Abstract
Cultivation of cells is usually performed under atmospheric oxygen tension; however, such a condition does not replicate the hypoxic conditions of normal physiological or pathological status in the body. Recently, the effects of hypoxia on bone marrow multipotent stromal cells (MSCs) have been investigated. In a long-term culture, hypoxia can inhibit senescence, increase the proliferation rate and enhance differentiation potential along the different mesenchymal lineages. Hypoxia also modulates the paracrine effects of MSCs, causing upregulation of various secretable factors, including the vascular endothelial growth factor and interleukin-6, and thereby promoting wound healing and diabetic fracture healing. Finally, hypoxia plays an important role in mobilization and homing of MSCs, primarily by its ability to induce stromal cell-derived factor-1 expression along with its receptor, CXCR4. After transplantation, an ischemic environment, that is the combination of hypoxia and lack of nutrition, can lead to apoptosis or cell death, which can be overcome by the hypoxic preconditioning of MSCs and overexpression of prosurvival genes like Akt, HO-1 and Hsp70. This review emphasizes that hypoxia is an important factor in all major aspects of stem cell biology, and the mechanism involved in the hypoxic inducible factor-1signaling pathway behind these responses is also discussed.
Keywords: Mesenchymal stem cells, hypoxia, hypoxic preconditioning, proliferation, differentiation potential, apoptosis, migration, engraftment, HIF-1
Multipotent stromal cells
Bone marrow contains several subpopulations of stem/progenitor cells that are capable of differentiating into various non-hematopoietic cells. Among the best studied subpopulations are the cells that are isolated by their adherence to tissue culture surfaces. Such cells are referred to as multipotent stromal cells (MSCs) [1-4]. MSCs have emerged as a promising tool for clinical applications such as tissue engineering and cell-based therapy. This is because they are readily isolated from a patient, can be expanded in culture with maintained differentiation potential and immune-modulating property, and have a limited tendency to form tumors.
Hypoxic niche of multipotent stromal cells
The bone marrow niche is vitally important to the survival of organism, which facilitates the maintenance of hematopoietic stem cells (HSCs) as undifferentiated, while supporting lineage commitment of the expanding blood populations [5]. MSCs, residing in the bone marrow, are associated with and maintain HSC functions, and give rise to mesenchymal committed cells such as osteoblast and adipocyte for maintaining bone structures. Thus, the roles of bone marrow niche in maintaining MSC properties and functions should be well investigated. Because bone marrow is hypoxic with the oxygen tension around 1 to 7% [6,7], the usual culture condition of 21% O2 is hyperoxic compared to the normal niche of MSCs. To know the effects of marrow niche on MSC properties and functions, MSCs should be cultured under hypoxic conditions and compared to that cultured under normoxic conditions (the air). It is not easy to recapture the in vivo hypoxic environment in culture, unless maintaining the culture under hypoxic laminar hood combined with hypoxic incubator. We actually use a modified method of hypoxic culture by using the hypoxic incubator combined with pre-equalization of media under hypoxic conditions, rather than the use of hypoxic laminar hood. However, we need to complete medium change or subculture procedure within 30 to 60 minutes; otherwise transient reoxygenation caused by these procedures will eliminate the proliferation and differentiation properties of the cultured cells [8]. This article is aimed to discuss the benefits of hypoxic culture on MSCs. Notably, hypoxia has been reported to enhance proliferation, survival, and dopaminergic differentiation of central nervous system precursors [9]. In parallel, hypoxia also determines the cell fate of embryonic development, neural crest stem cells, and HSCs [10,11]. These data suggest stem cells may exhibit a conserved response to reduced oxygen levels.
Involvement of HIF-1α in hypoxia-mediated effects
Hypoxia has been known to regulate several cellular processes and signal transductions. Under hypoxic conditions, the α regulatory subunits of hypoxia inducible factors, HIF-1α and HIF-2α, are constitutively stabilized from oxygen-dependent and von Hippel-Lindau (VHL) tumor suppressor-mediated ubiquitylation and proteasomal degradation [12-15], leading to increased levels of HIF-1 and HIF-2 and an extensive range of hypoxia-inducible mRNAs including those involved in energy metabolism, angiogenesis and apoptosis (e.g. glucose transporter (GLUT-1) and vascular endothelial growth factor (VEGF). Most of the effects of HIF-1α have been investigated for cancer cells. Hypoxia or hypoxic state mimicked by using cobalt chloride (CoCl2) and the iron chelator desferrioxamine (DFX) induced accumulation of wild-type p53 through HIF-1α-dependent association with and stabilization of p53 protein [16]. HIF-1α blocks neuronal and myogenic differentiation via recruitment to Notch-responsive promoters upon Notch activation under hypoxic conditions in neural stem cells and myogenic cells [17]. Recently, HIF-1α has been known to regulate MSC proliferation through the enhancement of TWIST expression, which downregulates the E2A-p21 pathway, and thereby inhibits senescence and increases proliferation [18]. Notably, HIF-1α upregulates CXCR4 and CX3CR1 in MSCs and thereby enhances their migration and engraftment after transplantation [19]. HIF-1α also plays a pivotal role in hypoxia-induced MSC mobilization into peripheral blood, possibly acting via its downstream genes VEGF and SDF-1α [20]. Recent studies also suggest that HIF-1α and hypoxia mimicking agents trigger the initiation and promotion of angiogenic-osteogenic cascade events and improve intraoral bone repair and regeneration procedures (reviewed by [21]). Besides, transcription factor networks related to HIF-1α and miR-124a in part control extensive changes of their global gene expression profile during the conversion of MSCs into neural stem cell-like cells [22]. Although, HIF-2α has been known to upregulate the expression of pluripotent gene such as Oct4 [23], thereby maintaining embryo development, however there is few studies mentioned the involvement of HIF-2α in regulating MSC properties. We have previously compared the protein levels of HIF-2α between MSCs cultured under hypoxic and normoxic conditions, and found there was no difference in the level of HIF-2α between these two conditions [18].
