Abstract
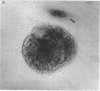
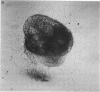
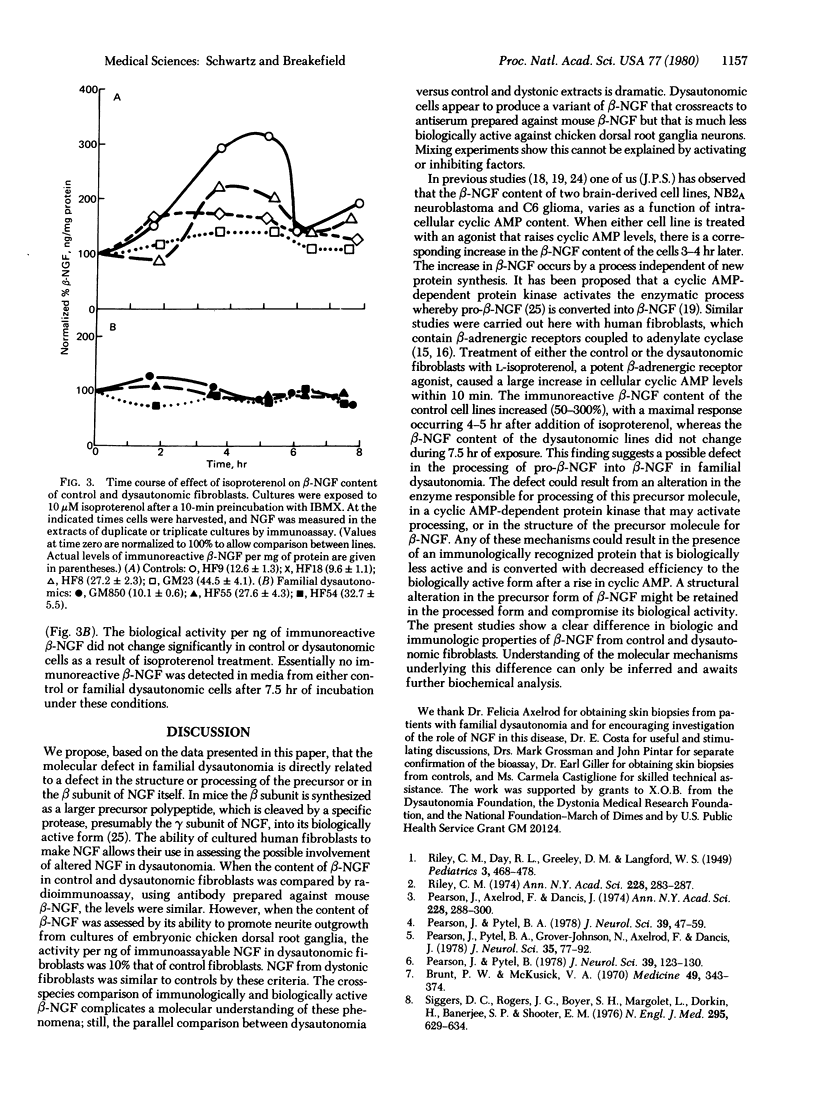
Nerve growth factor was measured in cultured human skin fibroblasts from controls and from patients with familial dysautonomia and dystonia musculoram deformans. Cells from these sources grown over a range of cell densities contained similar levels of beta-nerve growth factor as measured by radioimmunoassay. Results of bioassay demonstrated that the nerve growth factor from dysautonomic cells was only approximately 10% as active per ng of immunoreactive protein as that from control and dystonic cells. Treatment of fibroblasts with the beta-adrenergic agonist isoproterenol resulted in a 17- to 170-fold rise in the cyclic AMP content of both control and dysautonomic cells in 10 min. The content of immunoreactive beta-nerve growth factor in control fibroblasts increased 50--300% after 3--4 hr of exposure to isoproterenol. At no time, throughout a 7.5 hr period, was there a change in the amount of immunoreactive beta-nerve growth factor in the dysautonomic cells. These studies suggest that the molecular basis of the genetic defect in familial dysautonomia may lie in processing of the precursor or in the structure of the biologically active beta subunit of nerve growth factor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A., Shooter E. M. Evidence for pro-beta-nerve growth factor, a biosynthetic precursor to beta-nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3647–3651. doi: 10.1073/pnas.74.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt P. W., McKusick V. A. Familial dysautonomia. A report of genetic and clinical studies, with a review of the literature. Medicine (Baltimore) 1970 Sep;49(5):343–374. [PubMed] [Google Scholar]
- Crooks P. A., Breakefield X. O., Sulens C. H., Castiglione C. M., Coward J. K. Extensive conjugation of dopamine (3,4-dihydroxyphenethylamine) metabolites in cultured human skin fibroblasts and rat hepatoma cells. Biochem J. 1978 Oct 15;176(1):187–196. doi: 10.1042/bj1760187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groshong R., Gibson D. A., Baldessarini R. J. Monoamine oxidase activity in cultured human skin fibroblasts. Clin Chim Acta. 1977 Oct 1;80(1):113–120. doi: 10.1016/0009-8981(77)90270-4. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Goldstein S. Adenosine 3': 5'-cyclic monophosphate in young and senescent human fibroblasts during growth and stationary phase in vitro. Effects of prostaglandine E1 and of adrenaline. Biochem J. 1974 Nov;144(2):253–263. doi: 10.1042/bj1440253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M., Jr, Breakefield X. O. Monoamine oxidase A and B in cultured cells. J Neurochem. 1978 Jun;30(6):1391–1397. doi: 10.1111/j.1471-4159.1978.tb10471.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manganiello V. C., Breslow J. Effects of prostaglandin E1 and isoproterenol on cyclic AMP content of human fibroblasts modified by time and cell density in subculture. Biochim Biophys Acta. 1974 Oct 8;362(3):509–520. doi: 10.1016/0304-4165(74)90146-9. [DOI] [PubMed] [Google Scholar]
- Mellman W. J. A biochemical genetic view of human cell culture. Adv Hum Genet. 1971;2:259–306. [PubMed] [Google Scholar]
