Abstract
Platinum based drugs are widely used to treat various types of cancers by inducing DNA damage mediated cytotoxicity. However, acquirement of chemoresistance towards platinum based drugs is a common phenomenon and a major hurdle in combating the relapse of the disease. Oncogenesis and chemoresistance are multifactorial maladies which often involve deregulation of one of the prime cell survival pathways, the PI3K/Akt/mTOR signalling cascade. The genetic alterations related to this pathway are often responsible for initiation and/or maintenance of carcinogenesis. Molecular components of this pathway are long being recognized as major targets for therapeutic intervention and are now also have emerged as potential tools for diagnosis of cancer. To develop novel therapeutics against the key molecules of PI3K pathway, stringent validation is required using both in-vitro and in-vivo models. Repetitive and non-invasive molecular imaging techniques, a relatively recent field in biomedical imaging hold great promises for monitoring such diagnosis and therapy. In this review, we first introduced the PI3K/Akt/mTOR pathway and its role in acquirement of chemoresistance in various cancers. Further we described how non-invasive molecular imaging approaches are sought to use this PI3K signalling axis for the therapeutics and diagnosis. A theranostic approach using various imaging modalities should be the future of PI3K signalling based drug development venture.
Keywords: PI3K signalling, platinum based chemoresistance, repetitive and non-invasive molecular imaging techniques, PET imaging, bioluminescence imaging, Akt sensor, fluorescence imaging
Introduction
Cancer is one of the deadliest diseases in humans in which the cells undergo multiple genetic and epigenetic changes leading to uncontrolled growth. Cellular signalling that govern cell proliferation, motility and survival are often deregulated in cancer cells. To inhibit or restrict this unwanted growth, various cytotoxic, cytostatic or targeted drugs are employed in clinic. Chemotherapy with platinum-based drugs, cisplatin and carboplatin, is one of the major treatment modality in different cancers and development of chemoresistance is a widely observed clinical phenomenon [1-4]. Chemoresistance, a multifaceted problem, can be defined as a mechanism through which cells elude toxic effects of the chemotherapeutics and still remains a major hurdle to successful elimination of cancer cells and remedy of the disease. A profound understanding of the basic mechanisms which leads to evolution of the acquired chemoresistance in the progression of cancer is strongly desired [5]. Several excellent reviews have elaborated different mechanisms of platinum-based drug resistance in various carcinomas which is schematically represented in Figure 1 [6-11]. Mechanism underlying chemoresistance are alterations in drug transport, detoxification of drug, altered drug target, changes in DNA repair mechanisms, increased tolerance to drug damage and activation of genes involved in cell death and survival pathways, such as p53, Bcl-2, and PI3K/Akt/mTOR [12]. Among various survival pathways, the PI3K/Akt/mTOR signalling is often found activated and plays important role in development of platinum (cisplatin or carboplatin) and paclitaxel resistance [13-15]. This pathway therefore has emerged as a potential target for therapeutics and diagnosis of chemoresistance in cancer [13]. A major challenge to suppress or reverse chemoresistance lies in its multifactorial nature and thus intervention at early stages during resistance development could be more effective. Such interventions require repetitive and non-invasive monitoring to understand the efficacy and true reversal/suppression of chemoresistance.
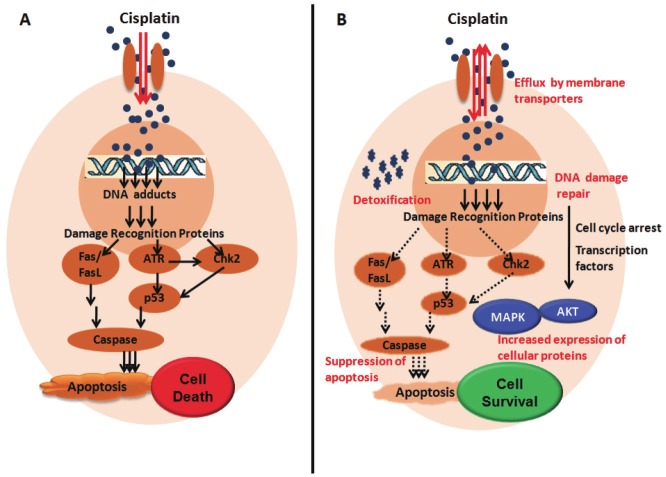
Figure 1.
Schematic representation of A: Mode of action of Platinum based drug cisplatin: Cisplatin enters cell through membrane transporters and form DNA adducts in the nucleus resulting in activation of DNA damage recognition proteins which causes cell cycle arrest. Apoptotic machinery is further activated with continuous exposure to cisplatin leading to cell death (arrows in black); B: Mechanisms of acquired resistance to cisplatin (text in red): Cisplatin entered in the resistant cells is either i) Effluxed by up regulation and overexpression of membrane transporters or ii) Detoxified by the Glutathione transferases. Formation of lower level of DNA adducts results in the (iii) Activation of DNA damage repair proteins and further global changes involve iv) Increased expression of cellular proteins like transcription factors, MAPK and Akt etc., which leads to cell survival and also v) Suppression of apoptosis eventually leading to chemoresistance.
Recently, an era of the novel imaging modalities used for monitoring tumor responses to treatment have emerged [16]. Non-invasive molecular imaging techniques are indispensable tools for the visualization, characterization and measurement of biological processes at the molecular and cellular levels in humans and other living systems [17]. Molecular imaging techniques typically include Magnetic Resonance Imaging (MRI), Computed Tomography (CT), Optical Bioluminescence and Fluorescence imaging, Ultrasound Imaging, Single Photon Emission Computed Tomography (SPECT), and Positron Emission Tomography (PET) [18]. Molecular imaging requires an imaging probe which is specific for a given molecular event and would generate specific molecular signature after interaction with its target in the body. These signatures can be radioactive rays (x-ray, gamma or beta), nuclear magnetic resonance, sound waves or light signals which can be captured by specific detectors and analysed to obtain molecular information. The most widely used imaging strategies are direct and in-direct imaging. In direct imaging, probes are used against endogenous targets like receptors or enzymes and the resultant image of probe intensity is directly proportional to the interaction with the target [19,20]. While in-direct imaging utilizes the introduction of reporter genes and reporter probes to follow the therapeutic gene expression in living subjects [21,22]. Though direct imaging techniques are most preferred for human use, it requires development and validation of probes against large number of endogenous biomolecules and therefore is extremely challenging. In contrary, in-direct imaging approach is more generalizable, generate useful information and easy to adapt with the limitation of restricted use in small animals. Currently, various reporter genes have emerged as powerful tool for non-invasive molecular imaging [23,24]. In-vivo imaging in living subjects like mice and primates enable smooth transfer of knowledge and molecular measurements between species thereby facilitating clinical translation of novel imaging agents and/or techniques [17]. The imaging modalities like MRI, CT and SPECT are not yet applied in diagnosis using the PI3K/Akt/mTOR signalling. This review is focused on the molecular imaging modalities revolving around PI3K signalling pathway with a brief description on the role of members of PI3K/Akt/mTOR pathway in cancer and drug resistance.
