Abstract
The phagocyte NADPH oxidase (NOX2) is known to be expressed in Epstein-Barr virus (EBV)-transformed human B lymphocytes. Phosphorylation of the NOX2 cytosolic subunit p47phox is required for phorbol myristate acetate (PMA)-induced NOX2 activation in EBV-transformed B lymphocytes, however the role of this process in receptor-mediated NOX2 activation is not known. Here, we used pansorbin which acts by cross linking cell surface IgG and transfected cells with mutated p47phox to address if the phosphorylation of this subunit is required for receptor-mediated NOX2 activation. We show that pansorbin induced NOX2 activation in a time and concentration-dependent manner, albeit at levels only of 20% of those induced by PMA. GF109203X, a PKC selective inhibitor, inhibited pansorbin as well as PMA-induced NOX2 activation. Using specific anti-phospho serine antibodies we showed that pansorbin induced p47phox phosphorylation on Ser304, 315, 320, 328, and 345 and kinetics of these phosphorylations preceed NOX2 activation. To determine whether the phosphorylation of p47phox is required for pansorbin-induced NOX2 activation, we transfected EBV-transformed lymphocytes deficent in p47phox with a plasmid expressing wild type p47phox or p47phox with all the phosphorylated serines mutated to alanines, p47phoxS(303-379)A. Results show that pansorbin-induced NOX2 activation was greatly decreased in lymphocytes expressing the mutant as compared to the wild-type p47phox. These results show that pansorbin induced p47phox phosphorylation on multiple sites in EBV-transformed B lymphocytes and this process is required for pansorbin-induced NADPH oxidase activation in these cells.
Keywords: NADPH oxidase, NOX2, p47phox, B lymphocytes, pansorbin, ROS, phosphorylation
Introduction
The phagocyte NADPH oxidase (NOX2) which produces reactive oxygen species (ROS) is a key enzyme for microbicidal and inflammatory activities of neutrophils, monocytes and macrophages [1-3]. The NADPH oxidase consists of a membrane bound flavocytochrome b558 (composed of p22phox and gp91phox/NOX2) and four cytosolic subunits, p47phox, p67phox, p40phox, and Rac1 or 2 [4-6]. Activation of the NADPH oxidase is initiated by two simultaneous mechanisms: the phosphorylation of p47phox on multiple sites and the activation of the small GTPase Rac1/2 followed by the migration of all the cytosolic components to the membrane where they associate with the membrane bound components to assemble the catalytically active oxidase [4-6]. The role of the p47phox phosphorylation sites in NOX2 activation was mainly studied in cells stimulated by the pharmacological PKC activator PMA [7-12]. Little information is available on the phosphorylation involved in receptor-mediated NOX2 activation.
NOX2 is also expressed in other cells such as Epstein-Barr virus (EBV)-transformed human B lymphocytes [13-15], but at lower levels than those found in neutrophils [16,17]. Phagocytes and EBV-transformed B lymphocytes from chronic granulomatous disease (CGD) patients are unable to produce superoxide in response to agents that induce NOX2 activation in normal cells [18,19]. EBV-transformed B lymphocytes are better suited than primary phagocytes or myeloid cell lines for transfecting NOX2 components. EBV-transformed B lymphocytes from CGD patients have been used to reconstitute NOX2 and to study the role of p47phox phosphorylation and p67phox protein domains in NOX2 assembly and activation [20-22]. However in most studies, NOX2 was activated by pharmacological PKC activators such as PMA [12,20]. The role of p47phox has yet to be explored in physiological conditions using receptor-mediated activation of NOX2. EBV-transformed B lymphocytes which express immunoglobulin (Ig) at their cell surface [13,23] represent a valuable model for such studies. Indeed, crosslinking of IgG by anti-IgG antibodies or by protein A-bearing inactivated S. aureus (pansorbin) resulted in ROS production [13,23]. In this study, we investigated the phosphorylation of p47phox and its role in pansorbin-induced NADPH oxidase/NOX2 activation in EBV-transformed B lymphocytes.
Materials and methods
Reagents
Pansorbin was from Calbiochem-Millipore (Molsheim, France). Luminol, protein kinase inhibitors, protease and phosphatase inhibitors, endotoxin-free buffers and salt solutions were from Sigma Chemical Co (Saint Louis, MO, USA). SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and Western blotting reagents were purchased from Bio-Rad (Richmond, CA, USA). The rabbit polyclonal antibodies against phospho-sites p47phox and p47phox have been described elsewhere [24,25].
