phenotype, whereas the Δrtn mutant produced the rdar phenotype (Fig. 2D). Thus, it appears that Rtn represses curli synthesis, while YciR may be involved in repression of PGA or cellulose synthesis.
Cellulose production was determined for all 32 mutants and wild-type E. coli CFT073 by observation of white fluorescence under UV light when strains are cultured for 24 h at either 30°C or 37°C on LB agar containing 0.02% Calcofluor white stain. Cellulose was not detected in wild-type E. coli CFT073 at either temperature; however, the ΔyciR mutant (Fig. 2E; Table 2) and the ΔyhdA mutant (Fig. 2F; Table 2) fluoresced at 30°C and at 37°C, respectively. These data suggest that E. coli CFT073, unlike UTI89 or K-12 strains, has developed additional regulatory elements that suppress cellulose and curli biosynthesis, resulting in a different extracellular matrix during biofilm formation.
Phosphodiesterases and diguanylate cyclases regulate E. coli CFT073 adherence to bladder epithelial cells.
Adherence to epithelial cells is a sessile phenotype that is vitally important during urinary tract infection. If E. coli cannot adhere to the bladder or kidney epithelium, the flow of urine may expel the invading pathogen. In general, c-di-GMP increases the synthesis of adhesins utilized for binding to epithelial cell surfaces (10, 20). We tested all 32 mutants for adherence to the immortalized bladder epithelial cell line, UM-UC-3. Thirteen mutants displayed quantitative adherence to bladder cells that was significantly different (P < 0.05) from wild-type E. coli CFT073 (Fig. 3A; Table 2): eight increased adherence (the ΔydiV, Δc1610, Δrtn, and ΔyhjH [PDE] mutants, the ΔyliF [DGC] mutant, and the ΔyfgF, ΔyhdA, and ΔyhjK [PDE/DGC] mutants), and five decreased adherence (the ΔydeH, ΔyeaJ, ΔyneF, and ΔyfiN [DGC] mutants and the ΔyegE [PDE/DGC] mutant).
FIG 3 .
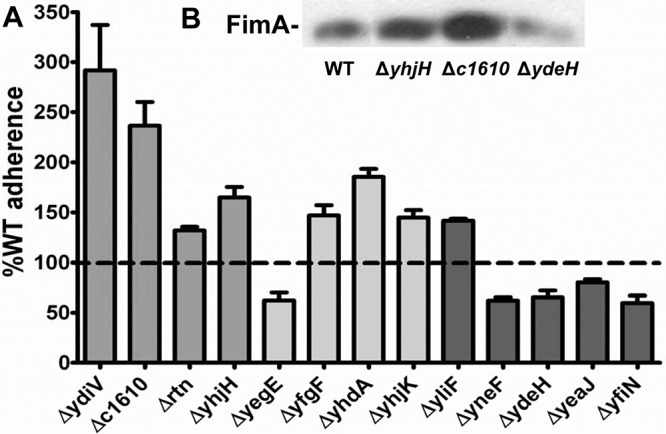
Deletion of PDEs increases and deletion of DGCs decreases adherence to bladder epithelial cells. (A) Relative adherence to the wild-type strain on UM-UC-3 immortalized bladder epithelial cells. For all data, P was <0.05 as assessed by Student’s t test. (B) Western blot of FimA, the main subunit of type 1 fimbriae, in the wild-type strain (WT) and the ΔyhjH, Δc1610, and ΔydeH mutants.
Exogenous phosphodiesterase or diguanylate cyclase activity and adherence of mutants.