Effects of hypoxic culture on the apoptosis of multipotent stromal cells
Significant apoptosis in MSCs has been demonstrated in the conditions associated with serum depletion, especially in a prolonged condition [24]. However, most studies have demonstrated that MSCs cultured under hypoxia or absolute hypoxia do not undergo apoptosis or change of immunophenotypes [25,26]. Moreover, combination of exposure to hypoxia partly inhibited apoptosis induced by serum depletion [24,27]. Some of the mechanisms that MSCs mediate to survive under hypoxic conditions or ischemia include the use of the glycolytic pathway [28] and the production of glucose-6-phosphatase for the use of glucose [29]. These results suggest that MSCs are characterized by metabolic flexibility, which enables them to survive from hypoxic and ischemic stress and retain their multipotent phenotype. These results highlight the potential utility of MSCs in the treatment of ischemic diseases.
It has been documented that only 0.44% of MSCs survive 4 days after transplantation into the ischemia heart [30]. Hypoxic preconditioning enhances the capacity of MSCs to survive after transplantation to the ischemic heart [31-33]. Similarly, hypoxic preconditioning of neural stem cells and MSCs also increases survival and decreases activation of caspase-3 after transplantation to the ischemic brain [34]. These data suggest short-term exposure of MSCs to hypoxia before transplantation improves their survival rates after transplantation. Although hypoxic preconditioning enhances survival, MSCs still cannot stay long in the ischemic tissues, conditions associated with hypoxia and lack of nutrition [24]. To these aims, several measures have been tested to help MSCs survive under these conditions, including transfection of MSCs with plasmids carrying integrin-linked kinase (ILK) [35], heme oxygenase-1 (HO-1) [36], angiogenin [37], angiopoietin-1 (Ang-1) and Akt [38], and Hsp70 [39] or pretreatment of MSCs with Cyclosporin A [40] or lysophosphatidic acid [41]. Most of these measures increased the survival ability of MSCs via downregualting the apoptosis-related pathways. Further, HO-1 may significantly affect the cell differentiation potential via suppressing miR-1, miR-133a, miR-133b, and miR-206 [42].
Effects of hypoxic culture on proliferation of multipotent stromal cells
The effects of hypoxic culture on MSC proliferation and expansion efficiency are still controversial. These effects depend on the sources of MSCs derived, the oxygen concentration, the seeding density, and the duration that were used to culture MSCs (Table 1). Some studies have found that hypoxia significantly inhibits proliferation [19,43-46], and others have found that hypoxia increases the proliferation capacity or increases the life span [25,46-52]. According to our previous results, the effect of hypoxia on proliferation was inhibitory within one passage of culture either at low-density or at high-density [19]. However, our recent experience with long-term culture has changed this conclusion (Table 2). We found that cell expansion efficiency at low-density culture decreased along with the increase of passage number under normoxic conditions while maintaining the same under hypoxic conditions [18]. For long-term expansion (up to 60 days) of MSCs at low-density, hypoxia significantly increased the expansion efficiency, inhibited senescence, increased proliferation and enhanced the in vitro and in vivo differentiation potential [18]. Bases on our and other studies, the effects of hypoxia on short-term MSC proliferation varied and depended on the oxygen tensions, seeding densities and MSC sources used (Table 1). However, the beneficial effects of hypoxia on long-term expansion of MSCs are consistent even with different culture conditions or MSC sources [18,25,51].
Table 1.
Comparisons of effect of hypoxia on MSC proliferation
| MSC source | Effect on proliferation | O2 (%) or hypxia mimicking; seeding density (cells/cm2); period tested | References |
|---|---|---|---|
| BM, murine | promotion | 8%; 10,000; 7-8 days | [46] |
| BM, murine | inhibition | CoCl2 or DFX; 10,000; 7-8 days | [46] |
| BM, human | inhibition | 1 %; 50-10,000; 10 days | [19] |
| BM, human | inhibition | 1-5 %; 6,250; 7 days | [43] |
| BM, human | promotion | 1-7 %; 50; 2-7 passages | [18] |
| AT, murine | promotion | 2 %; 10,000 ; 13 days | [101] |
| AT, human | promotion | 2 %; 3,000; 14 days | [52] |
| BM & AT, canine | inhibition | 1%, 5%; 5,000; 7 to 14 days | [44] |
| WJ, human | promotion | 2%; 5,000; 10 passages | [51] |
| PDB, human | inhibition | 1 %; 10,000; 2-4 days | [45] |
BM=bone marrow; AT=adipose tissue; PDB=placental decidua basalis; WJ=Wharton's jelly
Table 2.