EBV-transformed B lymphocytes preparation
EBV-transformed B lymphocytes were prepared as described [18,26]. Briefly, peripheral blood from healthy donors or p47phox deficient CGD patient, was fractionated by Ficoll centrifugation. Mononuclear cells were cultured in RPMI1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 ug/ml streptomycin in the presence of supernatants from B95-8, an EBV producing cell line. Three to five weeks later EBV-transformed B lymphocytes started to proliferate. P47phox deficient EBV-transformed B lymphocytes were transfected with a plasmid expressing wild type p47phox or a plasmid expressing p47phox mutated on phosphorylated serines S(303-379)A as previously described [11,12].
ROS production assay
ROS production was measured by the luminol and horse radish peroxidase (HRP)-enhanced chemiluminescence method. 2 × 106 EBV-transformed B lymphocytes were resuspended in 500 μL of HBSS containing 10 μM luminol and 10 U HRP, preheated at 37°C in the thermostated chamber of the luminometer (Biolumat LB937; Berthold) for 10 min, then stimulated by different concentrations of pansorbin (0.25-4 mg/ml) or PMA (10-400 ng/ml) and chemiluminescence was recorded for 30 min [12].
Western blotting analysis
EBV-transformed B lymphocytes were resuspended in HBSS (4 × 106/ml), preheated and then treated with pansorbin (2 mg/ml) or PMA (200 ng/ml) for the indicated times at 37°C with mild shaking. The reaction was stopped by adding 5 × concentrated modified Laemmli sample buffer [27] containing 5 mmol/L Na3VO4, 2.5 mmol/L p-NPP, 10 mmol/L NaF, 5 mmol/L EDTA, 5 mmol/L EGTA, 20 μg/L leupeptin, 20 μg/L pepstatin and 20 μg/L aprotinin. Samples were then incubated for 2 min in boiling water (100°C) and stored at -80° C until use. Cell lysates were sonicated and subjected to 10% SDS-PAGE (eq. of 1 × 106 cells/well), using standard techniques [27]. The separated proteins were transferred to nitrocellulose [28], which was blocked with 5% milk in Tris-buffered saline containing Tween 20 (TBS-T) for 1 hour. After blocking, the membranes were probed with the anti-phospho-Ser304-p47phox (1:2000), anti-phospho-Ser315-p47phox (1:2000), anti-phospho-Ser320-p47phox (1:2000), anti-phospho-Ser328-p47phox (1:2000), anti-phospho-Ser345-p47phox (1:10,000) and anti-p47phox (1:5000) antibodies. After 1 h, membranes incubation with HRP-labeled goat anti-rabbit antibody (1:5000). The protein bands were revealed by using enhanced chemiluminescence (Santa Cruz, CA, USA).
Statistical analysis
All results are expressed as means ± standard error of the mean (SEM). Significant differences (p < 0.05) were identified with Student’s t tests.
Results
Comparison of pansorbin- and PMA-induced ROS production by human EBV-transformed B lymphocytes
To compare the effect of pansorbin and PMA on EBV-transformed B lymphocytes ROS production, cells were incubated with different concentrations of these agonists and ROS production was measured by luminol- and HRP-amplified chemiluminescence. Results show that pansorbin (0.25-4 mg/ml) induced a clear increase of ROS production by EBV-transformed B lymphocytes (Figure 1A). The effect was concentration dependent and the maximal response (peak = 1.3 × 106 cpm) was obtained at 2 mg/ml. A lag time was present before pansorbin response and it was inversely proportionnal to pansorbin concentration. The PMA stimulatory effect was also concentration dependent with a maximal response (peak= 8 × 106 cpm) obtained at 400 ng/ml (Figure 1B). After a lag time of 2 min, the PMA induced a progressive rise of ROS production reaching a plateau which is proportional to PMA concentration. These results show that pansorbin induces NADPH oxidase activation to a lesser extent than PMA.
Figure 1.