To determine if the effect of the mutations on adherence to epithelial cells was due to the modulation of [c-di-GMP], we complemented each mutant with either exogenous PDE (VC1086) or DGC (WspR). Wild-type E. coli CFT073 adherence increased when WspR was induced (122% ± 4% of the adherence of the uninduced strain; P = 0.0146) (see Fig. S3A in the supplemental material), demonstrating that DGC activity promotes adherence to epithelial cells, and decreased when VC1086 was induced (82% ± 4% of the adherence of the VC1086* strain; P = 0.0193) (see Fig. S3B), due to PDE activity. The active PDE VC1086 complemented ΔydiV, ΔyhjH, Δc1610, ΔyfgF, and ΔyhjK, but ΔyhdA and ΔyegE were unaffected (see Fig. S3B). This suggests that YhdA and YegE do not act as PDEs, whereas YdiV, YhjH, C1610, YfgF, and YhjK affect c-di-GMP levels. Interestingly, ΔydiV, which increased motility, biofilm formation in minimal media, and adherence to bladder epithelial cells, was complemented only by VC1086 in adherence assays. This suggests that YdiV may indirectly affect the concentration of c-di-GMP, possibly by repression of an active PDE. The inactive PDE VC1086* did not affect adherence in wild-type E. coli CFT073 or the PDE mutants, with the exception of Δrtn; therefore, no conclusion could be made as to whether [c-di-GMP] contributes to the adherence phenotype of Δrtn.
When the exogenous DGC WspR was induced by 5 mM IPTG, the adherence of wild-type E. coli CFT073 increased as expected. Induction of WspR complemented ΔyfiN, ΔyliF, ΔyegE, ΔyfgF, ΔyhdA, ΔyneF, ΔydeH, and ΔyeaJ (see Fig. S3A). However, ΔyhjK was not complemented, indicating that YhjK is a PDE.
Type 1 fimbria expression is increased in the ΔyhjH and Δc1610 mutants but reduced in the ΔydeH mutant.
Western blots against FimA, the main subunit of type 1 fimbriae, were conducted to determine if expression of these fimbriae is affected in mutants with adherence phenotypes. Three of the 13 mutants revealed levels of FimA different from that of wild-type E. coli CFT073, with the ΔyhjH and Δc1610 strains expressing more and the ΔydeH strain expressing less FimA than the wild type (Fig. 3B). To determine if the adherence effects of ΔydeH, ΔyhjH, and Δc1610 were due to the aberrant expression of type 1 fimbriae, these mutants were constructed in the Fim L-ON background, and adherence of each mutant to UM-UC-3 bladder epithelial cells was compared to that of the Fim L-ON parental strain. In Fim L-ON, the ΔydeH (122% ± 23%, P = 0.1524), ΔyhjH (111% ± 29%, P = 0.5185), and Δc1610 (99% ± 17%, P = 0.9557) strains were not significantly different in adherence from the Fim L-ON parental strain, suggesting that all three mutations affect adherence through type 1 fimbriae.
DISCUSSION
The regulatory network of UPEC involving c-di-GMP is complex, partly due to the large number of DGCs and PDEs encoded in the genome. Although the role of DGCs and PDEs in regulating the switch between motile and sessile lifestyles is being elucidated, one must ask how all of these enzymes affect the concentration of one molecule yet have individualized effects within a single bacterium. That several of the proteins annotated as PDEs or DGCs are degenerate, and may not be enzymatically active, but still are involved in regulation of motility and sessility adds another complication to interpretation. To address these issues, we conducted a comprehensive screen of single-deletion mutants of each PDE-, DGC-, PDE/DGC-, and PilZ-encoding gene in E. coli CFT073 to determine which regulates motility, adherence, biofilm formation, curli production, and cellulose synthesis. Furthermore, complementation of these mutants with an exogenous PDE and/or DGC demonstrated that some of these proteins indeed act by affecting [c-di-GMP], while others act by a [c-di-GMP]-independent mechanism. Finally, strain differences between E. coli K-12 and uropathogenic E. coli CFT073 are apparent in the c-di-GMP regulatory network, suggesting that c-di-GMP signaling is regulated differently in pathogens than environmental or commensal bacteria.
E. coli CFT073 has 32 genes encoding PDE (n = 11), DGC (n = 13), PilZ (n = 2), and both PDE and DGC (n = 6) domains, whereas E. coli K-12 has 31 genes encoding PDE (n = 10), DGC (n = 12), PilZ (n = 2), and both PDE and DGC (n = 7) domains (21). While 29 of these genes are shared between the two strains, only E. coli CFT073 has c1246 (PDE), c1918, and c1919 (DGCs), and only E. coli K-12 has dos-yddU (PDE/DGC) and yddV (DGC). Several of the 29 shared proteins function differently in these two backgrounds to control motility and sessility. Thus, the role of c-di-GMP in regulation of the transition from motile to sessile states in E. coli CFT073 has diverged, in some cases, from that of E. coli K-12.