Efficiency of MSC expansion under different seeding densities and culture conditions; reference [18]
| Hyp/Nor | Seeding density (cells/cm2) | Subculture periods (days) | Passages for 60 days | Cell fold increase for each passage | Expected cell fold increase for 60 days | Ratio to Nor/4000 |
|---|---|---|---|---|---|---|
| Nor | 4,000 | 5~7 | 10 | 5 for each passage | 9,765,625 | 1 |
| Nor | 1,000 | ~10 | 6 | 18, 18, 16, 15, 14, 11 | 11,975,040 | 1.2 |
| Nor | 100 | 11~12 | 5 | 190, 121, 68, 45, 40 | 2,813,976,000 | 288.2 |
| Nor | 50 | ~12 | 5 | 252, 170, 80, 70, 48 | 11,515,392,000 | 1,179.2 |
| Hyp | 50 | ~12 | 5 | ~250 for each passage | 976,562,500,000 | 100,000 |
Differentiation of multipotent stromal cells under hypoxic conditions
It is well documented that hypoxic culture inhibits the osteogenic and adipogenic differentiation of MSCs [25,43,53]. The same effects have also been demonstrated in adipose-derived MSCs [54]. Compared to normoxic culture, hypoxic cultures of MSCs induced for osteogenic differentiation show decreased expression of osteogenic genes, such as Runx2 and osteocalcin, and reduced alkaline phosphatase activity as well as mineralization ability [25,26,43,53,55]. The mechanism is mediated through the downregulation of Runx2 transcription factor by the HIF-TWIST pathway (Figure 1) [56]. Notably, hypoxia or activation of HIF-1α also enhanced in vivo osteoclastogenesis both in malignant and non-malignant conditions [57,58]. Similarly, MSCs induced in adipogenic medium under hypoxic conditions show decreased expression of adipogenic genes, such as PPARγ and LPL, and reduced ability to accumulate fat droplets [25,43]. However, MSCs induced for chondrogenic differentiation under hypoxic conditions show increased expression of chondrogenic genes and proteins, such as Sox5, 6 and 9, aggrecan and type II collagen [59-63], decreased expression of genes associated with osteogenesis and endochondral ossification, such as Col-1a1 and Col-10a1, and suppression of IL-1β-induced loss of extracellular matrix proteins [64]. Moreover, hypoxic culture as well as hypoxia-mimicking agents have been demonstrated to increase ectodermal differentiation such as neuron-like cells [65] and skin-regenerative potential [66]. A prolyl hydroxylase inhibitor, FG-0041, in combination with a ROCK inhibitor, Y-27632, has been demonstrated to initiate differentiation of MSCs into neuron-like cells [67].
Figure 1.
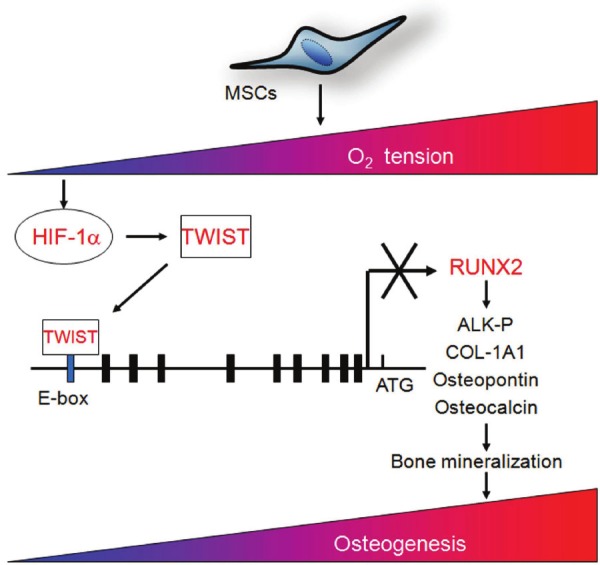
A schematic representation shows the underlying mechanism that hypoxia mediates to downregulate RUNX2 expression and inhibits osteogenesis in bone marrow MSCs [56].
Effects of hypoxic culture on maintaining stem cell properties of multipotent stromal cells
“Stemness” or stem cell properties, referred to as self-renewal and differentiation potential, is a prerequisite for success in stem cell application. Consistent with previous studies [68], we found that low-density culture provides a method for rapid expansion of MSCs. However, MSCs expanded at low-density culture gradually lost proliferation capacity and underwent senescence under normoxic conditions [18,69], while maintaining proliferation as well as differentiation potential, increasing the expression of pluripotent genes, such as Oct4, Nanog and Sox2 [70], and inhibiting senescence under hypoxic conditions [18]. Further, long-term expansion of MSCs under normoxic conditions adversely affects stem cell function when it comes to protecting myocardium [71]. On the other hand, MSCs with hypoxic preconditioning have been shown to increase in the lineage differentiation into bone [72], fat and cartilage [73] both in vitro and in vivo [18]. Increased telomere length and telomerase activity, normal karyotyping and chromosome integration have also been observed [18]. Moreover, MSCs with long-term hypoxic preconditioning do not form tumors when transplanted into immunedeficient mice. These results suggest hypoxic culture provides a method for efficiently expanding MSCs without losing stemness [70,74] and increasing tumorigenecity. Hypoxic culture may also be applied for future designs of expansion conditions for the clinical application of MSCs.