PI3K/Akt/mTOR signaling pathway
The phosphoinositide-3 kinases (PI3K) are the family of lipid kinases that transmit intracellular signals involved in cellular processes such as proliferation, growth, apoptosis and cytoskeleton rearrangement. PI3Ks consist of three classes, of which mutational inactivation and overexpression of Class I PI3K shows enhanced PI3K signalling and is often associated with oncogenic cellular transformation and cancer [25,26]. Class I PI3Ks phosphorylate phosphatidylinositol 4, 5 bisphosphate (PIP2) at the 3 position of the inositol ring to phosphatidylinositol 3, 4, 5 trisphosphate (PIP3), which acts as a second cellular messenger. Class I PI3Ks are heterodimeric proteins that consist of a catalytic and a regulatory subunit encoded by different genes. The catalytic subunit has four isoforms as p110α, p110β, p110γ and p110δ, which combines with one of the five isoforms of regulatory subunit p85 (p85α, p85β, p55α, p55γ and p50α) to generate an active kinase [26-28]. Class I PI3Ks are activated by signals transduced by upstream receptor tyrosine kinases including the EGFR (Epidermal growth factor receptor), HER2 (Human epidermal growth factor receptor 2), PDGFR (Platelet derived growth factor receptor), insulin growth like factor 1 receptor (IGF1R), insulin receptor (IR) and G-protein coupled receptors [25,29]. Class I PI3K product PIP3 generated at the inner leaflet of the plasma membrane directly binds to the PH domain in Akt and PDK1 (Phosphoinositide dependent kinase 1) and recruits them to the cell membrane. Akt is then activated by phosphorylation on Thr308 residue in the activation loop by PDK1 [30]. Further phosphorylation at the Ser473 residues in the C-terminal domain of Akt either by other AGC (Protein kinase A, Protein kinase G or Protein kinase G) kinase family or by auto-phosphorylation results in the complete activation of Akt [31]. Signals originating from Akt control the initiation of protein synthesis through a cascade of interactions that proceed through the tuberous sclerosis complex (TSC), Rheb (Ras homolog enriched in brain) and mTOR (mammalian target of rapamycin) proteins to two effector downstream targets, S6K (p70 S6 kinase) and 4EBP (eukaryotic initiation factor 4E-binding protein). Akt signals also regulate transcription by inducing phosphorylation-dependent degradation of FOXO1 (forkhead box-O- 1 transcription factor) and inactivation of GSK3β (glycogen synthase kinase-3β). Important targets of FOXO1 are the growth-attenuating p27 (Kip1) and p21 (Cip1) and pro-apoptotic BIM (Bcl-2 interacting mediator of cell death) proteins. GSK3β regulates the potentially oncogenic transcription factors Jun (cellular homolog of the Jun oncoprotein of avian retrovirus ASV17) and Myc (cellular homolog of the avian myelocytoma retroviral oncogene). The overall effect of the combined PI3K signals is to enhance the cellular replication and survival and to reduce growth inhibition and apoptosis [15,31-33] (Figure 2). Phosphatase and tensin homolog (PTEN), another member of PI3K pathway acts as a tumor suppressor gene with dual-specificity of protein phosphatase and lipid phosphatase activity. PTEN hydrolyzes the 3- phosphate on PIP3 to generate PIP2, thus acts as the catalytic antagonist of PI3K and inhibits the PI3K signaling [15,34] (Figure 2).
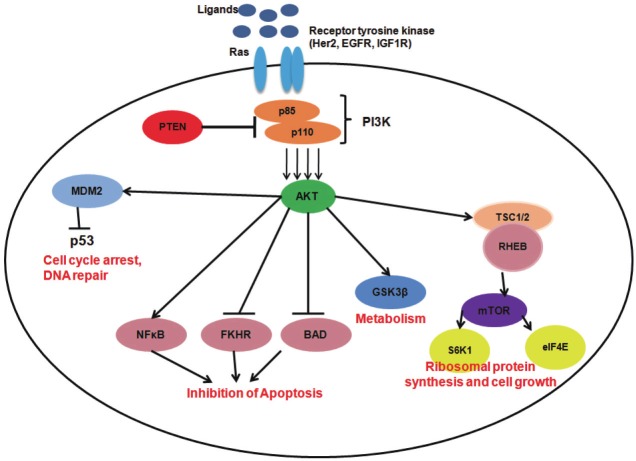
Figure 2.
Schematic representation of the PI3K/Akt/mTOR pathway: Ligands binds to the receptor tyrosine kinases like EGFR, IGF1R, Her2, and Ras leads to conformational changes and activates PI3K. PI3K is a heterodimeric protein consisting of a regulatory subunit p85 and a catalytic subunit p110. PI3K initiates activation of Akt by phosphorylation which acts as a major source of activation to further downstream signalling moieties involved in various cellular processes such as (text in red) ribosomal protein synthesis and cell growth (mTOR, S6K1 and eIF4E), loss of apoptosis (through inhibition of BAD, FKHR and activation of NFkB), metabolism (activation of GSK3β), cell-cycle arrest and DNA repair mediated by p53.