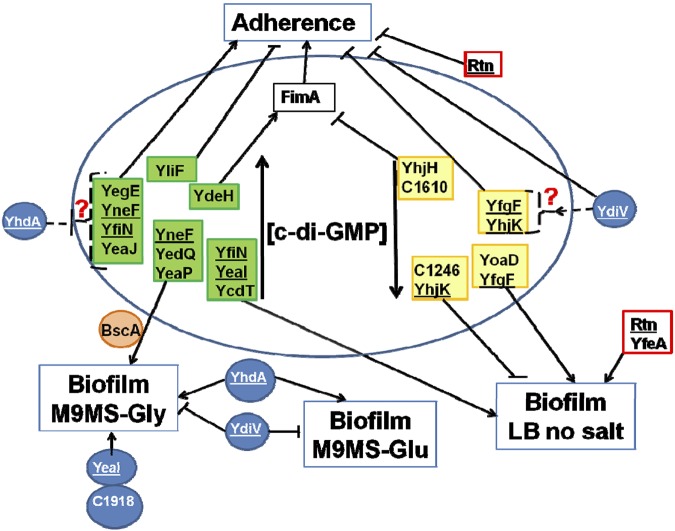
The enzymatic activity of some PDEs and DGCs in E. coli K-12 strains has been verified, and since these proteins are highly conserved (96 to 100% amino acid sequence identity) between E. coli K-12 and E. coli CFT073, their enzymatic activity can be inferred. YfgF, YhjH, and YciR, for example, are experimentally verified active phosphodiesterases (17, 22, 23), while YdeH, YfiN, YedQ, YeaP, YeaI, and YcdT have verified diguanylate cyclase activity (24–27). YhdA/CsrD is enzymatically inactive, and BscA and YcgR are known to bind c-di-GMP through their PilZ domains (28). By complementing each deletion that had a phenotype with either WspR (a known active DGC) or VC1086 (a known active PDE), we determined whether the effect of each deletion was due to enzymatic activity that caused a change in the [c-di-GMP]. We were able to complement ΔydeH, ΔyliF, ΔyegE, ΔyneF, ΔyfiN, ΔyeaJ, ΔyedQ, ΔyeaP, ΔyeaI, and ΔycdT with expression of WspR (Fig. 4). Of this group, all six DGCs with verified diguanylate cyclase activity were complementable, demonstrating that the phenotypes were likely mediated by a decrease in [c-di-GMP]. Expression of VC1086 complemented ΔyhjH, ΔyfgF, ΔyhjK, ΔyoaD, Δc1610/ycgG, and Δc1246 (Fig. 4), and since both YhjH and YfgF have verified PDE activity, this demonstrates that these are active phosphodiesterases.
FIG 4 .
Model of the regulatory network based on the finding of this study. The sessile phenotypes adherence and biofilm formation are regulated by both active PDEs and DGC affecting the level of c-di-GMP, as well as inactive enzymes acting through c-di-GMP-independent pathways. Green indicates active DGC, yellow indicates active PDE, and blue indicates inactive enzymes. Red boxes indicate unknown activity. Orange indicates the PilZ domain. Pointed arrows indicate activation, and blunt arrows indicate repression.
From a screening of all 32 deletions of PDE, DGC, PDE/DGC, and PilZ domain-encoding genes for effects on motility in E. coli CFT073, three were implicated in regulation of motility, all of which encode degenerate PDE domains. YhdA and YcgF promote motility, whereas YdiV acts as a repressor of motility. These proteins affect motility through a mechanism independent of effects on [c-di-GMP], as expression of an active PDE could not complement the observed effects. All three degenerate PDEs affect the expression of FliC, the main subunit comprising flagella. Epistatic analysis demonstrates that YcgF and YdiV affect motility in a shared regulatory pathway, with the increased motility phenotype of ΔydiV masking the decreased motility of ΔycgF. On the other hand, ΔyhdA had an additive effect on motility with ΔydiV, demonstrating that these mutations affect motility via different regulatory pathways. Interestingly, no functional DGCs or PDEs appear to be involved in regulation of motility, which is very different from E. coli K-12, in which deletion of the DGC-encoding genes ycdT, yegE, ydeH, yeaI, yeaJ, yneF, yfiN, and yedQ increased swimming motility (21, 23, 24, 26) and deletion of yhjH and ycgR reduced motility (29, 30). Thus, E. coli CFT073 diverged from E. coli K-12 to regulate motility in such a way that single deletions of active PDEs or DGCs do not affect motility.
