Abstract
The author reviewed pathologic features of 37 cases of malignant lymphoma in the gastrointestinal organs in the last 10 years in our pathology laboratory. The current WHO classification was adopted. The 37 cases consisted of 20 males and 17 female, and the age ranged from 46 to 89 years with a median of 69 years. Of the 37 cases, 25 cases (68%) were gastric lymphomas, 6 cases (16%) were small intestinal lymphomas, and 6 cases (16%) were colon lymphomas. Of the 37 cases, 35 cases (95%) were B-cell neoplasms and 2 cases (5%) were T-cell neoplasms. In the 25 gastric lymphomas (male:female=14:11, age range 46-84 years, median 70 years) 11 cases were diffuse large B-cell lymphomas, and 14 cases were extranodal marginal zone B-cell lymphomas (MALT lymphomas). The clinical endoscopic diagnosis was gastritis in 3, gastric ulcer in 3, gastric carcinoma in 7, carcinoid in 1, submucosal tumor in 1, malignant lymphoma in 2, and suspected MALT lymphoma in 8. In the 6 small intestinal lymphomas (male:female=2:4, age range 49-89 years, median 70 years), all cases were located in the ileum. Of the 6 cases, 4 were diffuse large B-cell lymphoma and 2 were peripheral T-cell lymphoma. One case showed multiple lymphomas, and one case was associated with rectal adenocarcinoma and one case with gastric MALT lymphoma. The clinical diagnosis was adenocarcinoma in 2, suspected lymphoma in 2, and ileal tumor in 2. In the 6 colon lymphomas (male:female=4:2, age range 69-86 years, median 74 years), 5 cases were diffuse large B-cell lymphomas and one case was follicular lymphoma. Clinical endoscopic diagnosis was GIST in 1, colon carcinoma in 4, and colon polyp in 1. Cases of Hodgkin’s disease, mantle cell lymphoma, Burkitt lymphoma were not recognized in the present series. In summary, the author reported pathologic features of 37 cases of gastrointestinal malignant lymphoma in our laboratory in the last 10 years.
Keywords: Malignant lymphoma, gastrointestinal organs
Introduction
Malignant lymphoid neoplasms are classified into Hodgkin’s disease and non-Hodgkin malignant lymphoma [1]. Hodgkin’s disease is uncommon in Japan. Non-Hodgkin malignant lymphomas are classified into nodal and extranodal lymphoma [1-5]. Gastrointestinal organs are most common sites of extra-nodal malignant lymphoma, accounting for 5-10% of all non-Hodgkin lymphomas [1-5]. Several comprehensive studies of gastrointestinal malignant lymphoma have been performed [6-11]. However, the interpretation of the data of previous studies is difficult because the classification of lymphomas varied in several times owing to advances in immunohistochemical and genetics analyses. The author herein reports 37 cases of gastrointestinal malignant lymphoma in the last 10 years in our pathology laboratory.
Materials and methods
The author surveyed and re-examined malignant lymphomas in the last 10 years in our pathology laboratory. In gastrointestinal malignant lymphoma cases, the histopathological and immunohistochemical studies were performed.
The immunohistochemical study was performed using Dako Envision methods (Dako Corp. Glostrup, Denmark), as previously described [12,13]. The antibododies used were anti-cytokeratin (AE1/3, Dako), anti-cytokeratin (polyclonal wide, Dako), CD3 (M7193, Dako), CD5 (DK23, Dako), CD10 (M0727, Dako), CD15 (M0733, Dako), CD30 (M0751, Dako), CD45 (M0855, DAKO), CD45RO (UCHL-1, Dako), CD79α (M7050, Dako), CD56 (MOC-1, Dako), kappa light chain (polyclonal, Dako), lamda light chain (polyclonal, Dako), bcl-2 (124, Dako), cyclin D1 (DCS-22, Dako), TdT (HT-1, Dako), p53 protein (DO-7, Dako), and Ki-67 antigen (MIB-1, Dako). In some cases, Giemsa stain was performed to identify Helicobactor pylori bacteria. Of these, CD10, CD20 and CD79α are B-cell markers, CD3 and CD45RO are T-cell markers, and CD56 is NK cell markers [1]. Anti-cytokeratin antibodies and CD45 were employed to differentiate between lymphoma and carcinoma. CD15 and CD30 were performed to identify Hodgkin cells and anaplastic large cell lymphoma. Kappa and lamda chains were done to identify light chain restriction. CD5, CD10, cyclinD1, and bcl-2 were used to determine low grade lymphomas, such as extranodal marginal zone B-cell lymphoma (MALT lymphoma), mantle cell lymphoma, and follicular lymphoma. TdT was employed to determine lymphoblastic lymphoma.