PI3K/Akt/mTOR: role in cancer and chemoresistance
PIK3CA: Genetic alterations in 110kD alpha catalytic subunit of PI3K (PIK3CA), has been found in diverse types of cancers [35]. Mutational activation and amplification of Class I PI3K and genetic or epigenetic inactivation of PTEN result in enhanced PI3K signalling which is associated with oncogenic cellular transformation and cancer. Somatic mutations in the PIK3CA gene are found in breast, endometriod, colorectal, urinary tract, thyroid and ovarian cancer [30,35,36]. These mutations are most frequently observed in two hotspots: either in the helical domain (E545K and E542K) and in the kinase domain (H1047R). The hot spot mutations induce a gain of function in p110α by which the lipid kinase activity of the mutant protein is significantly enhanced [26,36-38] and acts as oncogenic signals in in-vitro and in-vivo [36,39,40]. Several studies demonstrated that these hotspot mutations lead to hyperphosphorylation of Akt, S6K, 4EBP and GSK3β. Several cancer specific mutations other than these hot spot mutations are found to be widely distributed in the C2, helical, kinase and the adaptor binding domains of p110 catalytic subunit [33]. Amplification in PIK3CA gene located in chromosome 3q26.3 leading to constitutively active PI3K/Akt signalling is another common feature in malignancy and chemoresistance. According to the recent study by The Cancer Genome Atlas (TCGA), mutations in PIK3CA gene is a rare event in ovarian carcinomas and only found in endometriod and clear cell subtypes; while amplifications has been reported in 13-24 % of ovarian carcinomas and is associated with Tp53 mutant tumors. PIK3CA amplification is also associated with increased expression of phosphorylated Akt [39,41-43]. Frequent PIK3CA amplifications are observed in Non-small-cell lung carcinoma (NSCLCs) and gastric cancer and are associated with poor survival rate [44,45]. A recent study by Kolasa et al on ovarian carcinoma, suggested that patients with complete remission (CR) of the disease have lower frequency of PIK3CA amplification than those treated with taxane-platinum (TP) or platinum-cyclophosphamide (PC) regimens [41,46]. These observations for the first time indicated the role of PIK3CA amplification as an independent predictor of chemotherapy response in ovarian cancer during development of chemoresistance [41]. Overall the role of PIK3CA as a mediator of chemoresistance is less studied than Akt, the immediate downstream protein substrate of PIK3CA.
Akt: Akt, also known as protein kinase B, PKB, is a SH2-like (SRC homology 2-like) domain containing serine-threonine protein kinase and is activated by binding to the products of PI3K. Of the three isoforms of Akt, Akt 2 is frequently amplified in human malignancies, though alterations at protein and kinase levels are observed with all isoforms [47]. Amplifications in multiple Akt isoforms have been reported in pancreatic, ovarian, and head and neck cancers [48]. Akt is activated by deregulation of the upstream molecules like overproduction of growth factors, alterations in receptor tyrosine kinases, Ras, Src, PIK3CA and PTEN. Recently, a somatic missense mutation in the PH domain of Akt 1 (E17K) was identified in breast, colorectal and ovarian cancers [49]. This mutation in PH domain results in prolonged Akt activation and constitutive association with the plasma membrane and is sufficient to transform cells in culture [50]. Modulation in the Akt activity in response to chemotherapy has been frequently observed in chemoresistant cancers. Accumulating evidence indicate that activation of Akt promotes acquired resistance to treatment with radiation, chemotherapy, and/or targeted therapy [51]. Constitutively active Akt has also found in cisplatin resistant lung, glioma and ovarian cancer cell lines, compared to their sensitive parental counterparts [52]. Tsang et al had shown that cisplatin is able to induce both X-linked inhibitor of apoptosis (XIAP) and Akt cleavage by activating caspase-3 in chemosensitive (A2780-s and OV2008) but not in cisplatin resistant (A2780-cp and C13) ovarian cancer cells [53,54]. Gagnon et al demonstrated the expression of phosphorylated Akt even after treatment with PI3K inhibitor and studied the role of Akt and its isoforms in the resistance of uterine cancer cells to cisplatin [55]. However, cisplatin resistant intraheptic cholangiocaricnoma cells with activated Akt could revert back to chemo sensitive stage after PI3K inhibitor LY294002 treatment [56]. These studies emphasize the role of Akt in cisplatin resistance and as an important potential target for therapy.
mTOR: mTOR is one of the critical downstream effector of activated Akt. It is a large protein kinase that nucleates at least two distinct multi-protein complexes—mTORC1 and mTORC2 [57]. The second complex, mTORC2, contains rictor, mLST8 and sin1 and functions to phosphorylate Akt as well as to regulate actin cytoskeleton [58]. The mTORC1 pathway regulates growth through downstream effectors, such as the regulators of translation 4EBP1 (eukaryotic translation initiation factor 4E binding protein 1) and S6K1 (ribosomal S6 kinase 1) [58]. Dysregulation of mTOR activity is correlated with several hamartomas syndromes, including tuberous sclerosis complex, and Peutz–Jeghers syndromes. These genetic disorders are known to be caused by mutations in tumor-suppressor genes such as TSC1-TSC2, and LKB1 that negatively regulate mTOR [59-63]. Mutations in mTOR are also found in human cancer genome. Sato et al identified two point mutations (S2215Y and R2505P) in mTOR which confer constitutive activation of mTOR signalling [64]. Kim et al recently attempted to identify transcriptional alterations associated with acquired chemotherapy resistance from pre- and post- treatment biopsy samples of the same patient suffering from gastric cancer and uncovered potential molecular pathways involved in treatment. They demonstrated elevated levels of 4EBP1 and S6K1 involved in mTOR pathway in post treatment biopsy samples [65]. Wu et al demonstrated that the use of mTOR inhibitor CCI-779, an ester analog of rapamycin, can overcome cisplatin resistance and restore cisplatin sensitivity in small cell lung cancer cell lines. Thus, mTOR has turned out to be one of the major molecules in PI3K pathway responsible for chemoresistance [66].
With better understanding of PI3K/Akt/mTOR pathway and its involvement in the progression of cancer and acquirement of chemoresistance, the need to target the important molecules of this pathway for therapeutic use is increasing. It is evident that the molecular components of the PIK3CA signalling axis are potential tool for therapy and diagnosis and to explore these molecular alterations a system which would allow visualization of such molecular level interactions is required. Also such a system should be competent enough to quantify temporal changes to therapeutic responses and reflect the cellular processes non-invasively and repetitively in intact living subjects. In-vivo molecular imaging techniques serve the need by using them for diagnostic and therapeutic purpose. The molecular imaging modalities with its link-up with the PI3K signalling axis are described in the following part of the review (Table 1).
Table 1.