The focus of current research is on active PDEs and DGCs and the effect of fluctuations in [c-di-GMP] mediated by these enzymes; however, enzymatically inactive degenerate domains also clearly mediate phenotypic changes. For instance, in P. fluorescens, LapD, an enzymatically inactive PDE/DGC, binds c-di-GMP with the degenerate PDE domain and mediates signal transduction to cause the sequestration of a protease, LapG, thus preventing cleavage of an adhesin LapA (10, 31). Furthermore, YdiV, the degenerate PDE protein that inhibited motility in our study, also inhibits motility in Salmonella enterica serovar Typhimurium and E. coli K-12 by binding to FlhD, causing a decrease in fliA transcription (32–34). However, there are strain differences between E. coli K-12 and CFT073. In E. coli K-12, deletion of ydiV does not affect motility, since this strain does not naturally express ydiV (32), whereas E. coli CFT073 ΔydiV is significantly more motile than the wild type (reference 13 and this study). In E. coli K-12, ydiV expression is inhibited posttranscriptionally (15, 32), which was postulated to be due to a secondary structure formed in the 5′ untranslated region occluding the translational start site, since this structure does not occur in Salmonella, where ydiV is fully expressed (32). By alignment of the intergenic region between ydiV and the closest gene upstream in E. coli K-12 and CFT073, we found there is a 1-bp difference between the two strains that would improve the predicted secondary structure, not abolish it. Therefore, the absence of YdiV in E. coli K-12 cannot solely be due to the secondary structure in the transcript.
High [c-di-GMP] induces sessile phenotypes, such as adherence to epithelial cells or biofilm formation on abiotic surfaces in bacteria (35). In E. coli CFT073, 13 deletions affected adherence to the bladder epithelial cells, indicating that these proteins may play a role in regulation of adherence factors important for colonization of the human bladder. Of the eight mutations that increased adherence to bladder epithelial cells, five (ΔydiV, ΔyhjH, Δc1610, ΔyfgF, and ΔyhjK) were complemented by expression of a functional PDE, demonstrating that YhjH, C1610, YfgF, and YhjK inhibit adherence by directly decreasing [c-di-GMP] and that YdiV indirectly decreases [c-di-GMP] (Fig. 4). The five mutations that decreased adherence to epithelial cells, ΔydeH, ΔyeaJ, ΔyneF, ΔyfiN, and ΔyegE, were complemented by expression of an active DGC, suggesting that these enzymes promote adherence by increasing [c-di-GMP]. This is consistent with increased [c-di-GMP] in the bacterial cell inducing and decreased [c-di-GMP] repressing the sessile lifestyle. Furthermore, ΔydeH, Δc1610, and ΔyhjH affect adherence to bladder cells through regulation of type 1 fimbriae (Fig. 4). In the Crohn’s disease-associated adherent-invasive E. coli strain LF82, YhjH activates expression of type 1 fimbriae through the degradation of c-di-GMP (20). The mechanism(s) by which the ten other mutations affect adherence has yet to be elucidated.
Both degenerate and functional PDEs and DGCs affect biofilm formation in a medium-specific manner, as the analysis of the effect of the mutations on biofilm formation does not perfectly conform to dogma for all conditions tested. For example, in LB without salt, YcdT, YeaI, and YfiN (active DGCs) promote and C1246 and YhjK (active PDEs) inhibit biofilm formation, conforming to the hypothesis that increased [c-di-GMP] induces biofilm formation (Fig. 4). However, YoaD and YfgF (active PDEs) promote biofilm formation, contrary to this hypothesis. Carbon source had an effect in M9 minimal medium, where biofilm formation is regulated by YdiV and YhdA through a [c-di-GMP]-independent mechanism in glucose (Fig. 4). However, in glycerol, both [c-di-GMP]-independent regulation (YdiV inhibited and YhdA, C1918, and YeaI promoted biofilm formation) and a [c-di-GMP]-dependent mechanism occur (YneF, YedQ, and YeaP [active DGC] and BscA [PilZ] promoted biofilm formation) (Fig. 4). These findings are in direct contrast with results from E. coli K-12 strain BW25113, where YfiN, YedQ, and YeaI inhibit biofilm formation (26). Thus, these proteins have evolved to regulate the same phenotype by disparate mechanisms in UPEC and commensal E. coli.