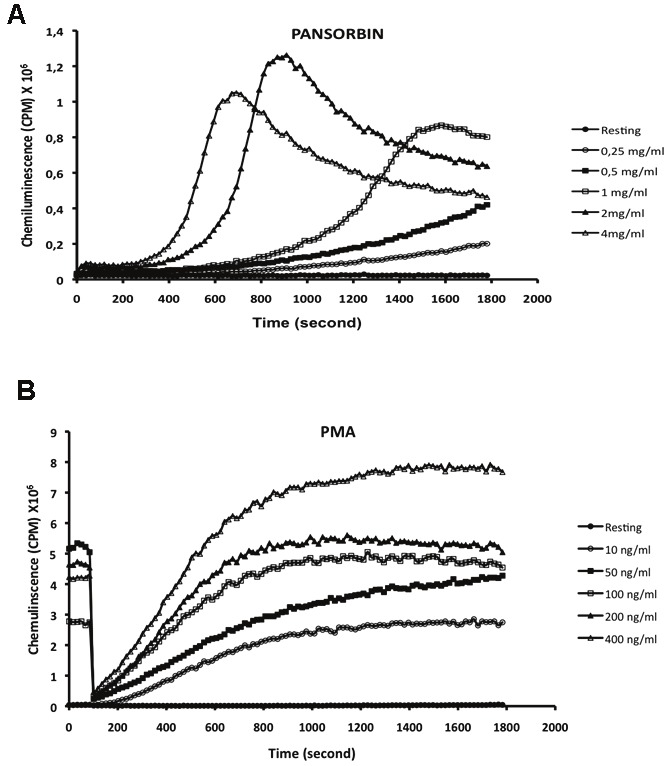
Pansorbin and PMA induce ROS production by human EBV-transformed B lymphocytes. EBV-transformed B lymphocytes (2 x 106 cells) were incubated in 0.5 ml Hanks buffer containing 10 μM luminol and 10 U HRPO at 37°C for 10 min. Pansorbin (at 0.25, 0.5, 1, 2 or 4 mg/ml) (A) or PMA ( at 10, 50, 100, 200 or 400 ng/ml) (B) were added and luminol- and HRPO amplified chemiluminescence was measured. Data are representative of 3 experiments.
The PKC inhibitor, GF109203X inhibited pansorbin-induced ROS production by human EBV-transformed B lymphocytes
Activation of NADPH oxidase by PMA is known to involve PKC-mediated protein phosphorylations and it is accompanied by the phosphorylation of the regulatory subunit p47phox [6]. Thus, we examined whether pansorbin-induced ROS production in EBV-tranformed B lymphocytes involves PKC. Cells were treated by GF109203X, a PKC inhibitor and ROS production was measured as described above. Results show that GF109203X at a concentration known to inhibit PKC, inhibited pansorbin-induced ROS production by EBV-transformed B lymphocytes (Figure 2). As expected GF109203X inhibited PMA-induced ROS production (data not shown). Wortmannin, a PI3Kinase inhibitor inhibited pansorbin response to a lesser extent than GF109203X (Figure 2).
Figure 2.
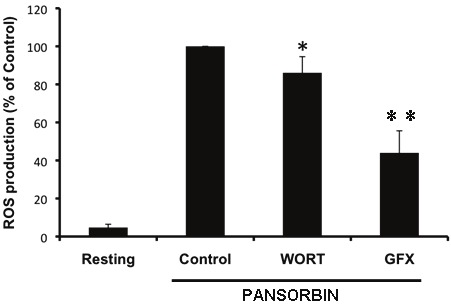
Effect of protein kinase inhibitors on Pansorbin-induced ROS production by EBV-transformed lymphocytes; B. Cells (4 x 106 cells/ml) were incubated for 20 min without or with Wortmannin (100 nM) or GF109203X (5 μM) then treated with pansorbin (2 mg/ml) and luminol-amplified chemiluminescence was measured. Results represent means ± SEM (n=3,*p<0.05 **p<0.01).
Pansorbin induces the phosphorylation of p47phox
Activation of NADPH oxidase in neutrophils and EBV-transformed B lymphocytes by PMA is accompanied by the phosphorylation of the regulatory subunit, p47phox, on serines 303-379 of the C-terminal region [6]. Thus, we examined whether pansorbin-induced ROS production was associated with p47phox phosphorylation using specific anti-phosphoSer antibodies developed in our laboratory [24,25]. Interestingly, Western blotting analysis indicated that pansorbin at 2 mg/ml induced a transient phosphorylation of p47phox on serines, Ser304, Ser315, Ser320, Ser328, and Ser345 (Figure 3A). It is noteworthy that pansorbin-induced p47phox phosphorylation preceeds ROS production. PMA induced the phosphorylation of p47phox on these sites (Figure 3B). After a lag time of 2 min, the PMA-effect was time-dependent reaching a plateau parallel to ROS production. These results suggest that pansorbin induces p47phox phosphorylation while being less effective than PMA.