Results
There are 181 cases of malignant lymphoma in that period. Of these, 37 cases (20%) were gastrointestinal malignant lymphomas. Hodgkin’s diseases were not present in the 37 gastrointestinal lymphomas. The WHO histological classification was adopted [1-5].
The 37 cases consisted of 20 males and 17 female, and the age ranged from 46 to 89 years with a median of 69 years. Of the 37 cases, 25 cases (68%) were gastric lymphomas, 6 cases (16%) were small intestinal lymphomas, and 6 cases (16%) were colon lymphomas. Of the 37 cases, 35 cases (95%) were B-cell neoplasms and 2 cases (5%) were T cell neoplasms. The presenting symptom was abdominal pain in 15 cases, abdominal discomfort in 6 cases, nausea and vomiting in 4 cases, diarrhea in 4 cases, constipation in 2 cases, occult blood in feces in 4 cases, and asymptomatic in 2 cases.
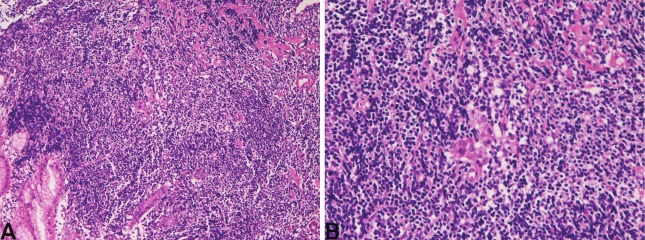
In t he 25 gastr i c l ymphomas (male:female=14:11, age range 46-84 years, median 70 years), 11 cases were diffuse large B-cell lymphomas, and 14 cases were extranodal marginal zone B-cell lymphomas (MALT lymphomas). The clinical endoscopic diagnosis was gastritis in 3, gastric ulcer in 3, gastric carcinoma in 7, carcinoid in 1, submucosal tumor in 1, malignant lymphoma in 2, and suspected MALT lymphoma in 8. Grossly, diffuse large B-cell lymphoma was characterized by ulcerated lesions in 8, submucosal lesions in 2, linitis plastica lesion in 1. Histologically, diffuse large B-cell lymphoma showed diffuse proliferation of large malignant lymphocytes with vesicular nuclei and nucleoli. Immunohistochemically, it is characterized by positive reactions for B-cell markers (CD20, CD79α) and negative reactions for T-cell markers (CD3 and CD45RO) and NK-cell markers (CD56). TdT was negative. Grossly, MALT lymphoma showed gastritis-like flat lesions, slightly depressed lesions, ulceration, and elevated lesions. Histologically, MALT lymphoma was characterized by proliferation of centrocyte-like cells, germinal centers, plasma cell infiltration, and lymphoepithelial lesions (Figures 1A and 1B). Infection of Helicobacter pylori was present in 7/8 (88%) examined. There were several surgical cases whose clinical diagnosis was gastric carcinoma but the pathologic diagnosis was malignant lymphoma. Of the 14 cases of MALT lymphoma, 10 developed in antrum and 4 in body. In the 11 cases of diffuse large B-cell lymphoma, the location was antrum in 7 cases, body in 4 cases.
Figure 1.
MALT lymphoma of the stomach. A: Centrocyte-like neoplastic cell proliferation is recognized. HE x40. B: Lymphoepithelial lesions are seen. HE x200.