In this work, we demonstrated that active PDEs and DGCs regulate adherence to host cells and biofilm formation but do not regulate motility. Instead, motility is regulated in a [c-di-GMP]-independent manner by three enzymatically inactive PDEs. The roles of enzymatically active and inactive PDEs and DGCs in the transition between sessile and motile lifestyles have diverged in E. coli CFT073 from E. coli K-12. Future studies will determine the molecular mechanism(s) by which these active and inactive enzymes modulate motility and sessility of UPEC.
MATERIALS AND METHODS
Construction and complementation of mutants for PDE, DGC, PDE/DGC, and PilZ domain-encoding genes.
Deletion mutants were constructed in E. coli CFT073 using the lambda red recombinase system as described by Datsenko and Wanner (36). Primers containing sequences homologous to the 5′ and 3′ ends of the target sequence were designed and used to amplify the resistance cassette from either template plasmid pKD3 or pKD4 (encoding chloramphenicol or kanamycin resistance, respectively). Lambda red-mediated recombination was used to replace each PDE-, DGC-, PDE/DGC-, or PilZ-encoding gene individually with these PCR products. Primers homologous to flanking regions of each gene were designed for confirmation of replacement.
Motility assays.
Motility was evaluated for each mutant in soft agar plates (1% tryptone, 0.5% NaCl, and 0.25% agar) and compared to the parental wild-type strain as described previously (37). Briefly, mutants were cultured overnight in LB broth, used to inoculate 5 ml of sterile LB broth, and incubated at 37°C with aeration to an OD600 of 1.0 to 1.2. Cultures were standardized to an OD600 of 1.0 and used to stab the center of soft agar plates with an inoculating needle. Plates were incubated for 16 h at 30°C, at which time the diameter of motility was measured. Diameters are directly correlated with bacterial motility (38). Motility of the complemented mutants was examined similarly, but the medium and the soft agar contained ampicillin (100 µg/ml) for maintenance of the plasmid. Wild-type E. coli CFT073 and each mutant transformed with the empty plasmid pGEN-MCS were included as controls.
Western blots to detect flagella and type 1 fimbriae.
All blots were developed using chemiluminescence according to the manufacturer’s instructions (Amersham ECL Plus; GE Healthcare Life Sciences).
Samples to detect FliC, the main subunit of flagella, were prepared as described previously (13) with the following modification. Sample lysates (10 µl) were electrophoresed on a 12.5% denaturing sodium dodecyl sulfate (SDS)-polyacrylamide gel before being transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). Blots were incubated with a 1:40,000 dilution of rabbit polyclonal antiserum to H1 flagella (Statens Serum Institute, Copenhagen, Denmark), followed by a 1:20,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma). Blots were developed using chemiluminescence according to the manufacturer’s instructions (Amersham ECL Plus; GE Healthcare Life Sciences).
Samples to detect FimA, the main subunit of type 1 fimbriae, were prepared as described previously (13) with the following modification. Sample lysates were electrophoresed on a 12.5% denaturing SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). Blots were incubated with a 1:40,000 dilution of rabbit polyclonal antiserum to FimA (Statens Serum Institute, Copenhagen, Denmark), followed by a 1:20,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma).
Detection of curli and cellulose production.
For detection of curli, bacteria were streaked onto YESCA agar (1 g/liter yeast extract, 10 g/liter Casamino Acids, 20 g/liter agar) containing 50 µg/ml Congo red (Sigma). Plates were incubated for 24 h at either 37° or 30°C. Red colonies were indicative of curli production (19). To detect cellulose production, bacteria were streaked onto low salt (0.5 g NaCl/liter) LB agar containing 0.02% Calcofluor white stain (Sigma). Plates were incubated for 24 or 48 h at either 37° or 30°C. White fluorescence of the colonies under UV light was indicative of cellulose production (19).