Summary of the imaging studies related to the PI3K/Akt/mTOR pathway
| Imaging Modality | Agents | Molecular component of PI3K/Akt/mTOR pathway involved and its application in in-vitro/ in-vivo | Reference |
|---|---|---|---|
| PET Imaging | [18F]FDG-PET for evaluating the Gamma Secretase Inhibitor(GSI) activity | ERBB2 (receptor involved in PI3K/ Akt signalling) transgenic mice showed reduced expression of Glut1 on treatment with GSI which correlated with decreased uptake of glucose ([18F]FDG) in tumor. | [73] |
| [18F]FLT-PET for evaluating everolimus, the mTOR inhibitor | [18F]-FLT uptake correlated with the level of mTOR inhibition by everolimus in the SKOV3 (cisplatin resistant cells) ovarian tumor model. | [74] | |
| Bioluminescence Imaging | BAR (Akt sensor) for evaluating the activity of the API2 and perifosine | Akt activity is inhibited by Akt inhibitor, API2 and a PI3K inhibitor, perifosine in a time- and dose-dependent manner. Increased bioluminescence activity in in-vitro and in-vivo model indicated Akt inhibition. | [76] |
| AST (Akt sensor) for evaluating the activity of perifosine and LY294002 | Inhibition of Akt activity by inhibitors like perifosine and LY29004, increased bioluminescence activities in temporal- and dose-dependent manner in in-vitro and in-vivo models. | [79] | |
| Fluorescence Imaging | Use of Intra-vital fluorescence microscopy for evaluating the action of PI3K inhibitor Wortmannin- NBD (Wm-NBD) | Wm-NBD in its active form arrest tumor growth in mice. Intra-vital fluorescence microscopy was used to calculate the concentration that activates neutrophils, required for tumor arrest. | [81] |
Molecular imaging
Molecular imaging allows the visualization of normal and abnormal cellular processes in living subjects at the molecular or genomic level along with the anatomic details [18]. Noninvasive molecular imaging technologies provide new opportunities to study small-animal models of human disease. Improvements in instrumentation, identification of better imaging targets by reporter based approaches and designing of better imaging probes offer an important role of molecular imaging techniques in disease diagnosis and therapy. In-vivo imaging modalities can be broadly classified into anatomical and functional imaging. Conventional anatomical imaging modalities include MRI and CT scanning which rely on the interaction of electromagnetic waves with the tissues and results in structural information. Functional molecular imaging requires an imaging probe that is specific for a given molecular event and produce physiological information. PET and SPECT, both require administration of radiolabeled imaging agents or reporter probes [67]. Optical imaging techniques such as fluorescence and bioluminescence imaging are of particular value for mapping specific molecular events in mice and for translating such studies to humans. Non invasive “multimodal” in-vivo imaging using the functional and anatomical imaging modalities is becoming standard practice in the clinic and is rapidly changing the evolving field of experimental imaging of gene expression (“molecular imaging”). In this brief review, we comprehensively describe the use of these novel modalities in the theranostic application of the PI3K/Akt/mTOR signalling.
Radionuclide imaging
PET is a nuclear medicine imaging technique that produces a three-dimensional image of molecular and cellular events after introduction of appropriate radiolabeled probes. With improvements in the technologies, a three dimensional PET image is often accomplished with an aid of a CT scan at the same time and in the same machine. The positron-emitting isotopes commonly used in biology are 11C, 13N, 15O, 124I, 64Cu and 18F. The most frequently used PET agent is 18F-fluorodeoxyglucose (FDG), a glucose analog that is selectively taken up by metabolically active cells, a distinguishing feature of the malignant cells (FDG is approved by FDA for monitoring malignancies and treatment response in patients). FDG-PET imaging has been approved for staging of breast cancer, colorectal cancer, oesophageal cancer, head and neck cancer, NSCLCs, melanoma, and lymphoma in USA, Germany and UK [68]. A plethora of reporter probes for PET scanning have already been described in many reviews [18,69-71]. These probes include small molecules, peptides, and antibodies labelled with radionuclides (e.g., 11C, 18F, 99mTc, and 123I), fluorochromes, or magnetic ligands.
One of the advantages of PET is that drugs or existing molecules known to interact with a specific target can be modified by tagging radioisotopes. Such radionuclide-labelled probes have been used to monitor, the expression of reporter genes such as ectopically expressed dopamine D2 receptor (D2R), or the herpes simplex virus type 1–thymidine kinase (HSV1- TK), Somatostatin receptor subtype II (SSTr2) in living mice. The PET reporter probes like acycloguanosines and 18F-fluoroethyl-spisperone (FESP) have lead to innovative analyses of gene expression in transgenic animals, methods to monitor the location, magnitude and duration of expression for gene therapy vectors and to the ability to non-invasively track the targeting, viability and expansion of cellular therapeutics [72]. PET is often used in conjunction with CT to provide an anatomical context to the relatively low-resolution PET image. In the clinic the fusion of X-ray CT and PET images has led to improvements in tumour detection. A recent study by Efferson et al showed that Gamma-Secretase inhibitor (GSI) attenuates the mTOR/Akt signalling. The antitumor activity of GSI demonstrated decreased Glut1 transporter expression in an ERBB2 (Erythroblastosis oncogene B2, also known as HER2) transgenic mouse model of breast cancer. microPET imaging showed that the functional consequences of decreased Glut1 transporter translated to reduced glucose uptake and correlated with antitumor effects. The changes in the tumor volume were measured by microCT imaging. This study suggest that [18F]FDG-PET imaging can be used monitoring clinical response and GSI can provide therapeutic benefit to a subset of ERBB2-positive breast cancers [73].
PET imaging may also be particularly useful in the field of molecularly targeted therapies, including drugs given either alone or in combination with conventional chemotherapies. Targeting the mammalian target of rapamycin (mTOR) pathway could be a potential method of overcoming cisplatin resistance in ovarian cancer patients. Since mTOR inhibition affects cell proliferation, a study by Aide et al showed that 39-deoxy-18F-fluorothymidine [18F]FLT-PET could be useful for monitoring early response to treatment with mTOR inhibitors (Figure 3). They demonstrated reduction in [18F]-FLT uptake correlates with the level of mTOR inhibition by everolimus in the SKOV3 (cisplatin resistant cells) ovarian tumor model. These studies suggest that early treatment monitoring by [18F] FLT-PET may be of use in future preclinical or clinical trials evaluating treatment of cisplatin resistant ovarian tumors by mTOR inhibitors [74].
