Abstract
Current therapies directed at controlling vascular abnormalities in cancers and neovascular eye diseases target VEGF and can slow the progression of these diseases. While the critical role of VEGF in development has been well described, the function of locally synthesized VEGF in the adult eye is incompletely understood. Here, we show that conditionally knocking out Vegfa in adult mouse retinal pigmented epithelial (RPE) cells, which regulate retinal homeostasis, rapidly leads to vision loss and ablation of the choriocapillaris, the major blood supply for the outer retina and photoreceptor cells. This deletion also caused rapid dysfunction of cone photoreceptors, the cells responsible for fine visual acuity and color vision. Furthermore, Vegfa deletion showed significant downregulation of multiple angiogenic genes in both physiological and pathological states, whereas the deletion of the upstream regulatory transcriptional factors HIFs did not affect the physiological expressions of angiogenic genes. These results suggest that endogenous VEGF provides critical trophic support necessary for retinal function. Targeting factors upstream of VEGF, such as HIFs, may be therapeutically advantageous compared with more potent and selective VEGF antagonists, which may have more off-target inhibitory trophic effects.
Introduction
Phototransduction by rod and cone photoreceptors in the retina converts light into electrical energy. This process, forming the basis for vision, is facilitated by the vascular plexus (the choriocapillaris) and retinal pigmented epithelial (RPE) cells, both of which underlie and are intimately associated with the photoreceptors. Degeneration of photoreceptors, RPE cells, and the choriocapillaris is associated with age-related macular degeneration (AMD), the leading cause of vision loss in industrialized nations. While VEGF clearly plays a role in physiological vascular development (1–4) and pathological neovascularization (5, 6) in the retina and the choroid, there is increasing evidence that it is also important for the trophic maintenance of neurons (7–9). Inhibition of VEGF has become the mainstay of therapy for treating AMD (10–12), and increasingly potent VEGF antagonists have recently been introduced into the clinics. In order to better understand the trophic activity of VEGF in the adult retina and choriocapillaris and to assess the effect of deleting its expression by RPE cells, we utilized inducible Cre/loxP technology to genetically delete floxed Vegf alleles in vivo with an RPE-specific VMD2-Cre transgenic line (13).
Results and Discussion
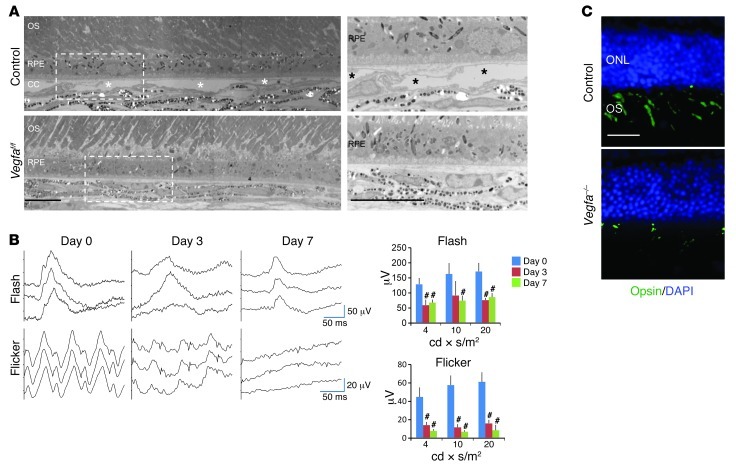
Three days after RPE-specific Vegf inactivation, we observed complete ablation of the choriocapillaris (Figure 1A). Since almost the entire blood supply to the outer two-thirds of the retina (including the photoreceptors) is provided by the choriocapillaris, we predicted that its loss would induce dramatic secondary effects on the retinal neurons. In fact, following Vegfa inactivation in RPE cells, we observed profound retinal dysfunction, especially in cone photoreceptors. Within 3 days after induction, rapid and progressive dysfunction of cone photoreceptors was detected using electroretinography (ERG) (Figure 1B) compared with multiple controls, including Vegfaf/f (no Cre), Vegfaf/f;VMD2-Cre, Vegfaf/+;VMD2-Cre with doxycycline, and Vegfaf/f;VMD2-Cre without doxycycline (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI65157DS1). Using immunohistochemistry to selectively stain for the cone photopigment opsin, we observed a nearly complete absence of cone photoreceptor outer segments (Figure 1C). Cone dysfunction was observed by at least 7 months after Vegfa deletion in RPE (Supplemental Figure 2). These data demonstrate that RPE-derived VEGF is required for maintenance of the choriocapillaris and for cone photoreceptors, the most metabolically demanding cells in the central nervous system (14, 15). Photoreceptors also express VEGF receptors (9, 16), and paracrine-derived VEGF may have a direct role in their maintenance. In humans, loss of cones results in legal blindness, since fine (or “reading”) visual acuity is maintained by cones located in the central retina, or macula.