Adherence assays.
Cell culture and adherence assays were performed as described in reference 39 using the immortalized bladder epithelial cell line UM-UC-3 (ATCC CRL-1749). Adherence was expressed as cell-associated CFU/initial CFU/well, and each mutant was normalized to the wild-type control. All assays were performed in triplicate.
Biofilm assays.
Briefly, the wild-type strain and the mutants were cultured statically overnight in LB and normalized to an OD600 of 1.0. Bacteria were collected by centrifugation and resuspended in M9 minimal salts medium (0.2% glucose or 0.4% glycerol as the carbon source) or LB medium without salt. Cultures were diluted 1:100 into fresh medium and cultured statically for 24 h at 37°C in 96-well polystyrene plates. The culture concentration for each strain was estimated by spectrophotometry at OD600 in a plate reader (μQuant; Bio-Tek Instruments, Inc.). Cultures were removed by aspiration, and the plates were washed once with sterile distilled H2O to remove unbound bacteria and stained with crystal violet (0.7% solution) for 3 min. Quantification was conducted by dissolving the crystal violet in 100% ethanol (100 µl) for 15 min, and plates were read at OD570 on a plate reader. To control for any growth defects in the mutant strains and normalize data between experiments, data are expressed as OD570/OD600 relative to the wild-type strain.
SUPPLEMENTAL MATERIAL
Complementation of biofilm phenotypes in M9 minimal medium by expression of the exogenous PDE VC1086 or DGC WspR. (A) Complementation of ΔyhdA by WspR in M9 minimal medium with 0.2% glucose as the carbon source. Asterisks indicate a statistically significant difference (P < 0.05) from the data for uninduced wild type. (B) Complementation of ΔyhdA and ΔydiV by VC1086 in M9 minimal medium with 0.2% glucose as the carbon source. Asterisks indicate a statistically significant difference (P < 0.05) from the wild type complemented with the inactive site directed mutant of VC1086 (EAL*). (C) Complementation by WspR of ΔyhdA, ΔyeaI, Δc1918, ΔyeaP, ΔyneF, and ΔyedQ in M9 minimal medium with 0.4% glycerol as the carbon source. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the uninduced wild type. Download Figure S1, TIF file, 0.6 MB.
Complementation of biofilm phenotypes in no-salt LB medium by expression of the exogenous DGC WspR (A) or PDE VC1086 (B). (A) Complementation of ΔyfiN, ΔyeaI, ΔycdT, ΔyhjK, ΔyfeA, and ΔyfgF by induction of WspR in no-salt LB medium. Asterisks indicate a statistically significant difference (P < 0.05) from the uninduced wild type. (B) Complementation of ΔyhjK, ΔyfeA, ΔyfgF, Δrtn, ΔyoaD, and Δc1246 by VC1086 in no-salt LB medium. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the wild type complemented with the inactive site-directed mutant of VC1086 (EAL*). Download Figure S2, TIF file, 0.9 MB.
Complementation of adherence phenotypes on UM-UC-3 bladder epithelial cells by expression of the exogenous DGC WspR (A) or PDE VC1086 (B). (A) Complementation of ΔyegE, ΔyfgF, ΔyhdA, ΔyhjK, ΔyliF, ΔyneF, ΔydeH, ΔyeaJ, and ΔyfiN by induction of WspR. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the uninduced wild type. (B) Complementation of ΔydiV, ΔyhjH, Δc1610, Δrtn, ΔyhdA, ΔyegE, ΔyfgF, and ΔyhjK by VC1086. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the value for the wild type complemented with the inactive site-directed mutant of VC1086 (EAL*). Download Figure S3, TIF file, 1.3 MB.
ACKNOWLEDGMENTS
We thank Christopher Waters for providing advice, quantification of c-di-GMP and plasmids, Stephen Lory for plasmids, and Sara Smith for conducting mouse infections.
This work was supported in part by Public Health Service grants AI059722 and DK094777 from the National Institutes of Health. We have no conflict of interest to report.
Footnotes
Citation Spurbeck RR, Tarrien RJ, and Mobley HLT. 2012. Enzymatically active and inactive phosphodiesterases and diguanylate cyclases are involved in regulation of motility or sessility in Escherichia coli CFT073. mBio 3(5):e00307-12. doi:10.1128/mBio.00307-12.