Effects of hypoxic culture on secretion of paracrine factors by multipotent stromal cells
The changes of MSC properties upon exposure to hypoxia or inflammation have been studied in the endogenous bone marrow MSCs of mice with a recent myocardial infarction (MI). Wang et al. demonstrate that recent MI impaired bone marrow cell therapeutic efficacy. MI led to myocardial inflammation and an increased inflammatory state in the bone marrow, changing the bone marrow cell composition and reducing their efficacy. Injection of a general antiinflammatory drug or a specific interleukin (IL)-1 inhibitor to donor mice after MI prevented this impairment [75]. In contrast, in vitro hypoxic preconditioning enhances the capacity of MSCs to repair infarcted myocardium or diabetic cardiomyopathy. This is attributable to reduced cell death and apoptosis of implanted cells, increased angiogenesis/vascularization, and paracrine effects [31-33]. It has been demonstrated that the conditioned medium (CM) from Akt-MSCs markedly inhibits hypoxia-induced apoptosis and triggers vigorous spontaneous contraction of adult rat cardiomyocytes in vitro [76]. Similarly, CM from MSCs has also been noted to reduce apoptosis or enhance tube formation in human endothelial cells [27]. Moreover, the effects were more obvious in a CM from hypoxic MSCs when compared to one from normoxic MSCs. This is partly due to its higher content of antiapoptotic and angiogenic factors, such as IL-6, VEGF [27], fibroblast growth factor 2 (FGF2), insulin-like growth factor 1 (IGF-1), or hepatocyte growth factor (HGF) [77]. A gene expression profile analyzed by a microarray study also demonstrated that hypoxic MSCs increase the expression of several growth factors involved in cell proliferation, apoptosis and angiogenesis [78]. Also, a CM from hypoxic MSCs activated the PI3K-Akt pathway in endothelial cells and thereby inhibited hypoxia-induced endothelial apoptosis and increased angiogenesis of endothelial cells [27]. The in vivo cardioprotection of CM from hypoxic MSCs was tested in a model of MI induced in Wistar male rats by permanent left coronary occlusion. Intramyocardial injection of 25× concentrated CM three hours after coronary occlusion was able to promote a significant reduction (35%) in left ventricular end-diastolic pressure and improvement of cardiac contractility (15%) and relaxation (12%) compared to non-conditioned medium 19-21 days after medium injection [79]. These results suggest that soluble factors released in vitro by MSCs are able to promote cardioprotection in vitro and improve cardiac function in vivo. Moreover, a CM from human hypoxic MSCs also enhanced healing of skin wound [80] as well as radiation-induced small intestine injury in mice [81], and promoted fracture healing in diabetic rats [82]. These results together suggest that the administration of hypoxic MSCs or their secreted factors may provide a therapeutic method for enhancing angiogenesis, epithelilization, wound healing and fracture healing (Figure 2). In addition to the increase in the secretion of paracrine factors, hypoxic MSCs have also been shown to increase total protein levels as well as different fibronectin expression patterns throughout the culture period, suggesting that oxygen levels can significantly affect tissue-development patterns [83]. The mechanism that hypoxic MSCs mediate to increase the release of angiogenic factor, such as VEGF is partly through the HIF-1α-mediated pathway [84]. Moreover, the advantages of using CM rather than cells include: (1) CM-based therapy circumvents some of the concerns and limitations in using viable replicating cells and does not compromise some of the advantages associated with using cells; (2) CM-based therapy is an ideal therapeutic approach because the complex cargo of proteins and genetic materials has the diversity and biochemical potential to participate in multiple biochemical and cellular processes, an important attribute in the treatment of complex disease; and others [85].
Figure 2.
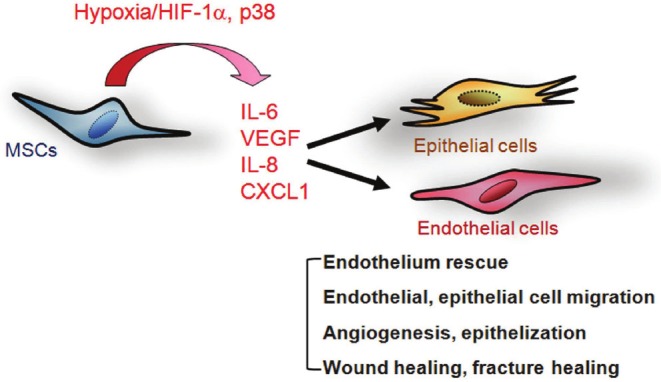
A schematic representation summarizes the effects of hypoxic culture on enhancing secretion of paracrine factors and promoting therapeutic effects in bone marrow MSC [27,80,82].