Figure 3.
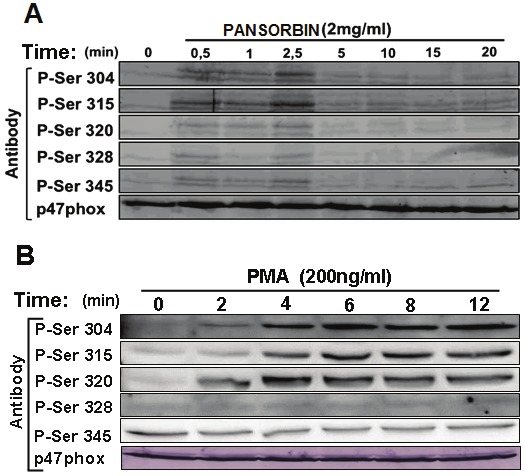
Pansorbin and PMA induce p47phox phosphorylation in EBV-transformed lymphocytes B. Cells (15 x 106) were incubated with pansorbin (2 mg/ml) (A) or PMA (200 ng/ml) (B) for different time periods and reaction was stopped by denaturation. Homogenates were subjected to SDS-PAGE and Western-blotting using different anti-phospho-p47phox antibodies: anti- phospho-Ser304, phospho-Ser315, phospho-Ser320, phospho-Ser328, phospho-Ser345 antibodies and anti-p47phox antibody. Data are representative of 3 experiments.
Phosphorylation of p47phox is required to Pansorbin-induced NOX2 activation in B lymphocytes
To ascertain whether the phosphorylation of p47phox is necessary for pansorbin-induced activation of NOX2, we used B lymphocytes from p47phox deficient CGD patient. The cells were transfected with a plasmid which encodes a p47phox mutant in which all the phosphorylated serines Ser(303-379) have been mutated to alanines Ser(303-379)A. Results show that transfection of p47phox-deficient B lymphocytes with a plasmid expressing wild type p47phox and stimulation with pansorbin or PMA resulted in NOX2 activation and ROS production (Figure 4). However transfection of p47phox-deficient B lymphocytes with a plasmid expressing the Ser (303-379)A-p47phox mutant resulted in a failure of NOX2 activation and ROS production in presence of pansorbin or PMA (Figure 4). Thus p47phox phosphorylation sites are required for pansorbin-induced NADPH oxidase activation.
Figure 4.
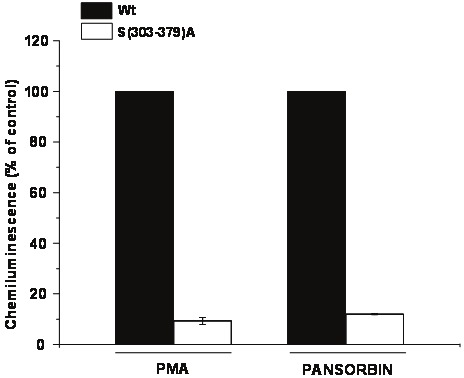
ROS production in EBV-transformed B lymphocytes expressing wild-type (Wt) and Ser(303-379) A mutant. P47phox-deficient B lymphocytes were transfected by wild-type or Ser(303-379)A p47phox mutant. ROS production was measured by luminol and HRPO-amplified chemiluminescence. Data are representative of 3 experiments.
Discussion
In the present study, we show that pansorbin induced NADPH oxidase activation in human EBV-transformed B lymphocytes. We also show that pansorbin induces the phosphorylation of the cytosolic NADPH oxidase subunit p47phox on Ser303, Ser315, Ser320, Ser328 and Ser345. Our results also suggest that PKC is involved in the phosphorylation of p47phox. Moreover, we show that p47phox phosphorylation is required for pansorbin-induced NOX2 activation in cells.