REFERENCES
- 1. Foxman B. 2003. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 49:53–70 [DOI] [PubMed] [Google Scholar]
- 2. Jenal U. 2004. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol. 7:185–191 [DOI] [PubMed] [Google Scholar]
- 3. Simm R, Morr M, Kader A, Nimtz M, Römling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123–1134 [DOI] [PubMed] [Google Scholar]
- 4. Tal R, et al. 1998. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J. Bacteriol. 180:4416–4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu X, Beyhan S, Lim B, Linington RG, Yildiz FH. 2010. Identification and characterization of a phosphodiesterase that inversely regulates motility and biofilm formation in Vibrio cholerae. J. Bacteriol. 192:4541–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolfe AJ, Visick KL. 2008. Get the message out: cyclic-di-GMP regulates multiple levels of flagellum-based motility. J. Bacteriol. 190:463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mobley HL, et al. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Welch RA, et al. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020–17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryan RP, Tolker-Nielsen T, Dow JM. 2012. When the PilZ don’t work: effectors for cyclic di-GMP action in bacteria. Trends Microbiol. 20:235–242 [DOI] [PubMed] [Google Scholar]
- 10. Newell PD, Monds RD, O’Toole GA. 2009. LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc. Natl. Acad. Sci. U. S. A. 106:3461–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burdette DL, et al. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yin Q, et al. 2012. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol. Cell 46:735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simms AN, Mobley HL. 2008. Multiple genes repress motility in uropathogenic Escherichia coli constitutively expressing type 1 fimbriae. J. Bacteriol. 190:3747–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hickman JW, Tifrea DF, Harwood CS. 2005. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. U. S. A. 102:14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waters CM, Lu W, Rabinowitz JD, Bassler BL. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 190:2527–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simm R, Lusch A, Kader A, Andersson M, Römling U. 2007. Role of EAL-containing proteins in multicellular behavior of Salmonella enterica serovar typhimurium. J. Bacteriol. 189:3613–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber H, Pesavento C, Possling A, Tischendorf G, Hengge R. 2006. Cyclic-di-GMP-mediated signalling within the sigma network of Escherichia coli. Mol. Microbiol. 62:1014–1034 [DOI] [PubMed] [Google Scholar]
- 18. Kostakioti M, Hadjifrangiskou M, Pinkner JS, Hultgren SJ. 2009. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol. Microbiol. 73:1020–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bokranz W, Wang X, Tschäpe H, Römling U. 2005. Expression of cellulose and curli fimbriae by Escherichia coli isolated from the gastrointestinal tract. J. Med. Microbiol. 54:1171–1182 [DOI] [PubMed] [Google Scholar]
- 20. Claret L, et al. 2007. The flagellar sigma factor FliA regulates adhesion and invasion of Crohn disease-associated Escherichia coli via a cyclic dimeric GMP-dependent pathway. J. Biol. Chem. 282:33275–33283 [DOI] [PubMed] [Google Scholar]
- 21. Sommerfeldt N, et al. 2009. Gene expression patterns and differential input into curli fimbriae regulation of all GGDEF/EAL domain proteins in Escherichia coli. Microbiology 155:1318–1331 [DOI] [PubMed] [Google Scholar]
- 22. Lacey MM, Partridge JD, Green J. 2010. Escherichia coli K-12 YfgF is an anaerobic cyclic di-GMP phosphodiesterase with roles in cell surface remodelling and the oxidative stress response. Microbiology 156:2873–2886 [DOI] [PubMed] [Google Scholar]
- 23. Pesavento C, et al. 2008. Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev. 22:2434–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jonas K, et al. 2008. The RNA binding protein CsrA controls cyclic di-GMP metabolism by directly regulating the expression of GGDEF proteins. Mol. Microbiol. 70:236–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 187:1792–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanchez-Torres V, Hu H, Wood TK. 2011. GGDEF proteins YeaI, YedQ, and YfiN reduce early biofilm formation and swimming motility in Escherichia coli. Appl. Microbiol. Biotechnol. 90:651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zähringer F, Massa C, Schirmer T. 2011. Efficient enzymatic production of the bacterial second messenger c-di-GMP by the diguanylate cyclase YdeH from E. coli. Appl. Biochem. Biotechnol. 163:71–79 [DOI] [PubMed] [Google Scholar]