- Pearson J., Axelrod F., Dancis J. Trophic functions of the neuron. V. Familial dysautonomis. Current concepts of dysautonomia: neuropathological defects. Ann N Y Acad Sci. 1974 Mar 22;228(0):288–300. doi: 10.1111/j.1749-6632.1974.tb20517.x. [DOI] [PubMed] [Google Scholar]
- Pearson J., Pytel B. A., Grover-Johnson N., Axelrod F., Dancis J. Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci. 1978 Jan;35(1):77–92. doi: 10.1016/0022-510x(78)90103-x. [DOI] [PubMed] [Google Scholar]
- Pearson J., Pytel B. A. Quantitative studies of sympathetic ganglia and spinal cord intermedio-lateral gray columns in familial dysautonomia. J Neurol Sci. 1978 Nov;39(1):47–59. doi: 10.1016/0022-510x(78)90187-9. [DOI] [PubMed] [Google Scholar]
- Pearson J., Pytel B. Quantitative studies of ciliary and sphenopalatine ganglia in familial dysautonomia. J Neurol Sci. 1978 Nov;39(1):123–130. doi: 10.1016/0022-510x(78)90193-4. [DOI] [PubMed] [Google Scholar]
- Riley C. M. Trophic functions of the neuron. V. Familial dysautonomia. Familial dysautonomia: clinical and pathophysiological aspects. Ann N Y Acad Sci. 1974 Mar 22;228(0):283–287. doi: 10.1111/j.1749-6632.1974.tb20516.x. [DOI] [PubMed] [Google Scholar]
- Roth J. A., Breakefield X. O., Castiglione C. M. Monoamine oxidase and catechol-O-methyltransferase activities in cultured human skin fibroblasts. Life Sci. 1976 Dec 1;19(11):1705–1710. doi: 10.1016/0024-3205(76)90077-1. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P. Catecholamine-mediated elevation of cyclic GMP in the rat C-6 glioma cell line. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):287–296. [PubMed] [Google Scholar]
- Schwartz J. P., Chuang D. M., Costa E. Increase in nerve growth factor content of C6 glioma cells by the activation of a beta-adrenergic receptor. Brain Res. 1977 Dec 2;137(2):369–375. doi: 10.1016/0006-8993(77)90349-3. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Costa E. Regulation of nerve growth factor content in C6 glioma cells by beta-adrenergic receptor stimulation. Naunyn Schmiedebergs Arch Pharmacol. 1977 Nov;300(2):123–129. doi: 10.1007/BF00505042. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Costa E. Regulation of nerve growth factor content in a neuroblastoma cell line. Neuroscience. 1978;3(4-5):473–480. doi: 10.1016/0306-4522(78)90051-9. [DOI] [PubMed] [Google Scholar]
- Siggers D. C., Rogers J. G., Boyer S. H., Margolet L., Dorkin H., Banerjee S. P., Shooter E. M. Increased nerve-growth-factor beta-chain cross-reacting material in familial dysautonomia. N Engl J Med. 1976 Sep 16;295(12):629–634. doi: 10.1056/NEJM197609162951201. [DOI] [PubMed] [Google Scholar]
- Smith A. P., Varon S., Shooter E. M. Multiple forms of the nerve growth factor protein and its subunits. Biochemistry. 1968 Sep;7(9):3259–3268. doi: 10.1021/bi00849a032. [DOI] [PubMed] [Google Scholar]
- Suda K., Barde Y. A., Thoenen H. Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4042–4046. doi: 10.1073/pnas.75.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon S., Nomura J., Shooter E. M. The isolation of the mouse nerve growth factor protein in a high molecular weight form. Biochemistry. 1967 Jul;6(7):2202–2209. doi: 10.1021/bi00859a043. [DOI] [PubMed] [Google Scholar]