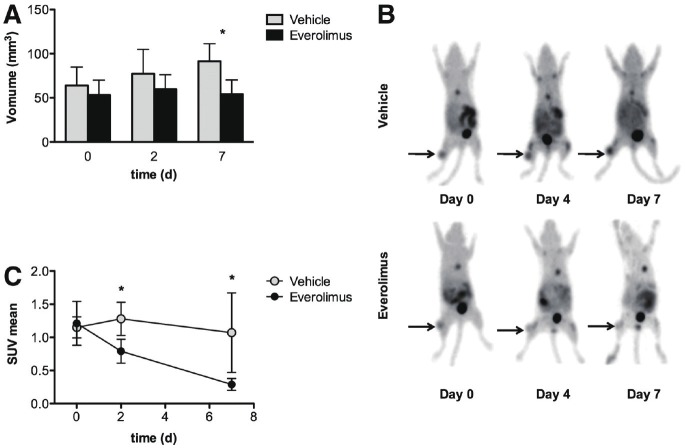
Figure 3.
[18F]-FLT imaging identifies early metabolic changes after treatment by everolimus: A. Tumor volume in everolimus and vehicle treated group: Graphical representation of the tumor volume in both groups measured for 7 days demonstrate that tumor volume increased in vehicle treated group but remain stable in everolimus treated mice. B. microPET imaging of representative animals in everolimus and vehicle treated groups: Increased uptake of [18F]-FLT in tumor xenograft mice were found by PET imaging in vehicle group while everolimus (mTOR inhibitor) treatment demonstrated reduced [18F]-FLT uptake. C. Mean standardized uptake value (SUV mean) changes of tumors in mice receiving either everolimus or vehicle treated groups: [18F]-FLT SUVmean of everolimus treated group showed significant decrease in 2nd and 7th day as compared to vehicle treated group. (Adapted from 74 with permission).
Optical imaging
Optical imaging, such as bioluminescence and fluorescence are emerging as powerful modalities for monitoring disease and therapy in small animals. Combining innovative molecular biology and chemistry, researchers have developed optical methods for imaging a variety of cellular and molecular processes in-vivo, including protein-protein interactions, protein degradation, and protease activity [18]. Optical imaging can primarily be categorized to bioluminescence and fluorescence based on the reporter genes and signals.
Bioluminescence imaging
Bioluminescence refers to light produced by the enzymatic reaction of a luciferase enzyme with its substrate. Firefly (Photinus pyralis) luciferase is the most frequently used luciferase for molecular imaging. This enzyme oxidizes its substrate, luciferin, in a reaction that requires oxygen and adenosine triphosphate (ATP), emitting light with a broad emission spectrum and a peak at 560 nm. Other luciferases such as Guassia luciferase and Renilla luciferase which are ATP independent and use coelentrazine as substrate are also widely used in in-vitro and in-vivo research. Bioluminescence imaging (BLI), is of low cost and non-invasive in nature and facilitates real-time analysis of disease processes at the molecular level in living organisms [75]. BLI is easy to execute and enables monitoring throughout the course of disease, allowing localization and serial quantification of biological processes without killing the experimental animal.
Recently, a new sensor system was developed whose bioluminescence activity within live cells and in mice can be used to measure modulation of Akt (Figure 4) [76]. This recombinant bioluminescent Akt reporter (BAR) was constructed by fusion of an Akt consensus substrate peptide and phospho-amino acid binding domain (FHA2) which were flanked by the amino- (N-Luc) and carboxyl- (C-Luc) terminal domains of the firefly luciferase reporter molecule respectively (Figure 4A and B). In the presence of Akt kinase activity, phosphorylation of the Akt consensus substrate sequences within the reporter results in its interaction with the FHA2 domain, thus sterically preventing reconstitution of a functional luciferase reporter molecule. In the absence of Akt kinase activity, release of this steric constraint allows reconstitution of the luciferase reporter molecule whose activity can be detected non-invasively by bioluminescent imaging. Inhibition of Akt activity using an Akt inhibitor, API2 and a PI3K inhibitor, perifosine resulted in an increase of bioluminescence activity in a time- and dosedependent manner, which indicated that BAR provides a surrogate for Akt activity in terms of quantity and dynamics (Figure 4C and D) [76]. BAR was also used to study upstream signalling events of Akt activation. For example, stimulation of EGFR could be evaluated using Akt activity as a surrogate and monitored by bioluminescent imaging. The use of an EGFR inhibitor, erlotinib in the erlotinib-sensitive and -resistant cell lines resulted in differential activation of the BAR reporter. BAR allows imaging of signaling leading to activation/inactivation of Akt in a quantitative, dynamic and non-invasive manner and this kinase imaging platform may be adapted for other kinases [76,77]
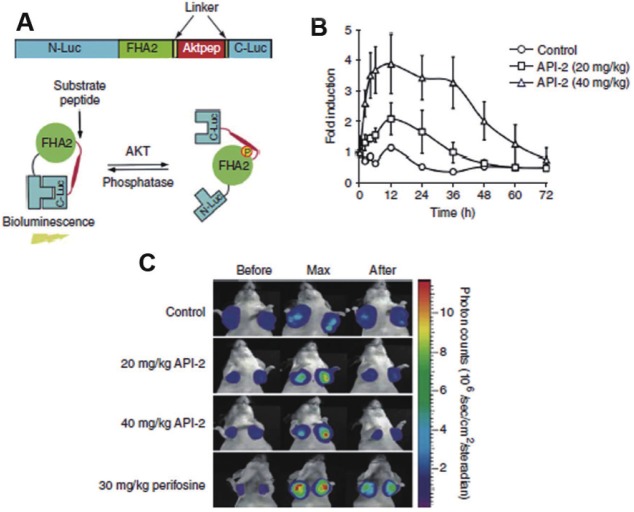
Figure 4.
A: Schematic representation of mechanism of action of Bioluminescent Akt reporter (BAR): BAR consist of an Akt consensus substrate peptide (Aktpep) and phospho-amino acid binding domain (FHA2) which are flanked by the N-terminal (N-Luc) and C-terminal (C-Luc) of the firefly luciferase reporter molecule respectively (upper panel). Akt dependent phosphorylation of the Aktpep domain induces interaction with the FHA2 domain (right) resulting minimal bioluminescence activity. In absence of Akt activity, the N-Luc and C-Luc domains reassociate restoring bioluminescence activity (left) (lower panel) B: Graphical representation of imaging Akt inhibition: D54-BAR (WT) stably transfected cells were implanted subcutaneously into nude mice. Tumor-specific bioluminescence activity was monitored 4 weeks after implantation, when tumors reached about 40–60 mm3 in size. Bioluminescence activity before treatment (time 0) and in response to treatment with vehicle control (20% DMSO in PBS) and API-2, Akt inhibitor (20 mg/kg or 40 mg/kg) was monitored at various times Bioluminescence activity remained flat over a 48-h period in vehicle-treated mice. In contrast, in mice treated with API-2 (40 mg/kg), bioluminescence activity increased within first 2h and reached a peak within 6h after treatment as compared to the small increase in activity on treatment with API-2 (20 mg/kg) The result suggest that dose in excess of 40 mg/kg of API-2 are required for maximal inhibition. C: Representative bioluminescence images of mice: Tumor bearing mice were treated with vehicle control (20%DMSO), API-2 (20mg/kg and 40mg/kg) and perifosine (PI3K inhibitor) (30 mg/kg). Images of representative mice are shown before treatment, during maximal luciferase signal upon treatment (Max), and after treatment. (Adapted from 76 with permission).
