Abstract
In this prospective, non-randomized phase-I clinical trial, we comparatively studied the performance of six laterally-directed biopsies or the modified fan-shaped biopsies (MFSB), midline sextant biopsies (MB), and transition zone biopsies (TZB) and examine their prostate cancer (PCa) detection rates. A total of 114 patients received combinations of MFSB, MB, and TZB based on prostate gland volume: those ≤15cc received 8 biopsies; those >15cc but ≤ 50cc received 14 biopsies; and those >50cc received 20 biopsies. The mean prostate-specific antigen (PSA) level, Gleason score, and prostate volume were 8.0 ng/ml, 6.4, and 47 cc, respectively. PCa detection rate of the MB was 25% while the MFSB was 22%. The overall PCa detection rate was 33.3% with all biopsies. PCa and high-grade prostatic intraepithelial neoplasia (HG-PIN) detection rates decrease as the size of the prostate increases. PCa detection rates were 50.0% for volumes ≤19.9cc and volumes of >50cc had a detection rate of 25.8%. PSA levels of <3.0 had PCa detection rates of 15% which increased to 58% with PSA levels >9.0. In a multivariate analysis, only TZB was significant for PCa diagnosed by PSA (β=7.4, p<0.01). Our study showed that it is important to perform both the lateral MFSB and the MB to improve overall PCa detections rates. Thus, we recommend performing MB, MFSB, and TZB based on prostate volume, as follows: 8 biopsies for ≤15 cc; 14 for those >15 cc but ≤50 cc, and 14-20 for those >50 cc.
Keywords: Prostate cancer, prostate gland volume, transrectal core biopsies, gleason score, PSA
Introduction
Advances in prostate cancer (PCa) screening via transrectal ultrasound (TRUS) guided needle biopsies have led to a progressive increase in the number of prostate biopsy cores. In 2011, approximately one million biopsy procedures were performed in the US, from which 240,890 were diagnosed with PCa [1]. The indications for a TRUS guided prostate needle biopsy are elevated serum prostate-specific antigen (PSA) level and/or abnormal digital rectal exam. However, both of them lack sensitivity and/or specificity to detect all clinically significant PCa, while at the same time they fail to identify PCa that would not progress during the patient’s lifetime [2]. Although serum PSA levels of ≤4.0 ng/mL are generally thought to be within normal reference range, there is a risk of PCa at all PSA values [3]; thus there is no ideal PSA level that yields both high sensitivity and high specificity. In the placebo arm of the Prostate Cancer Prevention Trial, the prevalence of PCa was 6.6% in men with PSA ≤0.5 ng/mL. This prevalence increased to 26.4% in men with PSA of 3.1-4.0 ng/mL [4]. Consequently, TRUS guided needle biopsy interpretation remains of paramount importance in clinical management of PCa [5,6].
The first sextant prostate needle biopsy scheme was developed by Hodge et al. in 1989 [7]. The sextant biopsy scheme consisted of biopsies of the prostate in the midline at the base, mid-gland, and apex. The midline sextant biopsies (MB) had a PCa detection rate of 20-30% [8-10]. However, 25-50% of aggressive PCa may remain undetected when using the MB scheme [11]. Stamey et al. in 1995 evaluated radical prostatectomy specimens and found that PCa had a higher likelihood of being found in the anterior horns of the peripheral zone and suggested that laterally directed biopsies may provide better detection [12]. Multiple studies have found that directing prostate needle biopsies more laterally increases the PCa detection rates [8-10]. Hence, the current recommendation is an extended-biopsy scheme with at least 8-12 cores including lateral biopsies, and transition zone biopsies (TZB) are not recommended on initial evaluation [13].
Due to the limitations of serum PSA levels to identify PCa patients, current biopsy protocols need to increase specificity for potentially aggressive PCa without reducing sensitivity. This may be accomplished by modifications to the number of cores and the biopsy location. Using computer simulations, it has been demonstrated that six laterally-directed biopsies, or the modified fan-shaped biopsy (MFSB) method, detect 20% more aggressive tumors than the MB scheme [14]. In the MFSB technique, the biopsy probe and needle are rotated 45 degrees to obtain more laterally directed biopsies. The study found that rotating the angle at which biopsies are taken can markedly improve the PCa detection rate without increasing the number of biopsies [14] .
In the present study, we evaluated 119 men who had an elevated PSA (>4.0ng/mL) and/or an abnormal digital rectal exam with a prostate needle biopsy. The participants were grouped according to prostate size. If the participant’s prostate was ≤15 cc then an 8-core biopsy was performed (Figure 1). If the prostate was >15 cc but <50 cc, a 14-core biopsy scheme was performed. Lastly, if the prostate was ≥50 cc, a 20-core biopsy scheme was performed. The biopsy schemes consisted of a combination of MB, MFSB, and TZB. We examined whether PCa detection rates were improved according to number and location of biopsies.
Figure 1.