Effects of hypoxic culture on expression of chemokine receptors, migration and engraftment of multipotent stromal cells
The ability of stem/progenitor cells to migrate and engraft into host tissues is the key to their potential use in cell-based therapy. Homing and engraftment of cells have been detected in rapidly growing embryos, including mouse [86], chick [87] and sheep [88], and following tissue injury, such as ischemic damage to heart [89,90] and brain [91]. However, various studies have shown that the degree of engraftment of MSCs in naive adult animals is very low [92-94]. Interestingly, it has been demonstrated that a 1-day exposure of MSCs to 1% O2 increased expression of the chemokine receptors CX3CR1and CXCR4 and promoted SDF-1α and Fractalkine-dependent and independent migration [19]. Similarly, MSCs cultured under hypoxic conditions have also shown increased VEGFR1 expression and VEGF- or PLGF-dependent migration [95]. Similarly, hypoxia-induced increase in migration has also been demonstrated in human umbilical cord blood MSCs [96]. Moreover, xenotypic transplantation into early chick embryos demonstrated that MSCs from hypoxic culture engraft more efficiently than cells from normoxic culture and generate a variety of cell types in host tissues [19] (Figure 3). Preconditioning with oxygen and glucose depletion also increased the survival of Sca-1+ cells via PI3K/Akt-dependent caspase-3 downregulation and thereby increased engraftment rate [97]. In contrast to the migration and engraftment of transplanted MSCs, it has also been demonstrated that mobilization of MSCs from bone barrow to the circulating blood in rats is consistently and dramatically increased (by almost 15-fold) when animals are exposed to chronic hypoxia [98]. In addition to the increase in migration and survival, MSCs with hypoxic preconditioning have also been shown to enhance revascularization after transplantation for hind limb ischemia [99]. These results suggest that short-term hypoxic preconditioning of MSCs may provide a general method of enhancing their survival, migration, angiogenesis, and engraftment in vivo into a variety of tissues.
Figure 3.
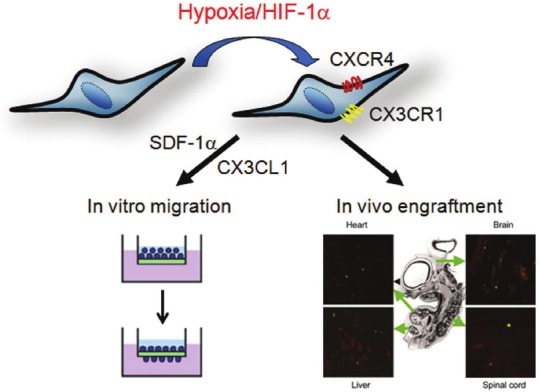
A schematic representation summarizes the effects of hypoxic exposure on enhancing the expression of chemokine receptors and increasing in vitro migration and in vivo engraftment in bone marrow MSCs [19].
Effects of hypoxic culture on glucose metabolism and oxidative stress of multipotent stromal cells
It has been demonstrated that exposure to hypoxia or the hypoxia mimetic agents such as CoCl2 induces the expression of HIF-1α and glucose-6-phosphate transporter (G6PT) in MSCs. Moreover, knockdown of HIF-1α inhibited G6PT expression in hypoxic MSCs, whereas constitutive expression of HIF-1α with a mutant oxygen-dependent degradation domain resulted in increased G6PT expression in normoxic MSCs, suggesting the involvement of the HIF pathway in the expression of G6PT [29]. Moreover, a potent G6PT inhibitor, AD4-015, specifically triggers cell death in MSCs constitutively expressing the HIF-1α mutant. Collectively, these data suggest that G6PT may account for the metabolic flexibility that enables MSCs to survive under conditions characterized by hypoxia [29]. MSCs cultured under hypoxia also induce higher specific consumption of nutrients, especially early in culture, but exhibit lower specific production of inhibitory metabolites. However, hypoxic culture of MSCs has been shown to increase cell division and favor expansion of the progeny of CFU-F, while maintaining MSC characteristics like immunophenotype and differentiation potential [100]. These data suggest hypoxia favors expanded MSCs increasing cellular metabolism efficiency, which leads toward the maximization of cell yield for application in clinical settings [100]. MSCs were also resistant to exposure to absolute hypoxia (0.5% O2), as well as inhibition of mitochondrial respiration with 2,4-dinitrophenol [28], indicating that in the absence of oxygen, MSCs can survive using anaerobic ATP production. Further studies have demonstrated that MSCs are capable of surviving hypoxia because of their ability to rely on glycolysis rather than mitochondrial respiration [28]. All together, these studies suggest that MSCs are characterized by metabolic flexibility, which enables them to survive under conditions of hypoxic and ischemic stress, and allows them to function in a reparative or regenerative capacity in the treatment of ischemic diseases.
Summary
All together, these studies suggest that hypoxic cultures have multiple effects on MSCs, which affect short-term proliferation, long-term expansion efficiency, differentiation potential , stemness or maintenance of stem cell properties, paracrine secretion capacity, the expression of chemokine receptors, migration and engraftment ability (Figure 4). Most of the effects are mediated through the HIF-1α related pathways. Moreover, MSCs are able to survive under hypoxia because of their metabolic flexibility in the synthesis of G6PT, anaerobic production of ATP, and dependence on glycolysis.