Figure 1. Inducible Vegfa deletion in adult RPE cells promotes rapid choriocapillaris degeneration and vision loss.
(A) Electron micrographs of control (upper panel, Vegfaf/f without Cre) and mutant (lower panels, Vegfaf/f with VMD2-Cre) murine retinas 3 days after Vegfa deletion in adult RPE. Right panels are enlargements of the boxed regions of the left panels. Note that Vegfa mutants lack choriocapillaris normally observed in controls (asterisks). (B) ERG of Vegfa mutant eyes shows loss of photopic and flicker signals. b-wave amplitudes from flash, or first peaks from flicker, ERG in photopic light-adapted conditions captured in Vegfa mutants are significantly attenuated compared with the same retina prior to Vegfa gene deletion (n = 4 for each time point). This dramatic attenuation is consistent with vision loss. (C) Immunohistochemical analyses for cone-opsin in Vegfa mutants and controls 7 days after induction. The absence of cone outer segments is apparent in the Vegfa mutants. #P < 0.01; 2-tailed Student’s t tests. Error bars indicate mean ± SD. Scale bars: 10 μm (A ); 20 μm (C). ONL, outer nuclear layer; OS, outer segments; CC,choriocapillaris.
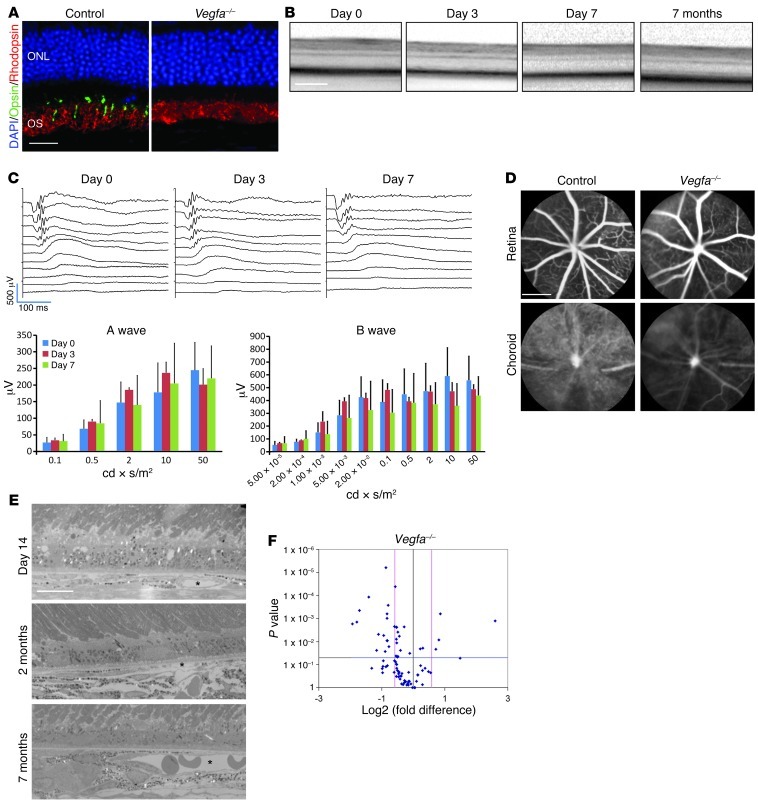
In contrast, rod photoreceptor function (responsible for achromatic and night vision) is insensitive to Vegfa inactivation at the same time points when we observe cone degeneration. Immunohistochemical analyses revealed that the layer of rod photoreceptor outer segments was as thick as that of controls (Figure 2A). Since the murine retina is composed largely of rods and the thickness of the entire retina does not change as late as 7 months after Cre recombinase induction (Figure 2B), this suggests that there is no significant rod photoreceptor atrophy. Scotopic (dark adapted) ERGs demonstrate that rod photoreceptors are fully functional, since there was no change in rod photoreceptor light responsiveness in recordings before and after induction in Vegfa mutant mice (Figure 2C). The preservation of rod photoreceptors for several months after gene deletion suggests that these cells are supported by a minimal vasculature not dependent on RPE-derived Vegfa for its survival. Indocyanine green angiography demonstrated 2 blood supply sources for photoreceptors in Vegfa mutants; intraretinal and outer, major choroidal vessels (Figure 2D). Utilizing electron microscopy, we determined that the choriocapillaris remained atrophic through 7 months and choroidal vessels were observed only sporadically (Figure 2E). These findings are consistent with results from a previous study that examined the effects of global deletion of the soluble VEGF isoforms (VEGF188/188 mice) (17).