NOX2 is mainly expressed in phagocytes such as neutrophils, eosinophils, monocytes, macrophages and dendritic cells [1-6]. Circulating B lymphocytes do not express NOX2, however transformation of B lymphocytes with EBV induces the expression of NADPH oxidase subunits although at lower levels than those found in phagocytes [18,26]. Because neutrophils are short-living cells and resistant to transfection, EBV-transformed B lymphocytes from CGD patients have been used for NADPH oxidase reconstitutions and studies [20-22]. NOX2 activation in EBV-transformed lymphocytes can be induced by several agonists receptor-mediated stimuli and receptor-independent stimuli [13,23,29,30]. Pansorbin, a known ligand for immunoglobulin G (IgG) expressed at the B cells surface induced ROS production by these cells. In this study, we show that p47phox phosphorylation is required for this process.
NADPH oxidase activation and regulation are controled by phosphorylation of its cytosolic component p47phox on serines located between Ser303 and Ser379 [6]. It is clear that stimulation of neutrophils by high concentrations of the chemotactic peptide fMLF or by the PKC agonist PMA induces complete phosphorylation of p47phox [11-13]. PMA induced the phosphorylation of p47phox in EBV-transformed lymphocytes [11,12] and this phosphorylation is required for NADPH oxidase activation [12]. Here we further show that p47phox phosphorylation is required for pansorbin-mediated NADPH oxidase activation, which is a physiological and receptor-mediated stimulus. It was also shown that Rap1A and Rac1 are required for NADPH oxidase activation in EBV-transformed B lymphocytes [31,32].
The results presented in this manuscript clearly show that pansorbin, an IgG agonist induces NADPH oxidase activation and p47phox phosphorylation in EBV-transformed B lymphocytes. Further, the results suggest that PKC is involved in this process and that phosphorylation of p47phox is necessary for Pansorbin-induced ROS production.
Acknowledgments
This work was supported by grants from INSERM, CNRS, University Paris7 and the Labex Inflammex.
References
- 1.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 2.El-Benna J, Dang PMC, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 2005;53:199–206. [PubMed] [Google Scholar]
- 3.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 4.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Benna J, Dang PMC, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 6.El-Benna J, Dang PMC, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nauseef WM, Volpp BD, McCormick S, Leidal KG, Clark RA. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991;266:5911–5917. [PubMed] [Google Scholar]
- 8.Curnutte JT, Erickson RW, Ding J, Badwey JA. Reciprocal interactions between protein kinase C and components of the NADPH oxidase complex may regulate superoxide production by neutrophils stimulated with a phorbol ester. J Biol Chem. 1994;269:10813–10819. [PubMed] [Google Scholar]
- 9.Nixon JB, McPhail LC. Protein kinase C (PKC) isoforms translocate to Triton-insoluble fractions in stimulated human neutrophils: correlation of conventional PKC with activation of NADPH oxidase. J Immunol. 1999;163:4574–4582. [PubMed] [Google Scholar]
- 10.El Benna J, Faust LP, Babior BM. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J Biol Chem. 1994;269:23431–23436. [PubMed] [Google Scholar]
- 11.El Benna J, Faust RP, Johnson JL, Babior BM. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J Biol Chem. 1996;271:6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- 12.Faust LR, el Benna J, Babior BM, Chanock SJ. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest. 1995;96:1499–1505. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maly FE, Cross AR, Jones OT, Wolf-Vorbeck G, Walker C, Dahinden CA, De Weck AL. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988;140:2334–2339. [PubMed] [Google Scholar]
- 14.Maly FE, Nakamura M, Gauchat JF, Urwyler A, Walker C, Dahinden CA, Cross AR, Jones OT, de Weck AL. Superoxide-dependent nitroblue tetrazolium reduction and expression of cytochrome b-245 components by human tonsillar B lymphocytes and B cell lines. J Immunol. 1989;142:1260–1267. [PubMed] [Google Scholar]