Figure 4.
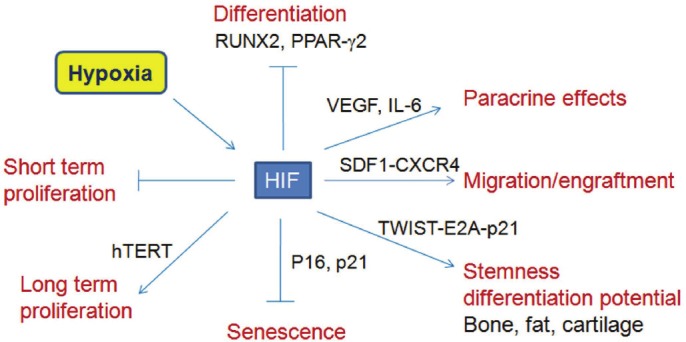
A schematic representation summarizes the effects of hypoxic culture on proliferation, expansion, differentiation, engraftment and stem cell properties in bone marrow MSCs [18,19,27,56,70,80,82].
Acknowledgements
Grants supported by Veterans General Hospital-Taipei (V99E1-011, V100E1-011); National Science Council (98-2628-B-010-001-MY3; 100-2321-B-010-022) and National Yang-Ming University, Ministry of Education.
References
- 1.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100:11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81:685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yew TL, Chang MC, Hsu YT, He FY, Weng WH, Tsai CC, Chiu FY, Hung SC. Efficient expansion of mesenchymal stem cells from mouse bone marrow under hypoxic conditions. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1491. [DOI] [PubMed] [Google Scholar]
- 9.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Q, Kim Y, Alarcon RM, Yun Z. Oxygen and Cell Fate Decisions. Gene Regul Syst Bio. 2008;2:43–51. doi: 10.4137/grsb.s434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 13.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 14.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 16.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 19.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Yu Q, Lin J, Lai X, Cao W, Du K, Wang Y, Wu K, Hu Y, Zhang L, Xiao H, Duan Y, Huang H. Hypoxia-inducible factor-1alpha is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev. 2011;20:1961–1971. doi: 10.1089/scd.2010.0453. [DOI] [PubMed] [Google Scholar]
- 21.Mamalis AA, Cochran DL. The therapeutic potential of oxygen tension manipulation via hypoxia inducible factors and mimicking agents in guided bone regeneration. A review. Arch Oral Biol. 2011;56:1466–1475. doi: 10.1016/j.archoralbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Maisel M, Habisch HJ, Royer L, Herr A, Milosevic J, Hermann A, Liebau S, Brenner R, Schwarz J, Schroeder M, Storch A. Genome-wide expression profiling and functional network analysis upon neuroectodermal conversion of human mesenchymal stem cells suggest HIF-1 and miR-124a as important regulators. Exp Cell Res. 2010;316:2760–2778. doi: 10.1016/j.yexcr.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- 25.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 26.Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–40016. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- 27.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 28.Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F, Szegezdi E. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008;26:1325–1336. doi: 10.1634/stemcells.2007-1072. [DOI] [PubMed] [Google Scholar]
- 29.Lord-Dufour S, Copland IB, Levros LC Jr, Post M, Das A, Khosla C, Galipeau J, Rassart E, Annabi B. Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1alpha: Targeting G6PT with mumbaistatin analogs in hypoxic mesenchymal stromal cells. Stem Cells. 2009;27:489–497. doi: 10.1634/stemcells.2008-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 32.Li JH, Zhang N, Wang JA. Improved antiapoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic preconditioning in diabetic cardiomyopathy. J Endocrinol Invest. 2008;31:103–110. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- 33.Wang JA, He A, Hu X, Jiang Y, Sun Y, Jiang J, Gui C, Wang Y, Chen H. Anoxic preconditioning: a way to enhance the cardioprotection of mesenchymal stem cells. Int J Cardiol. 2009;133:410–412. doi: 10.1016/j.ijcard.2007.11.096. [DOI] [PubMed] [Google Scholar]
- 34.Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Song SW, Chang W, Song BW, Song H, Lim S, Kim HJ, Cha MJ, Choi E, Im SH, Chang BC, Chung N, Jang Y, Hwang KC. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009;27:1358–1365. doi: 10.1002/stem.47. [DOI] [PubMed] [Google Scholar]
- 36.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxiaregulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 37.Liu XH, Bai CG, Xu ZY, Huang SD, Yuan Y, Gong DJ, Zhang JR. Therapeutic potential of angiogenin modified mesenchymal stem cells: angiogenin improves mesenchymal stem cells survival under hypoxia and enhances vasculogenesis in myocardial infarction. Microvasc Res. 2008;76:23–30. doi: 10.1016/j.mvr.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 39.Chang W, Song BW, Lim S, Song H, Shim CY, Cha MJ, Ahn DH, Jung YG, Lee DH, Chung JH, Choi KD, Lee SK, Chung N, Jang Y, Hwang KC. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27:2283–2292. doi: 10.1002/stem.153. [DOI] [PubMed] [Google Scholar]
- 40.Chen TL, Wang JA, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X, Cao J. Cyclosporin A preincubation attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Scand J Clin Lab Invest. 2008;68:585–593. doi: 10.1080/00365510801918761. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Baydoun AR, Xu R, Deng L, Liu X, Zhu W, Shi L, Cong X, Hu S, Chen X. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells. 2008;26:135–145. doi: 10.1634/stemcells.2007-0098. [DOI] [PubMed] [Google Scholar]