Figure 2. Cone but not rod photoreceptor dysfunction in RPE-specific Vegfa mutants.
(A) Immunohistochemistry for rhodopsin (rods) and cone opsin (cones) 45 days after induction. Note that rhodopsin expression in Vegfa mutants is comparable to that observed in controls. (B) Optical coherence tomographic analysis indicates that retinal thicknesses remain relatively unchanged at all stages through 7 months. (C) Scotopic dark-adapted ERG captured in Vegfa mutants 3 or 7 days after induction. Note that no significant reduction in the a- and b-wave amplitudes is observed (n = 4). (D) Indocyanine green angiography for Vegfa mutants 45 days after induction. Note that choroidal circulation is significantly diminished in the mutants, while the retinal circulation is normal. (E) Electron micrograph of a cross-sectioned Vegfa mutant retina 14 days, 2 months, and 7 months after induction. Note that a few deep choroidal vessels are observed (asterisk). (F) mRNA array for angiogenic genes in Vegfa mutant RPE/choroids compared with controls 3 days after injection (n = 3). Fold-change (x axis) and P value (y axis) of gene expression compared with controls are shown. Note that 32 of 84 genes are significantly downregulated. Error bars indicate mean ± SD. Scale bars: 2,000 μm (D); 200 μm (B); 20 μm (A); 10 μm (E).
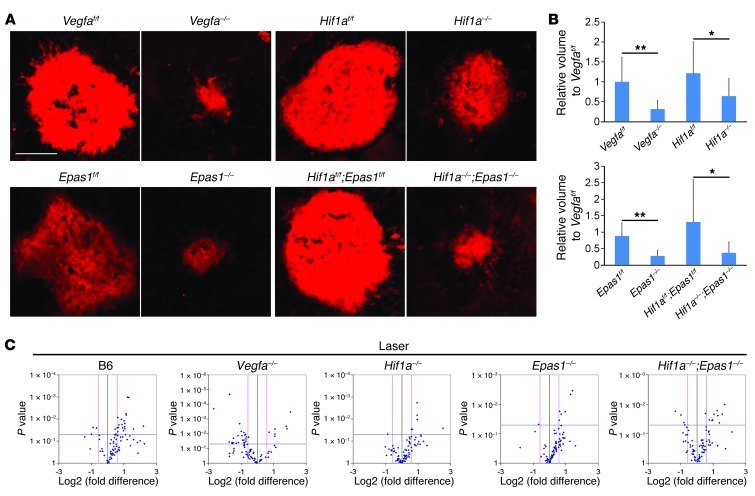
Atrophy and thinning of the choriocapillaris is a known age-related event in humans (18). To compensate for choriocapillaris thinning, the RPE is known to synthesize proangiogenic factors that maintain the choriocapillaris (19, 20). Our findings suggest that VEGF may act both directly and indirectly to regulate this phenomenon. Using gene-profiling assays, we demonstrated that the loss of Vegfa affects the expression of multiple angiogenesis-related genes (most are downregulated) in isolated RPE/choroid preparations (Figure 2F and Supplemental Table 1). Vegfa is tightly regulated by HIFs during development and pathologically in disease states (21, 22). To determine the role for HIFs in maintenance of the choriocapillaris, we genetically ablated Hif1a (encoding HIF-1α protein), Epas1 (HIF-2α), and Hif1a/Epas1 using VMD2-Cre in adult RPE. No obvious morphological, functional, or transcriptional differences, including Vegfa mRNA, were observed in naive-state adult HIF mutants (Supplemental Figures 3 and 4, and Supplemental Table 1). Several pathways, such as NF-κB/JunB and PGC1α/ERRα are known to induce Vegfa gene expression independently of HIFs (23, 24). These factors, or others, rather may maintain VEGF levels at sufficient levels in healthy retinas. Using laser photocoagulation to induce choroidal neovascularization (CNV), we determined that the extent of CNV was significantly reduced in Vegfa and Epas1 mutants (and partially reduced in Hif1a mutants; Figure 3, A and B). The upregulation of angiogenic genes was observed in wild-type C57BL/6 mice, but normal expression of these genes was observed in HIF mutants and expression was attenuated in Vegfa mutants compared with naive controls (Figure 3C and Supplemental Table 2). These data strongly suggest that HIFs become activated in pathological states to promote VEGF-mediated neovascularization, but are not required to maintain physiological vasculature.