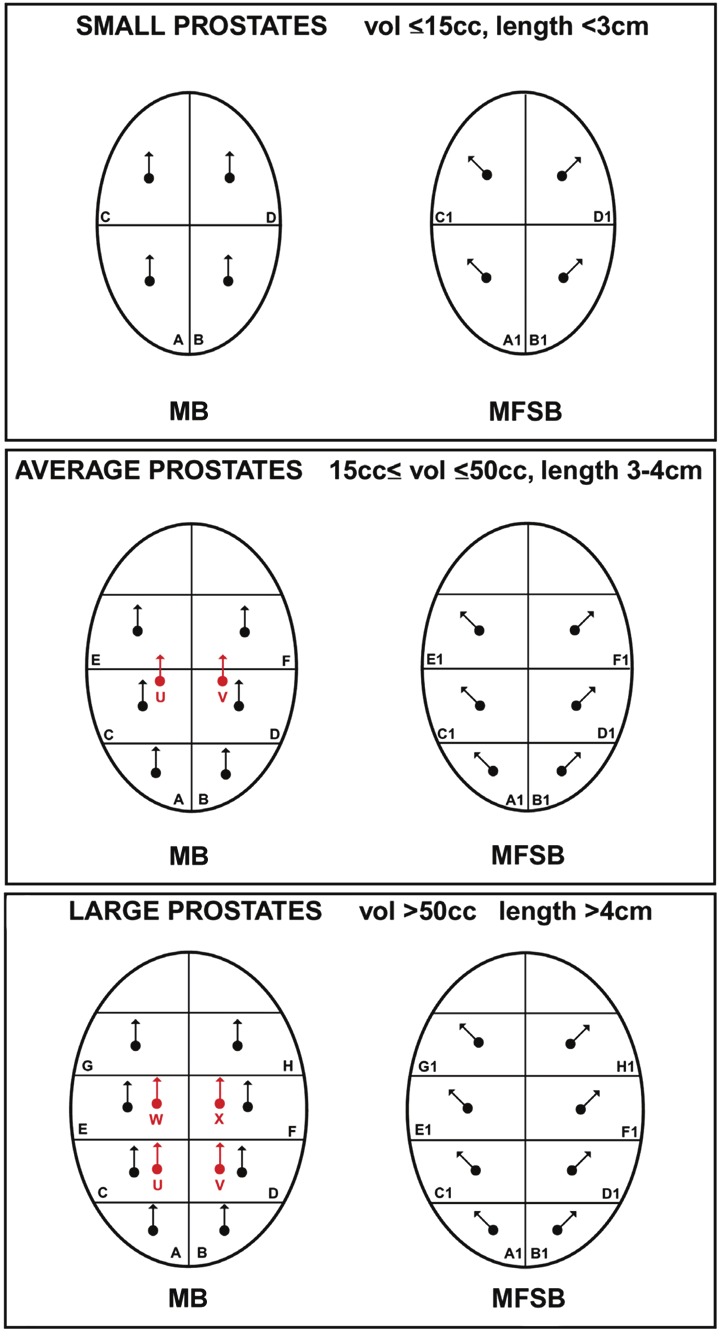
Proposed biopsy scheme based on size of the prostate where midline sextant biopsy (MB) is denoted by A-H, modified fan-shaped biopsy (MFSB) is denoted by A1-H1, and transition zone biopsy (TZB) is denoted by U-W.
Materials and methods
This was a prospective, non-randomized study conducted from November 2002 to March 2008. 119 patients were selected and agreed to participate in the study. Patients qualified for the study based on having either an elevated PSA for their age, increasing PSA velocity, or abnormal digital rectal examination. It was not required that this biopsy be the patient’s first prostate biopsy. The prostate biopsies were performed by two urologists: one at the University of Colorado Hospital (EDC) and one at the Denver Heath and Hospital Administration (FJK).
All patients received fluoroquinolones before the biopsy and an enema on the morning of the biopsy. All patients were then lain in the left lateral decubitus position and were administered local anesthesia with 1% lidocaine using a spinal needle under ultrasound guidance [15]. An 18-gauge biopsy needle was used to perform the biopsies.
Prostate gland volume was calculated using TRUS and participants were divided into three groups based on the size of their prostate. The number of biopsies each participant received varied based on the size of the prostate. Prostates that were ≤15 cc had eight biopsies (Figure 1). The eight biopsies were taken from the prostate base and apex bilaterally, and then two more, laterally oriented, MFSB were taken at the base and apex, bilaterally (Figure 1). Prostates that were >15 cc but ≤50 cc received 14 biopsies. The fourteen biopsies were taken from the following locations: the base, mid and apex, bilaterally (normal MB pattern described by Hodge et al.); three more MFSB bilaterally at the base, mid and apex; and bilateral TZB (Figure 1). Prostates that were >50 cc received 20 biopsies, taken from base, proximal mid gland, distal mid gland and apex, bilaterally; four more MFSB at the base, proximal mid gland, distal mid gland and apex, bilaterally; and finally two TZB, bilaterally (Figure 1). Additional biopsies were performed if there was a palpable lesion on rectal exam or a lesion visualized by ultrasound.