- 15.Hancock JT, Maly FE, Jones OT. Properties of the superoxide-generating oxidase of B-lymphocyte cell lines. Determination of Michaelis parameters. Biochem J. 1989;262:373–375. doi: 10.1042/bj2620373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chetty M, Thrasher AJ, Abo A, Casimir CM. Low NADPH oxidase activity in Epstein-Barr-virus-immortalized B-lymphocytes is due to a post-transcriptional block in expression of cytochrome b558. Biochem J. 1995;306:141–145. doi: 10.1042/bj3060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batot G, Paclet MH, Doussière J, Vergnaud S, Martel C, Vignais PV, Morel F. Biochemical and immunochemical properties of B lymphocyte cytochrome b558. Biochim Biophys Acta. 1998;1406:188–202. doi: 10.1016/s0925-4439(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 18.Morel F, Cohen Tanugi Cholley L, Brandolin G, Dianoux AC, Martel C, Champelovier P, Seigneurin JM, Francois P, Bost M, Vignais PV. The O2- generating oxidase of B lymphocytes: Epstein-Barr virus-immortalized B lymphocytes as a tool for the identification of defective components of the oxidase in chronic granulomatous disease. Biochim Biophys Acta. 1993;1182:101–109. doi: 10.1016/0925-4439(93)90159-x. [DOI] [PubMed] [Google Scholar]
- 19.Kannengiesser C, Gérard B, El Benna J, Henri D, Kroviarski Y, Chollet-Martin S, Gougerot-Pocidalo MA, Elbim C, Grandchamp B. Molecular epidemiology of chronic granulomatous disease in a series of 80 kindreds: identification of 31 novel mutations. Hum Mutat. 2008;29:E132–149. doi: 10.1002/humu.20820. [DOI] [PubMed] [Google Scholar]
- 20.Chanock SJ, Faust LR, Barrett D, Bizal C, Maly FE, Newburger PE, Ruedi JM, Smith RM, Babior BM. O2- production by B lymphocytes lacking the respiratory burst oxidase subunit p47phox after transfection with an expression vector containing a p47phox cDNA. Proc Natl Acad Sci USA. 1992;89:10174–10177. doi: 10.1073/pnas.89.21.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thrasher A, Chetty M, Casimir C, Segal AW. Restoration of superoxide generation to a chronic granulomatous disease-derived B-cell line by retrovirus mediated gene transfer. Blood. 1992;80:1125–1129. [PubMed] [Google Scholar]
- 22.Maly FE, Schuerer-Maly CC, Quilliam L, Cochrane CG, Newburger PE, Curnutte JT, Gifford M, Dinauer MC. Restitution of superoxide generation in autosomal cytochrome-negative chronic granulomatous disease (A22(0) CGD)-derived B lymphocyte cell lines by transfection with p22phax cDNA. J Exp Med. 1993;178:2047–2053. doi: 10.1084/jem.178.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leca G, Benichou G, Bensussan A, Mitenne F, Galanaud P, Vazquez A. Respiratory burst in human B lymphocytes. Triggering of surface Ig receptors induces modulation of chemiluminescence signal. J Immunol. 1991;146:3542–3549. [PubMed] [Google Scholar]
- 24.Dang PMC, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boussetta T, Gougerot-Pocidalo MA, Hayem G, Ciappelloni S, Raad H, Arabi Derkawi R, Bournier O, Kroviarski Y, Zhou XZ, Malter JS, Lu PK, Bartegi A, Dang PM, El-Benna J. The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils. Blood. 2010;116:5795–5802. doi: 10.1182/blood-2010-03-273094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condino-Neto A, Newburger PE. NADPH oxidase activity and cytochrome b558 content of human Epstein-Barr-virus-transformed B lymphocytes correlate with expression of genes encoding components of the oxidase system. Arch Biochem Biophys. 1998;360:158–164. doi: 10.1006/abbi.1998.0958. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancock JT, Henderson LM, Jones OT. Superoxide generation by EBV-transformed B lymphocytes. Activation by IL-1 beta, TNF-alpha and receptor independent stimuli. Immunology. 1990;71:213–217. [PMC free article] [PubMed] [Google Scholar]
- 30.Leca G, Joly F, Vazquez A, Galanaud P, Ninio E. Paf-acether-induced superoxide anion generation in human B cell line. FEBS Lett. 1990;269:171–173. doi: 10.1016/0014-5793(90)81146-f. [DOI] [PubMed] [Google Scholar]
- 31.Maly FE, Quilliam LA, Dorseuil O, Der CJ, Bokoch GM. Activated or dominant inhibitory mutants of Rap1A decrease the oxidative burst of Epstein-Barr virus-transformed human B lymphocytes. J Biol Chem. 1994;269:18743–18746. [PubMed] [Google Scholar]
- 32.Dorseuil O, Vazquez A, Lang P, Bertoglio J, Gacon G, Leca G. Inhibition of superoxide production in B lymphocytes by rac antisense oligonucleotides. J Biol Chem. 1992;267:20540–20542. [PubMed] [Google Scholar]