Since Akt requires recruitment to cell membrane for phosphorylation, this construct was further modified to another membrane targeted reporter molecule (MyrPalm-BAR) whose bioluminescence activity can be used to monitor Akt activity at the cell membrane. Utilizing changes in Akt activation status with small molecule inhibitors, Rehemtulla et al demonstrated that the membrane targeted Akt reporter was more sensitive and quantitative. Akt activity was modulated in response to activation or inhibition of phosphoinositide 3-kinase or direct inhibition of Akt. These studies emphasized on the usefulness of this sensor for rapid dose and schedule optimization in the Akt targeted drug development process [76,78].
In another study, Chan et al developed a genetically encoded, generalizable split firefly luciferase (FL)-assisted complementation system named as Akt sensor (AST). Split-reporter complementation strategy involves two interacting proteins: Protein A connected with N-terminal of one-half a split reporter (N-Reporter) and Protein B connected with C-terminal of the rest of the reporter (C-Reporter). Interaction between protein A and protein B recovers the reporter activity through protein complementation. This sensor was constructed to monitor Akt phosphorylation in presence of PI3K (LY294002) and Akt (perifosine) inhibitors. Specificity of AST was determined using a non-phosphorylable mutant sensor containing an alanine substitution (ASA). The authors demonstrated that temporal- and dose-dependent increase in complemention of FL activities in 293T human kidney cancer cells stably expressing AST (293T/AST) but not in 293T/ASA cells. Treatment of nude mice bearing 293T/AST xenografts with perifosine led to a 2-fold increase in complemented FL activities compared to that of 293T/ASA xenografts. This sensor was developed for non-invasive monitoring of phosphorylation and efficacies of kinase inhibitors in cell culture and in small living subjects by optical bioluminescence imaging [79].
These above-mentioned generalizable approaches for non-invasive monitoring of Akt activity by bioluminescence imaging hold great promise for accelerated discovery and validation of novel inhibitors in the PI3K/Akt/mTOR pathway.
Fluorescence imaging
In fluorescence imaging, an external light source of appropriate wavelength is used to excite a target fluorescent molecule, followed almost immediately by release of longer-wavelength, lower-energy light for imaging. In vivo fluorescence imaging uses a sensitive camera to detect fluorescence emission from fluorophores in whole-body living small animals [80]. Targets for fluorescence imaging may be endogenous molecules (such as collagen or hemoglobin), exogenous fluorescent proteins (green fluorescent protein [GFP] and related molecules), or optical contrast agents tagged with fluorescent molecules [18]. Activatable probes are commonly used for functional fluorescence imaging of enzyme activity. They often contain two or more identical or different flurochromes joined in close proximity to each other by an enzyme specific peptide linker. In the absence of the target protease, the fluorochrome is quenched by the close proximity of a second fluorochrome molecule. In the presence of the protease, the peptide substrate is cleaved releasing the fluorochromes and resulting in fluorescence. An emerging new class of probes for in-vivo fluorescence imaging are semiconductor nanocrystals or quantum dots which are gaining popularity for wide variety of applications. Quantum dots (QDs) typically have a core/ shell structure of 2–8 nm in diameter with size-dependent fluorescence emission. Fluorescent QDs require excitation from external illumination sources to fluoresce. Such quantum dots can be conjugated to chemotherapeutic drugs to visualize drug activity.
Recently, Smith et al demonstrated the inhibitory action of Wortmannin (Wm), a PI3K inhibitor after labelling with a fluorochrome, NBD on its C20 position. Wm-NBD in its active form resulted in arrest of tumor growth when injected in a tumor xenograft model. The pharmacokinetics of this Wm-NBD was studied using intra vital fluorescence microscopy in a mice model and the concentrations of Wm-NBD to activate neutrophils were calculated. This is the only demonstration of in vivo fluorescence imaging of chemotherapy by a potential PI3K inhibitor [81,82].
Conclusion
In this short review we attempted to elucidate the role of molecular imaging techniques to non-invasively monitor the PI3K/Akt/mTOR pathway as one of the key regulator of development of carcinogenesis and chemoresistance. Both Akt and mTOR have shown to be prime responsible molecules of this signalling cascade to initiate and maintain cisplatin resistance by cell growth and proliferation, metabolism and suppression of apoptosis. To detect acquirement of resistance at an earlier phase, non invasive imaging approaches are important and essential. Till to date these novel techniques are seldom utilized to monitor the therapeutic and diagnostic aspects of PI3K/Akt signalling in cancer particularly during recurrence of the disease. Few Akt based sensors were developed to validate different drugs using bioluminescence imaging and [18F]FLT based PET imaging was used to evaluate mTOR inhibitor in mouse models. The nature of the targeted therapy depends upon designing of novel inhibitors against the kinase activities of PI3K, Akt or mTOR. Molecular alteration with the novel inhibitors can be studied using various molecular imaging modalities. Fluorescence imaging technique can be further enhanced and used in therapeutic and diagnosis if coupled with the use of FRET/BRET (Fluorescence resonance energy transfer and Bioluminescence resonance energy transfer). On the other hand promoter based sensors can be developed to monitor transcriptional alteration of the key genes. The achievements obtained using these novel techniques to asses with PI3K/Akt/mTOR targeted therapy must be continued and improved for basic, pre-clinical and translational research. We can anticipate further incorporation of these non-invasive imaging strategies for use with in-vivo high throughput screening, multiplexed imaging within or across PI3K/Akt/mTOR pathway and pursue its application in therapy and diagnosis of acquired chemoresistance.
Acknowledgements
This work is supported by ACTREC start up fund, IRG-2698 and CSIR 27(232)/10(PR). SMG acknowledges fellowship funding from Council of Scientific and Industrial Research (India).