Figure 3. Attenuated angiogenic gene regulation in pathological states of Vegfa conditional mutants.
(A) Representative Z projections of laser-CNV lesions (stained with GS lectin). (B) Quantified lesion volumes of laser-CNV (n = 8–21). Note that laser-CNV is only partially reduced in the Hif1a mutants, while it is completely inhibited in the Epas1 and Hif1a;Epas1 mutants (comparable to that observed in the Vegfa mutants). (C) mRNA array for angiogenic genes in Hif1a, Epas1, and Hif1a;Epas1 mutant RPE/choroids compared with controls in laser-irradiated C57BL/6 wild-type, Vegfa, Hif1a, Epas1, and Hif1a;Epas1 mutants compared with naive state controls (n = 3). Fold-change (x axis) and P value (y axis) of gene expression compared with controls. Note that few or no significantly dysregulated genes are observed in naive state Hif1a, Epas1, or Hif1a;Epas1 mutants. Twenty-two upregulated and 3 downregulated angiogenic genes are observed in laser-irradiated B6 wild-type mice, whereas we observed 7 (up) and 23 (down) in Vegfa mutants, 6 (up) and 1 (down) in Hif1a mutants, 6 (up) and 1 (down) in Epas1 mutants, and 10 (up) and 4 (down) in Hif1a;Epas1 mutants. *P < 0.05; **P < 0.01; 2-tailed Student’s t tests. Error bars indicate mean ± SD. Scale bar: 100 μm.
VEGF-mediated neovascularization is associated with vision loss in diseases such as AMD (10–12). The majority of patients receiving currently approved anti-VEGF drugs to treat AMD experience no adverse events. However, long-term safety studies of the effects of VEGF antagonism in the eye have not yet been completed, and there are isolated clinical reports showing progressive RPE/photoreceptor atrophy in patients on anti-VEGF therapies (25). Due to the differences in rod and cone photoreceptor distribution in humans and mice, it is difficult to speculate on what the effect of near-complete VEGF antagonism may be in human subjects. However, if a certain threshold of VEGF could be neutralized, the fovea would be very susceptible and the loss of macular function could result in the devastating loss of central vision. These observations, coupled with reported renal, gastrointestinal (GI), and mucosal complications in patients on systemic VEGF inhibition, contribute to the concern that VEGF antagonism may not be completely effective or safe (26, 27). The data in this study reinforce this concept and suggest that HIF-targeted or combination therapies targeting multiple angiogenic pathways may be safer and more effective than increased VEGF suppression therapy. Newer VEGF antagonists that bind VEGF with higher affinities and less rapid clearing from the eye are coming into use to treat a variety of neovascular diseases (28). Our results suggest that “off-target” effects of potent, persistent VEGF antagonism in the eye may exacerbate degeneration of the choriocapillaris, RPE, and photoreceptors due to the diminished trophic effect of RPE-produced VEGF.
