Abstract
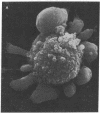
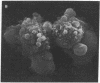
The role of Ca2+ in toxic liver cell death was studied with primary cultures of adult rat hepatocytes. Within 1 hr of exposure to phalloidin, a bicyclic heptapeptide isolated from the mushroom Amanita pahlloides, at 50 micrograms/ml, 60--70% of the cells were dead (trypan blue stainable). There was no loss of viability of the same cells exposed to phalloidin in culture medium devoid of Ca2+. A marked structural alteration of the surface of the phalloidin-treated hepatocytes characterized by innumerable evaginations seen by scanning electron microscopy occurred in the presence or absence of Ca2+. Pretreatment of the cells with cytochalasin B at 10 micrograms/ml prevented the surface alteration and the death of the cells in Ca2+ medium. Exposure of the cells to phalloidin in the absence of Ca2+ followed by exposure to cytochalasin B and then to Ca2+ also prevented the cell death. These results suggest a two-step mechanism by which phalloidin causes liver cell death. Initially phalloidin interacts in a Ca2+-independent process with cell membrane-associated actin. The second step is a Ca2+-dependent process that most likely represents an increased influx of Ca2+ across a compromised cell membrane permeability barrier and down the steep concentration gradient that exists between the outside and inside of the cell. These results strengthen the hypothesis that disturbances in Ca2+ homeostasis induced in vivo by a variety of hepatotoxins are causally related to liver cell death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dancker P., Löw I., Hasselbach W., Wieland T. Interaction of actin with phalloidin: polymerization and stabilization of F-actin. Biochim Biophys Acta. 1975 Aug 19;400(2):407–414. doi: 10.1016/0005-2795(75)90196-8. [DOI] [PubMed] [Google Scholar]
- El-Mofty S. K., Scrutton M. C., Serroni A., Nicolini C., Farber J. L. Early, reversible plasma membrane injury in galactosamine-induced liver cell death. Am J Pathol. 1975 Jun;79(3):579–596. [PMC free article] [PubMed] [Google Scholar]
- Farber J. L., Gill G., Konishi Y. Prevention of galactosamine-induced liver cell necrosis by uridine. Am J Pathol. 1973 Jul;72(1):53–62. [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Schäfer A. J., Weckauf M. The dissociation of the phalloidin-actin complex. Hoppe Seylers Z Physiol Chem. 1977 Feb;358(2):181–184. doi: 10.1515/bchm2.1977.358.1.181. [DOI] [PubMed] [Google Scholar]
- Frimmer M. The role of plasma membranes in hepatotoxic effects. Naunyn Schmiedebergs Arch Pharmacol. 1977;297 (Suppl 1):S15–S19. doi: 10.1007/BF00587764. [DOI] [PubMed] [Google Scholar]
- Frimmer M. Toxic cyclopeptides of the toadstool Amanita phalloides and related species. Naunyn Schmiedebergs Arch Pharmakol. 1971;269(2):152–163. doi: 10.1007/BF01003033. [DOI] [PubMed] [Google Scholar]
- GALLAGHER C. H., GUPTA D. N., JUDAH J. D., REES K. R. Biochemical changes in liver in acute thioacetamide intoxication. J Pathol Bacteriol. 1956 Jul;72(1):193–201. doi: 10.1002/path.1700720125. [DOI] [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. 1. The effect of insulin. In Vitro. 1976 Jul;12(7):521–532. doi: 10.1007/BF02796495. [DOI] [PubMed] [Google Scholar]