Conflict of interest statement
No conflicts declared.
References
- 1.Fallica B, Makin G, Zaman MH. Bioengineering approaches to study multidrug resistance in tumor cells. Integr Biol (Camb) 2011;3:529–539. doi: 10.1039/c0ib00142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling KS, Chen GD, Tsai HJ, Lee MS, Wang PH, Liu FS. Mechanisms involved in chemoresistance in Ovarian cancer. Taiwanese J Obstet Gynecol. 2005;44:209–217. [Google Scholar]
- 3.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 4.Kelland L. Broadening the clinical use of platinum drug-based chemotherapy with new analogues. Satraplatin and picoplatin. Expert Opin Investig Drugs. 2007;16:1009–1021. doi: 10.1517/13543784.16.7.1009. [DOI] [PubMed] [Google Scholar]
- 5.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 7.Fuertes MA, Alonso C, Perez JM. Biochemical modulation of Cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev. 2003;103:645–662. doi: 10.1021/cr020010d. [DOI] [PubMed] [Google Scholar]
- 8.Fuertes MA, Castilla J, Alonso C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10:257–266. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 10.Siddik ZH. Biochemical and molecular mechanisms of cisplatin resistance. Cancer Treat Res. 2002;112:263–284. doi: 10.1007/978-1-4615-1173-1_13. [DOI] [PubMed] [Google Scholar]
- 11.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 12.El-Deiry WS. Role of oncogenes in resistance and killing by cancer therapeutic agents. Current Opinion in Oncology. 1997;9:79–87. doi: 10.1097/00001622-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Peng DJ, Wang J, Zhou JY, Wu GS. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem Biophys Res Commun. 2010;394:600–605. doi: 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 16.Brindle K. New approaches for imaging tumour responses to treatment. Nat Rev Cancer. 2008;8:94–107. doi: 10.1038/nrc2289. [DOI] [PubMed] [Google Scholar]
- 17.Hong H, Yang Y, Zhang Y, Cai W. Non-invasive cell tracking in cancer and cancer therapy. Curr Top Med Chem. 2010;10:1237–1248. doi: 10.2174/156802610791384234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 19.Blasberg RG. Molecular imaging and cancer. Mol Cancer Ther. 2003;2:335–343. [PubMed] [Google Scholar]
- 20.Blasberg RG. In vivo molecular-genetic imaging: multi-modality nuclear and optical combinations. Nucl Med Biol. 2003;30:879–888. doi: 10.1016/s0969-8051(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 21.Ray P. Multimodality molecular imaging of disease progression in living subjects. J Biosci. 2011;36:499–504. doi: 10.1007/s12038-011-9079-0. [DOI] [PubMed] [Google Scholar]
- 22.Ray P. The pivotal role of multimodality reporter sensors in drug discovery: from cell based assays to real time molecular imaging. Curr Pharm Biotechnol. 2011;12:539–546. doi: 10.2174/138920111795163977. [DOI] [PubMed] [Google Scholar]
- 23.Gambhir SS, Herschman HR, Cherry SR, Barrio JR, Satyamurthy N, Toyokuni T, Phelps ME, Larson SM, Balatoni J, Finn R, Sadelain M, Tjuvajev J, Blasberg R. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–138. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massoud TF, Paulmurugan R, De A, Ray P, Gambhir SS. Reporter gene imaging of proteinprotein interactions in living subjects. Curr Opin Biotechnol. 2007;18:31–37. doi: 10.1016/j.copbio.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci USA. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geering B, Cutillas PR, Vanhaesebroeck B. Regulation of class IA PI3Ks: is there a role for monomeric PI3K subunits? Biochem Soc Trans. 2007;35:199–203. doi: 10.1042/BST0350199. [DOI] [PubMed] [Google Scholar]
- 29.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 32.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denley A, Kang S, Karst U, Vogt PK. Oncogenic signaling of class I PI3K isoforms. Oncogene. 2008;27:2561–2574. doi: 10.1038/sj.onc.1210918. [DOI] [PubMed] [Google Scholar]
- 34.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 36.Samuels Y, Waldman T. Oncogenic mutations of PIK3CA in human cancers. Curr Top Microbiol Immunol. 2010;347:21–41. doi: 10.1007/82_2010_68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, Ohta M, Jazag A, Guleng B, Tateishi K, Asaoka Y, Matsumura M, Kawabe T, Omata M. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–4567. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 38.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 39.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 40.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 41.Kolasa IK, Rembiszewska A, Felisiak A, Ziolkowska-Seta I, Murawska M, Moes J, Timorek A, Dansonka-Mieszkowska A, Kupryjanczyk J. PIK3CA amplification associates with resistance to chemotherapy in ovarian cancer patients. Cancer Biol Ther. 2009;8:21–26. doi: 10.4161/cbt.8.1.7209. [DOI] [PubMed] [Google Scholar]
- 42.Network TCGA. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, Swisher EM. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–613. doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Ji M, Guan H, Gao C, Shi B, Hou P. Highly frequent promoter methylation and PIK3CA amplification in non-small cell lung cancer (NSCLC) BMC Cancer. 2011;11:147. doi: 10.1186/1471-2407-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, Yao D, Liu W, Wang N, Lv H, Zhang G, Ji M, Xu L, He N, Shi B, Hou P. Highly frequent PIK3CA amplification is associated with poor prognosis in gastric cancer. BMC Cancer. 2012;12:50. doi: 10.1186/1471-2407-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang N, Huang J, Greshock J, Liang S, Barchetti A, Hasegawa K, Kim S, Giannakakis A, Li C, O’Brien-Jenkins A, Katsaros D, Butzow R, Coukos G, Zhang L. Transcriptional regulation of PIK3CA oncogene by NF-kappaB in ovarian cancer microenvironment. PLoS One. 2008;3:e1758. doi: 10.1371/journal.pone.0001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Ning J, He L, Yang H, Sun M, Nicosia SV, Cheng JQ. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 48.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 49.Tibes R, Kornblau SM, Qiu Y, Mousses SM, Robbins C, Moses T, Carpten JD. PI3K/AKT pathway activation in acute myeloid leukaemias is not associated with AKT1 pleckstrin homology domain mutation. Br J Haematol. 2008;140:344–347. doi: 10.1111/j.1365-2141.2007.06920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 51.Huang WC, Hung MC. Induction of Akt activity by chemotherapy confers acquired resistance. J Formos Med Assoc. 2009;108:180–194. doi: 10.1016/S0929-6646(09)60051-6. [DOI] [PubMed] [Google Scholar]