- 42.Kozakowska M, Ciesla M, Stefanska A, Skrzypek K, Was H, Jazwa A, Grochot-Przeczek A, Kotlinowski J, Szymula A, Bartelik A, Mazan M, Yagensky O, Florczyk U, Lemke K, Zebzda A, Dyduch G, Nowak W, Szade K, Stepniewski J, Majka M, Derlacz R, Loboda A, Dulak J, Jozkowicz A. Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal. 2012;16:113–127. doi: 10.1089/ars.2011.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Muller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung DJ, Hayashi K, Toupadakis CA, Wong A, Yellowley CE. Osteogenic proliferation and differentiation of canine bone marrow and adipose tissue derived mesenchymal stromal cells and the influence of hypoxia. Res Vet Sci. 2012;92:66–75. doi: 10.1016/j.rvsc.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Huang YC, Chen XH, Wang J, Li XQ, Xie HQ, Tang L, Deng L. [Effects of hypoxia on human placental decidua basalis-mesenchymal stem cells proliferation, apoptosis and VEGF expression. ] . Sheng Li Xue Bao. 2008;60:783–789. [PubMed] [Google Scholar]
- 46.Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, Jia M, Cai H, Han ZC, Yang R, Chen G, Zhao RC. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- 47.Buravkova LB, Grinakovskaia OS, Andreeva EP, Zhambalova AP, Kozionova MP. [Characteristics of human lipoaspirate-isolated mesenchymal stromal cells cultivated under a lower oxygen tension] . Tsitologiia. 2009;51:5–11. [PubMed] [Google Scholar]
- 48.Carrancio S, Lopez-Holgado N, Sanchez-Guijo FM, Villaron E, Barbado V, Tabera S, Diez- Campelo M, Blanco J, San Miguel JF, Del Canizo MC. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp Hematol. 2008;36:1014–1021. doi: 10.1016/j.exphem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Buravkova LB, Anokhina EB. Effect of hypoxia on stromal precursors from rat bone marrow at the early stage of culturing. Bull Exp Biol Med. 2007;143:411–413. doi: 10.1007/s10517-007-0143-6. [DOI] [PubMed] [Google Scholar]
- 50.Huang YC, Yang ZM, Jiang NG, Chen XH, Li XQ, Tan MY, Zhou KP, Tang L, Xie HQ, Deng L. Characterization of MSCs from human placental decidua basalis in hypoxia and serum deprivation. Cell Biol Int. 2010;34:237–243. doi: 10.1042/CBI20090044. [DOI] [PubMed] [Google Scholar]
- 51.Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M. Increased proliferation and analysis of differential gene expression in human Wharton's jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci. 2010;6:499–512. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valorani MG, Montelatici E, Germani A, Biddle A, D'Alessandro D, Strollo R, Patrizi MP, Lazzari L, Nye E, Otto WR, Pozzilli P, Alison MR. Preculturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45:225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–888. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 54.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 55.Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 56.Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965. doi: 10.1371/journal.pone.0023965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudanov IP, Andreev Iu V, Sobolev VE, Mezhenin DA, Smirnov DB, Kozlov KL. [Postoperative complications of gallstone disease in elderly and aged patients] . Adv Gerontol. 2007;20:79–82. [PubMed] [Google Scholar]
- 58.Bozec A, Bakiri L, Hoebertz A, Eferl R, Schilling AF, Komnenovic V, Scheuch H, Priemel M, Stewart CL, Amling M, Wagner EF. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature. 2008;454:221–225. doi: 10.1038/nature07019. [DOI] [PubMed] [Google Scholar]
- 59.Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 60.Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassir Y, Simchen G. Monitoring meiosis and sporulation in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:94–110. doi: 10.1016/0076-6879(91)94009-2. [DOI] [PubMed] [Google Scholar]
- 62.Markway BD, Tan GK, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;19:29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 63.Muller J, Benz K, Ahlers M, Gaissmaier C, Mollenhauer J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplant. 2011 doi: 10.3727/096368910X564094. [DOI] [PubMed] [Google Scholar]
- 64.Felka T, Schafer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17:1368–1376. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 65.Pacary E, Legros H, Valable S, Duchatelle P, Lecocq M, Petit E, Nicole O, Bernaudin M. Synergistic effects of CoCl(2) and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci. 2006;119:2667–2678. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- 66.Chung HM, Won CH, Sung JH. Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol Ther. 2009;9:1499–1508. doi: 10.1517/14712590903307362. [DOI] [PubMed] [Google Scholar]
- 67.Pacary E, Petit E, Bernaudin M. Concomitant inhibition of prolyl hydroxylases and ROCK initiates differentiation of mesenchymal stem cells and PC12 towards the neuronal lineage. Biochem Biophys Res Commun. 2008;377:400–406. doi: 10.1016/j.bbrc.2008.09.145. [DOI] [PubMed] [Google Scholar]