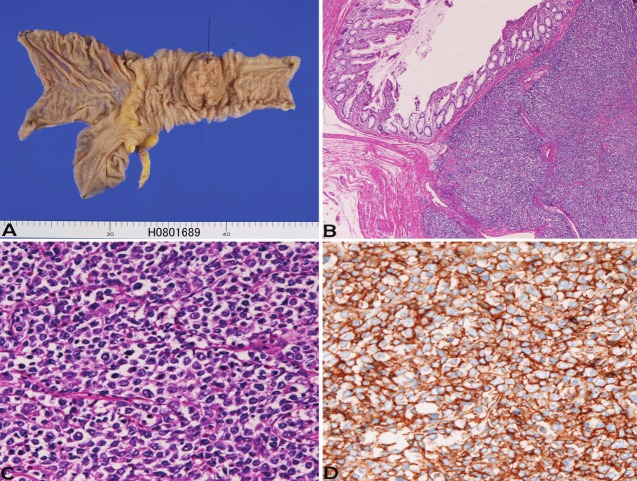
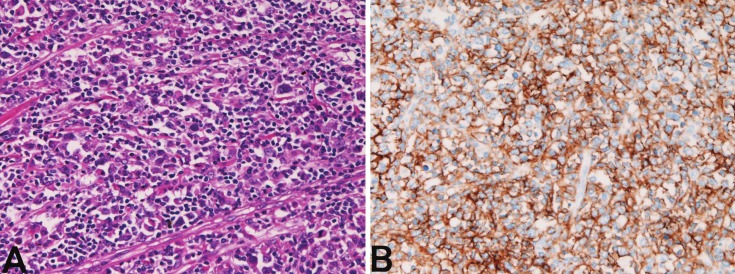
In the 6 small intestinal lymphomas (male:female=2:4, age range 49-89 years, median 70 years), all cases were located in the ileum. Of the 6 cases, 4 were diffuse large B-cell lymphoma and 2 were peripheral T-cell lymphoma. One case showed multiple lymphomas, one case was associated with rectal adenocarcinoma, and one case was associated with gastric MALT lymphoma. The clinical diagnosis was adenocarcinoma in 2, suspected lymphoma in 2, and ileal tumor in 2. Grossly, all the 6 cases with small intestinal lymphomas showed large ulcerated lesions similar to carcinoma (Figure 2A). The morphologies of diffuse large B-cell lymphoma are shown in Figures 2B and 2C. The 2 cases of peripheral T-cell lymphoma showed large irregular nuclei and positive for T-cell markers (Figure 3A and 3B).
Figure 2.
Diffuse large B-cell lymphoma of terminal ileum. A: Gross features indicate ulcerated large tumor (arrow). B: Low power view of the lymphoma. HE x20. C: Tumor cells have vesicular hyperchromatic nuclei and nucleoli. HE x200. D: Tumor cells are positive for CD20. Immunostaining x200.
Figure 3.
Peripheral T-cell lymphoma of the small intestine. A: Tumor cells with atypia. HE x200. B: Tumor cells arepositive for CD3. Immunostaining, x200.
In the 6 colon lymphomas (male:female=4:2, age range 69-86 years, median 74 years), five cases were diffuse large B-cell lymphomas, and one case was follicular lymphoma (Figure 4) in 1. Clinical endoscopic diagnosis was GIST in 1, colon carcinoma in 4, and colon polyp in 1. The locations were ascending colon in 1, sigmoid colon in 3, and rectum in 2. Grossly, 4 cases were ulcerated lesions and 2 cases were polypoid lesions. Histologically, diffuse large B-cell lymphoma and follicular lymphoma showed typical features. The follicular lymphoma was unique. The lymphoma showed multiple polyps (Figure 4A). Histologically, follicular pattern was recognized (Figure 4B), and bcl-2 was positive (Figure 4C). This case of follicular lymphoma was reported elsewhere [14].
Figure 4.