Methods
Animals.
Transgenic mice carrying the human vitelliform macular dystrophy-2 (VMD2) promoter-directed reverse tetracycline-dependent transactivator (rtTA) and the tetracycline-responsive element–directed (TRE-directed) Cre recombinase (VMD2-Cre mice) (13) (provided by Y.Z. Le of University of Oklahoma Health, Sciences Center, Oklahoma City, Oklahoma, USA) were mated with Vegfaf/f mice (29), Hif1af/f mice (30), or Epas1f/f mice (31) (provided by R.S. Johnson of University of Cambridge, Cambridge, United Kingdom).
Laboratory measures.
Electron microscopy, ganzfeld ERG, immunohistochemistry, in vivo imaging, and laser-induced CNV were performed as previously described (32–34). mRNA PCR arrays for 84 angiogenic genes (Mouse Angiogenesis RTβ Profiler PCR Array, PAMM-024; QIAGEN) were performed according to the manufacturer’s instructions. Please see Supplemental Methods for more details.
Statistics.
Comparisons between the mean variables of 2 groups were performed by a 2-tailed Student’s t test. P < 0.05 was considered significant. Please see Supplemental Methods for more details.
Study approval.
All procedures involving animals were approved by the Scripps Research Institute Animal Care Committee, which ensures that all federal animal experimentation guidelines are met.
Supplementary Material
Acknowledgments
We would like to thank Malcolm Wood, Melissa S. Henrie, and Mollie S. Friedlander at the Scripps Research Institute for the excellent technical assistance, and Yun-Zheng Le at University of Oklahoma Health Sciences Center and Randall S. Johnson at University of Cambridge for providing transgenic mice. This work was supported by grants to M. Friedlander from the National Eye Institute (EY-11254) and the Lowy Medical Institute (MacTel). T. Kurihara is supported by a fellowship from the Manpei Suzuki Diabetes Foundation and The Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad. P.D. Westenskow is a Ruth L. Kirschstein Fellow of the NIH (EY021416).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(11):4213–4217. doi:10.1172/JCI65157.
See the related Commentary beginning on page 3849.
References
- 1.Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43(11):3500–3510. [PubMed] [Google Scholar]
- 2.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010;112(6):1584–1592. doi: 10.1111/j.1471-4159.2010.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marneros AG, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167(5):1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krzystolik MG, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120(3):338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 6.Aiello LP, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med. 2011;17(11):1445–1447. doi: 10.1038/nm.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson GS, et al. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J. 2001;15(7):1215–1217. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- 10.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 11.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Le YZ, et al. Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2008;49(3):1248–1253. doi: 10.1167/iovs.07-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ames A. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992;12(3):840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JE, et al. Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Mol Vis. 2007;13:887–919. [PMC free article] [PubMed] [Google Scholar]
- 16.Saint-Geniez M, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106(44):18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43(6):1986–1993. [PubMed] [Google Scholar]
- 19.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120(9):3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355(14):1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 21.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365(6):537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 23.Arany Z, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt D, et al. Critical role for NF-kappaB-induced JunB in VEGF regulation and tumor angiogenesis. Embo J. 2007;26(3):710–719. doi: 10.1038/sj.emboj.7601539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–530. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Eremina V, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gressett SM, Shah SR. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother. 2009;43(3):490–501. doi: 10.1345/aph.1L426. [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulos N, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;2012(2):3. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber HP, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 30.Ryan HE, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60(15):4010–4015. [PubMed] [Google Scholar]
- 31.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104(7):2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorrell MI, et al. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009;119(3):611–623. doi: 10.1172/JCI35977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurihara T, Westenskow PD, Krohne TU, Aguilar E, Johnson RS, Friedlander M. Astrocyte pVHL and HIF-alpha isoforms are required for embryonic-to-adult vascular transition in the eye. J Cell Biol. 2011;195(4):689–701. doi: 10.1083/jcb.201107029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izumi-Nagai K, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170(6):2149–2158. doi: 10.2353/ajpath.2007.061018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.