- 68.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 69.Jin Y, Kato T, Furu M, Nasu A, Kajita Y, Mitsui H, Ueda M, Aoyama T, Nakayama T, Nakamura T, Toguchida J. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun. 2010;391:1471–1476. doi: 10.1016/j.bbrc.2009.12.096. [DOI] [PubMed] [Google Scholar]
- 70.Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Molecular Cell. 2012;47:169–182. doi: 10.1016/j.molcel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 71.Crisostomo PR, Wang M, Wairiuko GM, Morrell ED, Terrell AM, Seshadri P, Nam UH, Meldrum DR. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 72.Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 73.Martin-Rendon E, Hale SJ, Ryan D, Baban D, Forde SP, Roubelakis M, Sweeney D, Moukayed M, Harris AL, Davies K, Watt SM. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–1012. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 74.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Takagawa J, Lam VC, Haddad DJ, Tobler DL, Mok PY, Zhang Y, Clifford BT, Pinnamaneni K, Saini SA, Su R, Bartel MJ, Sievers RE, Carbone L, Kogan S, Yeghiazarians Y, Hermiston M, Springer ML. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Sci Transl Med. 2011;3:100ra190. doi: 10.1126/scitranslmed.3002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 77.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 78.Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007;25:1166–1177. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- 79.Fidelis-de-Oliveira P, Werneck-de-Castro JP, Pinho-Ribeiro V, Shalom BC, Nascimento-Silva JA, Souza RH, Cruz IS, Rangel RR, Goldenberg RC, Campos-de-Carvalho AC. Soluble factors from multipotent mesenchymal stromal cells have antinecrotic effect on cardiomyocytes in vitro and improve cardiac function in infarcted rat hearts. Cell Transplant. 2012;21:1011–1021. doi: 10.3727/096368911X623916. [DOI] [PubMed] [Google Scholar]
- 80.Yew TL, Hung YT, Li HY, Chen HW, Chen LL, Tsai KS, Chiou SH, Chao KC, Huang TF, Chen HL, Hung SC. Enhancement of wound healing by human multipotent stromal cell conditioned medium: the paracrine factors and p38 MAPK activation. Cell Transplant. 2011;20:693–706. doi: 10.3727/096368910X550198. [DOI] [PubMed] [Google Scholar]
- 81.Gao Z, Zhang Q, Han Y, Cheng X, Lu Y, Fan L, Wu Z. Mesenchymal stromal cell-conditioned medium prevents radiation-induced small intestine injury in mice. Cytotherapy. 2012;14:267–273. doi: 10.3109/14653249.2011.616194. [DOI] [PubMed] [Google Scholar]
- 82.Wang CY, Yang HB, Hsu HS, Chen LL, Tsai CC, Tsai KS, Yew TL, Kao YH, Hung SC. Mesenchymal stem cell-conditioned medium facilitates angiogenesis and fracture healing in diabetic rats. J Tissue Eng Regen Med. 2012;6:559–569. doi: 10.1002/term.461. [DOI] [PubMed] [Google Scholar]
- 83.Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 84.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 85.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 86.Chou SH, Kuo TK, Liu M, Lee OK. In utero transplantation of human bone marrow-derived multipotent mesenchymal stem cells in mice. J Orthop Res. 2006;24:301–312. doi: 10.1002/jor.20047. [DOI] [PubMed] [Google Scholar]
- 87.Pochampally RR, Neville BT, Schwarz EJ, Li MM, Prockop DJ. Rat adult stem cells (marrow stromal cells) engraft and differentiate in chick embryos without evidence of cell fusion. Proc Natl Acad Sci USA. 2004;101:9282–9285. doi: 10.1073/pnas.0401558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 89.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 91.Jin K, Sun Y, Xie L, Mao XO, Childs J, Peel A, Logvinova A, Banwait S, Greenberg DA. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis. 2005;18:366–374. doi: 10.1016/j.nbd.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 92.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 93.Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 95.Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marce M, Semenza GL. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- 96.Lee SH, Lee YJ, Song CH, Ahn YK, Han HJ. Role of FAK phosphorylation in hypoxia-induced hMSCS migration: involvement of VEGF as well as MAPKS and eNOS pathways. Am J Physiol Cell Physiol. 2010;298:C847–856. doi: 10.1152/ajpcell.00418.2009. [DOI] [PubMed] [Google Scholar]
- 97.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rochefort GY, Delorme B, Lopez A, Herault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–2208. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 99.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 101.Valorani MG, Germani A, Otto WR, Harper L, Biddle A, Khoo CP, Lin WR, Hawa MI, Tropel P, Patrizi MP, Pozzilli P, Alison MR. Hypoxia increases Sca-1/CD44 co-expression in murine mesenchymal stem cells and enhances their adipogenic differentiation potential. Cell Tissue Res. 2010;341:111–120. doi: 10.1007/s00441-010-0982-8. [DOI] [PubMed] [Google Scholar]