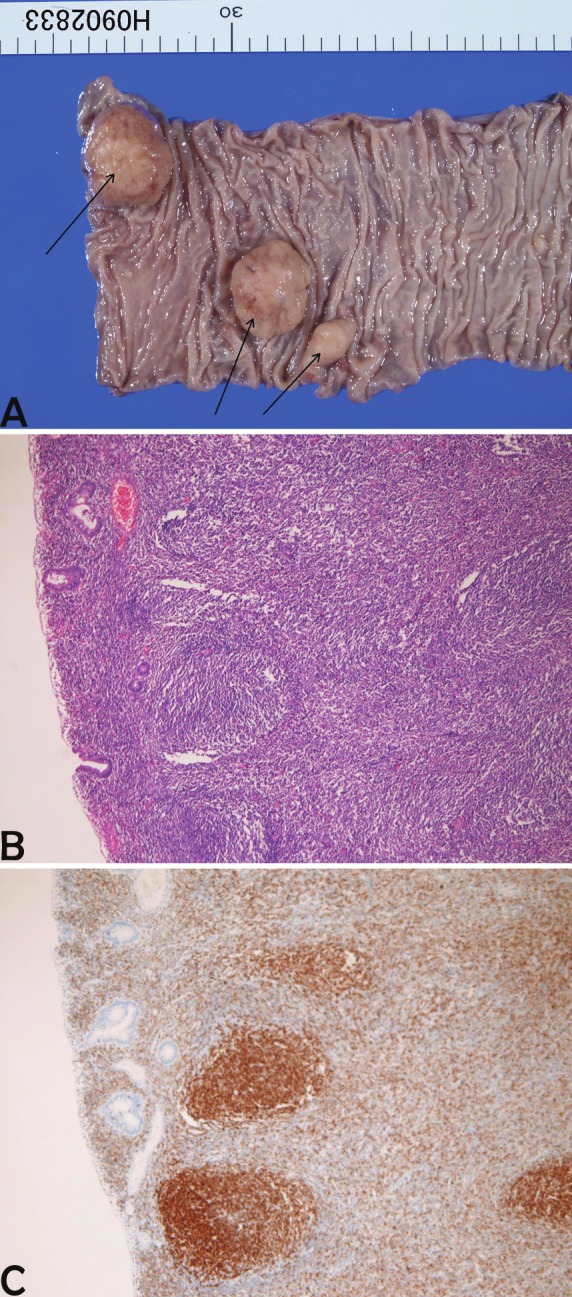
Follicular lymphoma of the colon. A: multiple polyps (arrows) are present. B: The polyp consists of lymphoid proliferation of follicular pattern. HE, x100. C: The follicles are positive for bcl-2. Immunostaining, x100.
There were no cases of Hodgkin’s disease, Mantle cell lymphoma, lymphoblastic lymphoma and Burkitt lymphoma in this series.
Discussion
In the present study, of the 37 cases with gastrointestinal lymphoma, 25 cases (68%) were gastric lymphomas, 6 cases (16%) were small intestinal lymphomas, and 6 cases (16%) were colon lymphomas. No lymphomas were observed in the esophagus and vermiform appendix. Lewin et al. [8], who examined 117 cases of gastrointestinal lymphomas, stated the following incidence: stomach 48/117, small intestine 37/117, ileocecal region 13/117, appendix 2/117, and colon 11/117. Taken together, the most common site of gastrointestinal lymphomas are stomach, followed in order by small intestine, colon, and appendix.
In the present study, of the 37 cases, 35 cases was B-cell lymphoma, and 2 cases were peripheral T-cell lymphomas. No NK/T cell lymphoma was recognized in the present series. Most of gastrointestinal lymphoma has been reported of B-cell type. Grody et al. [6] stated that B cell lymphomas accounted for 84% (21/25) in gastrointestinal lymphomas. Mantle cell lymphoma, lymphoblastic lymphoma, Burkitt lymphoma, and Hodgkin disease [2-5] were not observed in the present study, suggesting that these lymphomas are rare in Japan.
The age of the patients ranged from 46 to 89 years with a median of 69 years in the present study. Other reports also showed that the mean age was around 65 years [1-5]. The presenting symptom was abdominal pain in 15 cases, abdominal discomfort in 6 cases, nausea and vomiting in 4 cases, diarrhea in 4 cases, constipation in 2 cases, occult blood in feces in 4 cases, and asymptomatic in 2 cases, being compatible with other studies [1-5].
The prognosis of gastrointestinal lymphoma was not good [1-5]. The prognosis of diffuse large B-cell lymphoma was poorer than MALT lymphoma [1-5,11]. The present study did not examine the patient’s outcome.