- 52.Lee RS, House CM, Cristiano BE, Hannan RD, Pearson RB, Hannan KM. Relative Expression Levels Rather Than Specific Activity Plays the Major Role in Determining In Vivo AKT Isoform Substrate Specificity. Enzyme Res. 2011:720985. doi: 10.4061/2011/720985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraser M, Leung B, Jahani-Asl A, Yan X, Thompson WE, Tsang BK. Chemoresistance in human ovarian cancer: the role of apoptotic regulators. Reprod Biol Endocrinol. 2003;1:66. doi: 10.1186/1477-7827-1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fraser M, Leung BM, Yan X, Dan HC, Cheng JQ, Tsang BK. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 2003;63:7081–7088. [PubMed] [Google Scholar]
- 55.Gagnon V, Mathieu I, Sexton E, Leblanc K, Asselin E. AKT involvement in cisplatin chemoresistance of human uterine cancer cells. Gynecol Oncol. 2004;94:785–795. doi: 10.1016/j.ygyno.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 56.Yoon H, Min JK, Lee JW, Kim DG, Hong HJ. Acquisition of chemoresistance in intrahepatic cholangiocarcinoma cells by activation of AKT and extracellular signal-regulated kinase (ERK)1/2. Biochem Biophys Res Commun. 2011;405:333–337. doi: 10.1016/j.bbrc.2010.11.130. [DOI] [PubMed] [Google Scholar]
- 57.Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 59.Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJ, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316:1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- 60.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 61.Kwon G, Marshall CA, Liu H, Pappan KL, Remedi MS, McDaniel ML. Glucose-stimulated DNA synthesis through mammalian target of rapamycin (mTOR) is regulated by KATP channels: effects on cell cycle progression in rodent islets. J Biol Chem. 2006;281:3261–3267. doi: 10.1074/jbc.M508821200. [DOI] [PubMed] [Google Scholar]
- 62.Parry L, Maynard JH, Patel A, Clifford SC, Morrissey C, Maher ER, Cheadle JP, Sampson JR. Analysis of the TSC1 and TSC2 genes in sporadic renal cell carcinomas. Br J Cancer. 2001;85:1226–1230. doi: 10.1054/bjoc.2001.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Sato T, Nakashima A, Guo L, Coffman K, Tamanoi F. Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene. 2010;29:2746–2752. doi: 10.1038/onc.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HK, Choi IJ, Kim CG, Kim HS, Oshima A, Michalowski A, Green JE. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS One. 2011;6:e16694. doi: 10.1371/journal.pone.0016694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu C, Wangpaichitr M, Feun L, Kuo MT, Robles C, Lampidis T, Savaraj N. Overcoming cisplatin resistance by mTOR inhibitor in lung cancer. Mol Cancer. 2005;4:25. doi: 10.1186/1476-4598-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah K, Jacobs A, Breakefield XO, Weissleder R. Molecular imaging of gene therapy for cancer. Gene Ther. 2004;11:1175–1187. doi: 10.1038/sj.gt.3302278. [DOI] [PubMed] [Google Scholar]
- 68.Salminen E, Hogg A, Binns D, Frydenberg M, Hicks R. Investigations with FDG-PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 2002;41:425–429. doi: 10.1080/028418602320405005. [DOI] [PubMed] [Google Scholar]
- 69.Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293:855–862. doi: 10.1001/jama.293.7.855. [DOI] [PubMed] [Google Scholar]
- 70.Yuan H, Luo J, Field S, Weissleder R, Cantley L, Josephson L. Synthesis and activity of C11-modified wortmannin probes for PI3 kinase. Bioconjug Chem. 2005;16:669–675. doi: 10.1021/bc049714f. [DOI] [PubMed] [Google Scholar]
- 71.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500–516. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herschman HR. PET reporter genes for noninvasive imaging of gene therapy, cell tracking and transgenic analysis. Crit Rev Oncol Hematol. 2004;51:191–204. doi: 10.1016/j.critrevonc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE, Buser C, Yeatman T, Coppola D, Winter C, Clark EA, Draetta GF, Strack PR, Majumder PK. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res. 2010;70:2476–2484. doi: 10.1158/0008-5472.CAN-09-3114. [DOI] [PubMed] [Google Scholar]
- 74.Aide N, Kinross K, Cullinane C, Roselt P, Waldeck K, Neels O, Dorow D, McArthur G, Hicks RJ. 18F-FLT PET as a surrogate marker of drug efficacy during mTOR inhibition by everolimus in a preclinical cisplatin-resistant ovarian tumor model. J Nucl Med. 2010;51:1559–1564. doi: 10.2967/jnumed.109.073288. [DOI] [PubMed] [Google Scholar]
- 75.Sadikot RT, Blackwell TS. Bioluminescence imaging. Proc Am Thorac Soc. 2005;2:537–540. 511–532. doi: 10.1513/pats.200507-067DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L, Lee KC, Bhojani MS, Khan AP, Shilman A, Holland EC, Ross BD, Rehemtulla A. Molecular imaging of Akt kinase activity. Nat Med. 2007;13:1114–1119. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- 77.Dothager RS, Piwnica-Worms D. Molecular imaging of pulmonary disease in vivo. Proc Am Thorac Soc. 2009;6:403–410. doi: 10.1513/pats.200901-004AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Bhojani MS, Ross BD, Rehemtulla A. Enhancing Akt imaging through targeted reporter expression. Mol Imaging. 2008;7:168–174. [PMC free article] [PubMed] [Google Scholar]
- 79.Chan CT, Paulmurugan R, Reeves RE, Solow-Cordero D, Gambhir SS. Molecular imaging of phosphorylation events for drug development. Mol Imaging Biol. 2009;11:144–158. doi: 10.1007/s11307-008-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol. 2007;18:17–25. doi: 10.1016/j.copbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Smith A, Blois J, Yuan H, Aikawa E, Ellson C, Figueiredo JL, Weissleder R, Kohler R, Yaffe MB, Cantley LC, Josephson L. The antiproliferative cytostatic effects of a self-activating viridin prodrug. Mol Cancer Ther. 2009;8:1666–1675. doi: 10.1158/1535-7163.MCT-08-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen HH, Yuan H, Josephson L, Sosnovik DE. Theranostic Imaging of the Kinases and Proteases that Modulate Cell Death and Survival. Theranostics. 2012;2:148–155. doi: 10.7150/thno.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]