- Lengsfeld A. M., Löw I., Wieland T., Dancker P., Hasselbach W. Interaction of phalloidin with actin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2803–2807. doi: 10.1073/pnas.71.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz F., Glossmann H., Frimmer M. Binding of 3 H-desmethylphalloin to isolated plasma membranes from rat liver. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(4):341–351. doi: 10.1007/BF00499668. [DOI] [PubMed] [Google Scholar]
- Löw I., Dancker P. Effect of cytochalasin B on formation and properties of muscle F-actin. Biochim Biophys Acta. 1976 May 14;430(2):366–374. doi: 10.1016/0005-2728(76)90092-x. [DOI] [PubMed] [Google Scholar]
- Löw I., Dancker P., Wieland T. h. Stabilization of F-actin by phalloidin. Reversal of the destabilizing effect of cytochalasin B. FEBS Lett. 1975 Jun 15;54(2):263–265. doi: 10.1016/0014-5793(75)80088-3. [DOI] [PubMed] [Google Scholar]
- Löw I., Dancker P., Wieland T. h. Stabilization of F-actin by phalloidin. Reversal of the destabilizing effect of cytochalasin B. FEBS Lett. 1975 Jun 15;54(2):263–265. doi: 10.1016/0014-5793(75)80088-3. [DOI] [PubMed] [Google Scholar]
- Löw I., Wieland T. The interaction of phalloidin. Some of its derivatives, and of other cyclic peptides with muscle actin as studied by viscosimetry. FEBS Lett. 1974 Aug 30;44(3):340–343. [PubMed] [Google Scholar]
- MCLEAN A. E., AHMED K., JUDAH J. D. CELLULAR PERMEABILITY AND THE REACTION TO INJURY. Ann N Y Acad Sci. 1964 Aug 27;116:986–989. doi: 10.1111/j.1749-6632.1964.tb52563.x. [DOI] [PubMed] [Google Scholar]
- McLean A. E., McLean E., Judah J. D. Cellular necrosis in the liver induced and modified by drugs. Int Rev Exp Pathol. 1965;4:127–157. [PubMed] [Google Scholar]
- Moore L., Rodman Davenport G., Landon E. J. Calcium uptake of a rat liver microsomal subcellular fraction in response to in vivo administration of carbon tetrachloride. J Biol Chem. 1976 Feb 25;251(4):1197–1201. [PubMed] [Google Scholar]
- Nicholls D. G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978 Nov 15;176(2):463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- REES K. R., SINHA K. P., SPECTOR W. G. The pathogenesis of liver injury in carbon tetrachloride and thioacetamide poisoning. J Pathol Bacteriol. 1961 Jan;81:107–118. [PubMed] [Google Scholar]
- REYNOLDS E. S. LIVER PARENCHYMAL CELL INJURY. I. INITIAL ALTERATIONS OF THE CELL FOLLOWING POISONING WITH CARBON TETRACHLORIDE. J Cell Biol. 1963 Oct;19:139–157. doi: 10.1083/jcb.19.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. LIVER PARENCHYMAL CELL INJURY. II. CYTOCHEMICAL EVENTS CONCERNED WITH MITOCHONDRIAL DYSFUNCTION FOLLOWING POISONING WITH CARBON TETRACHLORIDE. Lab Invest. 1964 Nov;13:1457–1470. [PubMed] [Google Scholar]
- REYNOLDS E. S., THIERS R. E., VALLEE B. L. Mitochondrial function and metal content in carbon tetrachloride poisoning. J Biol Chem. 1962 Nov;237:3546–3551. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Smuckler E. A. Studies on carbon tetrachloride intoxication. IV. Effect of carbon tetrachloride on liver slices and isolated organelles in vitro. Lab Invest. 1966 Jan;15(1 Pt 1):157–166. [PubMed] [Google Scholar]
- Spudich J. A., Lin S. Cytochalasin B, its interaction with actin and actomyosin from muscle (cell movement-microfilaments-rabbit striated muscle). Proc Natl Acad Sci U S A. 1972 Feb;69(2):442–446. doi: 10.1073/pnas.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Weisburger E. K., Weisburger J. H. Isolation and long-term cell culture of epithelial-like cells from rat liver. Exp Cell Res. 1971 Nov;69(1):106–112. doi: 10.1016/0014-4827(71)90316-8. [DOI] [PubMed] [Google Scholar]