MALT lymphoma is prevalent in the stomach [11]. This relatively new entity (11) was in the past called reactive lymphoid hyperplasia [1-5,11]. This condition is often associated with Helicobacter pylori infection, as was the case of the present study. Eradication of these bacteria often leads to cure of the MALT lymphoma [1-5,11].
It is well known that site of origin and histological subtypes of gastrointestinal lymphoma vary among races and geographical population. For example, it is well known that gastrointestinal lymphomas predominantly affect the stomach and intestinal lymphoma is relatively small in number (Western type), while they predominantly involves the intestine (large and small) but relatively infrequent the stomach in Middle East (Middle Eastern type) [7]. Our hospital is a central hospital covering 300,000 persons in Shizuoka-Shimizu District of Japan. There are no particular referral centers nearby. Patients at first consult our hospital with or without consulting to private clinic. Therefore, there appears no bias of the patients in the present study. Geographical studies of primary malignant lymphoma are very rare. In Kyushu district of Japan [15], Hong Kong [16], Thailand [17] and India [18,] the sites of origin and histological subtypes of gastrointestinal lymphoma are similar to those of the present study.
For example, Nakamura et al [15] in Kyushu University of Japan reported that the location of 455 gastrointestinal lymphomas was stomach in 342 cases (75%), intestine in 96 cases (22%) stomach and intestine in 17 cases (4%). The intestinal lymphoma involved small intestine in 72 cases, and large intestine in 24 cases [15]. This location site is similar to that of the present study.
In their study, the histological subtypes were MALT lymphoma in 200 cases (44%), follicular lymphoma in 27 cases (6%), mantle cell lymphoma in 2 cases (0.4%), plasmacytoma in 2 cases (0.4%), diffuse large B-cell lymphoma in 175 cases (39%), Burkitt lymphoma in 6 cases (1.3%), lymphoblastic lymphoma in 4 cases (0.8%), and T-cell lymphoma in 39 cases (9%) [15]. No Hodgkin’s disease was seen [14]. Thus, the results of their study [15] are similar to those of the present study. The absence of mantle cell lymphoma, plasmacytoma, Burkitt lymphoma, and lymphoblastic lymphoma in the present series is due to the rarity of these lymphomas and due to the small number of cases examined. The locations and histological subtypes of other oriental countries, such as Hong Kong [16], Thailand [17] and India [18] indicated the similar results. As is well known, Hodgkin’s disease is very rare in Orient. Thus, primary gastrointestinal lymphoma of oriental countries belongs to Western type.
In Western countries, gastrointestinal lymphoma is of course of Western type; gastric lymphoma predominates over intestinal lymphoma. For example, a study from UK [19] showed that the most common location of lymphoma is stomach (63%), followed in order by small intestine (23%) and large intestine (13%), findings similar to the present study. The histological type of lymphoma is mostly MALT lymphoma (57%) and diffuse large B-cell lymphoma (21%), and other types of lymphoma, such as Burkitt lymphoma, mantle cell lymphoma, follicular lymphoma, T-cell lymphoma are scant (23%) (19). Similar findings have been reported in Greece [20], Denmark [9], France [21], and USA [11,22].
In summary, the author reported pathologic features of 37 cases of gastrointestinal malignant lymphoma.