- 28. Ryjenkov DA, Simm R, Römling U, Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281:30310–30314 [DOI] [PubMed] [Google Scholar]
- 29. Ko M, Park C. 2000. Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J. Mol. Biol. 303:371–382 [DOI] [PubMed] [Google Scholar]
- 30. Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. 2010. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol. Cell 38:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Navarro MV, et al. 2011. Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol. 9:e1000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wada T, Hatamoto Y, Kutsukake K. 2012. Functional and expressional analyses of the anti-FlhD4C2 factor gene ydiV in Escherichia coli. Microbiology 158:1533–1542 [DOI] [PubMed] [Google Scholar]
- 33. Wada T, et al. 2011. EAL domain protein YdiV acts as an anti-FlhD4C2 factor responsible for nutritional control of the flagellar regulon in Salmonella enterica serovar typhimurium. J. Bacteriol. 193:1600–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wozniak CE, Lee C, Hughes KT. 2009. T-POP array identifies EcnR and PefI-SrgD as novel regulators of flagellar gene expression. J. Bacteriol. 191:1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Lünsdorf H, Ehrén I, Brauner A, Römling U. 2010. Characteristics of biofilms from urinary tract catheters and presence of biofilm-related components in Escherichia coli. Curr. Microbiol. 60:446–453 [DOI] [PubMed] [Google Scholar]
- 36. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lane MC, et al. 2005. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 73:7644–7656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper LA, Simmons LA, Mobley HL. 2012. Involvement of mismatch repair in the reciprocal control of motility and adherence of uropathogenic Escherichia coli. Infect. Immun. 80:1969–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spurbeck RR, et al. 2011. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect. Immun. 79:4753–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complementation of biofilm phenotypes in M9 minimal medium by expression of the exogenous PDE VC1086 or DGC WspR. (A) Complementation of ΔyhdA by WspR in M9 minimal medium with 0.2% glucose as the carbon source. Asterisks indicate a statistically significant difference (P < 0.05) from the data for uninduced wild type. (B) Complementation of ΔyhdA and ΔydiV by VC1086 in M9 minimal medium with 0.2% glucose as the carbon source. Asterisks indicate a statistically significant difference (P < 0.05) from the wild type complemented with the inactive site directed mutant of VC1086 (EAL*). (C) Complementation by WspR of ΔyhdA, ΔyeaI, Δc1918, ΔyeaP, ΔyneF, and ΔyedQ in M9 minimal medium with 0.4% glycerol as the carbon source. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the uninduced wild type. Download Figure S1, TIF file, 0.6 MB.
Complementation of biofilm phenotypes in no-salt LB medium by expression of the exogenous DGC WspR (A) or PDE VC1086 (B). (A) Complementation of ΔyfiN, ΔyeaI, ΔycdT, ΔyhjK, ΔyfeA, and ΔyfgF by induction of WspR in no-salt LB medium. Asterisks indicate a statistically significant difference (P < 0.05) from the uninduced wild type. (B) Complementation of ΔyhjK, ΔyfeA, ΔyfgF, Δrtn, ΔyoaD, and Δc1246 by VC1086 in no-salt LB medium. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the wild type complemented with the inactive site-directed mutant of VC1086 (EAL*). Download Figure S2, TIF file, 0.9 MB.
Complementation of adherence phenotypes on UM-UC-3 bladder epithelial cells by expression of the exogenous DGC WspR (A) or PDE VC1086 (B). (A) Complementation of ΔyegE, ΔyfgF, ΔyhdA, ΔyhjK, ΔyliF, ΔyneF, ΔydeH, ΔyeaJ, and ΔyfiN by induction of WspR. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the uninduced wild type. (B) Complementation of ΔydiV, ΔyhjH, Δc1610, Δrtn, ΔyhdA, ΔyegE, ΔyfgF, and ΔyhjK by VC1086. Asterisks indicate a statistically significant difference (P < 0.05) from the value for the value for the wild type complemented with the inactive site-directed mutant of VC1086 (EAL*). Download Figure S3, TIF file, 1.3 MB.