References
- 1.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours. Pathology and genetics of tumours of hematopoietic and lymphoid tissues. Ryon: IARC press; 2001. [Google Scholar]
- 2.Wotherspoon A, Chott A, Gascoyne RD, Muller-Hermelink HK. Lymphomas of the stomach. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 57–61. [Google Scholar]
- 3.Gascoyne RD, Muller-Hermelink HK, Chott A, Wotherspoon A. B-cell lymphoma of the small intestine. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 83–86. [Google Scholar]
- 4.Gascoyne RD, Muller-Hermelink HK, Chott A, Wotherspoon A. Intestinal T-cell lymphoma. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 87–89. [Google Scholar]
- 5.Muller-Hermelink HK, Chott A, Gascoyne RD, Wotherspoon A. B-cell lymphoma of the colon and rectum. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 139–141. [Google Scholar]
- 6.Grody WW, Magidson JG, Weiss LM, Hu E, Warnke HA, Lewin KJ. Gastrointestinal lymphomas: Immunohistochemical studies on the cell of origin. Am J Surg Pathol. 1985;9:328–337. [PubMed] [Google Scholar]
- 7.Almasri NM, al-Abbadi M, Rewaily E, Abulkhail A, Tarawneh MS. Primary gastrointestinal lymphomas in Jordan are similar to those in Western countries. Mod Pathol. 1997;10:137–141. [PubMed] [Google Scholar]
- 8.Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tracts: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42:693–707. doi: 10.1002/1097-0142(197808)42:2<693::aid-cncr2820420241>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.d’Amore F, Brincker H, Gronbaek K, Thorling K, Pedersen M, Jensen MK, Andersen E, Pedersen NT, Mortensen LS. Non-Hodgkin’s lymphoma of the gastrointestinal tract. J. Clin. Oncol. 1994;12:1678–1684. doi: 10.1200/JCO.1994.12.8.1673. [DOI] [PubMed] [Google Scholar]
- 10.ZinZani PL, Magagnoli M, Pagliani G, Bendandi M, Gherlinzoni F, Merla E, Salvucci M, Tuna S. Primary intestinal lymphoma: clinical and therapeutic features of 32 patients. Haematologica. 1997;82:305–308. [PubMed] [Google Scholar]
- 11.Isaacson PG. Gastrointestinal lymphomas of T- and B-cell types. Mod Pathol. 1999;12:151–158. [PubMed] [Google Scholar]
- 12.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 13.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 14.Hiraide T, Shoji T, Higashi H, Matsuda I, Terada T. Primary multiple polypoid follicular lymphoma of the sigmoid colon. Gastrointestinal Endoscopy. 2011;73:182–184. doi: 10.1016/j.gie.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: A clinicopathologic analysis of 455 patients with special regerence to its time trend. Cancer. 2003;97:2462–2473. doi: 10.1002/cncr.11415. [DOI] [PubMed] [Google Scholar]
- 16.Liang R, Todd D, Chan TK, Chiu E, Lie A, Kwong YL, Choy D, Ho FC. Prognostic factors for primary gastrointestinal lymphoma. Hematol Oncol. 1995;13:153–163. doi: 10.1002/hon.2900130305. [DOI] [PubMed] [Google Scholar]
- 17.Sukpanichnant S. Analysis of 1983 cases of malignant lymphoma in Thailand according to World Health Organization classification. Hum Pathol. 2004;35:224–230. doi: 10.1016/j.humpath.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Chandran RR, Raj EH, Chaturvedi HK. Primary gastrointestinal lymphoma: 30-year experience at the Cancer Institute, Madros, India. J Surg Oncol. 1995;60:41–49. doi: 10.1002/jso.2930600109. [DOI] [PubMed] [Google Scholar]
- 19.Koh PK, Horsman JM, Radstone CR, Hancock H, Goepel JR, Hancock BW. Localised extranodal non-Hodgkin’s lymphoma of the gastrointestinal tract: Sheffield Lymphoma Group Experience. Int I Oncol. 2001;18:743–748. doi: 10.3892/ijo.18.4.743. [DOI] [PubMed] [Google Scholar]
- 20.Paraxoinis G, Parageogiou S, Rontogianni D, Kaloutsi V, Fountzillas G, Pavlidis N, Dimopoulos M, Tsatalas C, Xiros N, Economopoulos T. Primary gastrointestinal non-Hodgkin’s lymphoma; a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG) Leuk Lymphoma. 2006;47:2140–2146. doi: 10.1080/10428190600709226. [DOI] [PubMed] [Google Scholar]
- 21.Azab MB, Henry-Amar M, Rougier P, Bognel C, Theodore C, Carde P, Lasser P, Cosset JM, Caillou B, Droz JP. Prognostic factor in primary gastrointestinal non-Hodgkin’s lymphoma; a multivariate analysis, report of 106 cases, and review of the literature. Cancer. 1989;64:1208–1217. doi: 10.1002/1097-0142(19890915)64:6<1208::aid-cncr2820640608>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphoma. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]