The MFSB approach was developed to take lateral biopsies. To take MFSB, the rectal ultrasound probe is manipulated up and down, using the anal sphincter as a fulcrum. In the left lateral decubitus position, angling the ultrasound probe handle down 45 degrees from midline will image the right side of the prostate and aid in biopsying the right lateral prostate. Angling the ultrasound probe handle up 45 degrees from midline will image the left side of the prostate and aid in biopsying the left lateral prostate.
Five patients were excluded from the study. One was excluded because the biopsy specimens were mislabeled and the locations of the biopsy cores were not correct. Four other participants were excluded because more than half of their biopsies were fibromuscular tissue without any identifiable prostate tissue.
The biopsy specimens were separately labeled and reviewed by one pathologist at the University of Colorado Hospital (FGLR). Adequate biopsy samples were diagnosed as positive for PCa, high grade prostatic intraepithelial neoplasia (HG-PIN), or benign. Three cores that showed lesions considered as atypical small acinar proliferations were further evaluated between two pathologists to classify them as either HG-PIN or PCa (FGLR, MSL). Biopsy cores that were positive for PCa had the Gleason score (GS) and extent of core involvement recorded for each core.
The diagnostic performances of MB, MFSB, and TZB techniques were analyzed statistically using SAS statistical software.
Results
A total of 114 patients were included in the analyses since 5 patients were excluded due to incomplete data. The mean age of the patient population was 62 years. The majority of the participants were white (56.1%), followed by Hispanic (18.4%), African American (15.7%), and Asian (1%). 8.8% of the participants did not identify race. The mean PSA was 8.0 ng/mL while the mean prostate gland volume was 47 cc; the average GS was 6.4 (Table 1).
Table 1.
Demographics
| Age (years), mean | 62 | |
|---|---|---|
| Race | White | 64 (56.1%) |
| Black | 18 (15.7%) | |
| Hispanic | 21 (18.4%) | |
| Asian | 1 (1%) | |
| Unknown | 10 (8.8%) | |
| Serum PSA, mean (ng/mL) | 8 | |
| Prostate volume, mean (cc) | 47 | |
| Gleason score, mean | 6.4 | |
Overall PCa detection was 33.3% using the biopsy techniques described above. PCa detection rate using the laterally directed fan-shaped biopsy scheme was 22%, versus 25% using the sextant scheme. Of the 38 total patients with PCa, 25 of the patients had positive cores using the laterally-directed MFSB technique (66%) and 29 patients had positive cores using the MB technique (76%). However, using just the MB technique, the PCa detection rate was 25.4% but adding the additional MFSB biopsies increased the PCa detection rate to 30.9% (Table 2). Significant differences were observed between unique PCa detected by MB versus TZB and MFSB versus TZB (McNemar’s test p<0.01 and p<0.02, respectively). However, there was no significant difference between unique cancers diagnosed by MB versus MFSB.
Table 2.
Prostate cancer detection according to biopsy location
| Location | Cancer Detection Rate |
|---|---|
| Overall (N=38) | 33.3% |
| MFSB (N=25) | 21.9% |
| MB (N=29) | 25.4% |
| TZB (N=15) | 13.2% |
PCa detection according to prostate size is shown in Figure 2. Overall, the PCa detection rate decreased as the size of the prostate increased. There was no significant relationship between prostate size and PCa diagnosed by MB, MFSB, or TZB for any PSA value. The 50-59.9 cc group had an increased PCa detection rate (57%) and is most likely an outlier. HG-PIN detection rate also decreased with increasing volume of the prostate. Prostates with volume of ≤19.9 cc had the highest HG-PIN detection rate at 57.1%.
Figure 2.
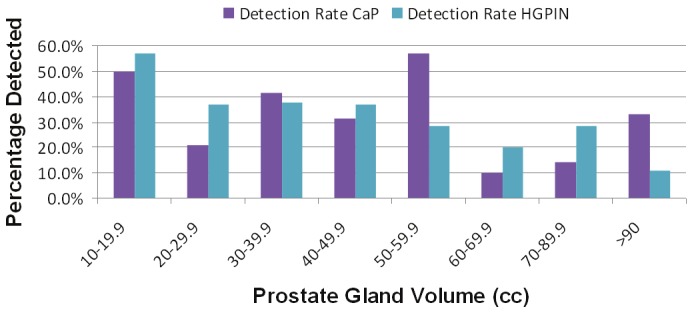
Prostate Cancer and high-grade prostatic intraepithelial neoplasia (HG-PIN) diagnosed according to prostate gland volume in 20 cc increments.
Figure 3 shows PCa detection rates by the three categories of prostate size, which determined which biopsy scheme was performed. Prostates that were ≤15 cc had eight cores taken with a PCa detection rate of 40%. Prostates between 15 cc and 50 cc had fourteen cores taken with a PCa detection rate of 36%. Prostate volumes that were ≥50 cc had 20 cores taken with a PCa detection rate of 25.8%. HG-PIN detection rate was inversely proportional to prostate volume.
Figure 3.
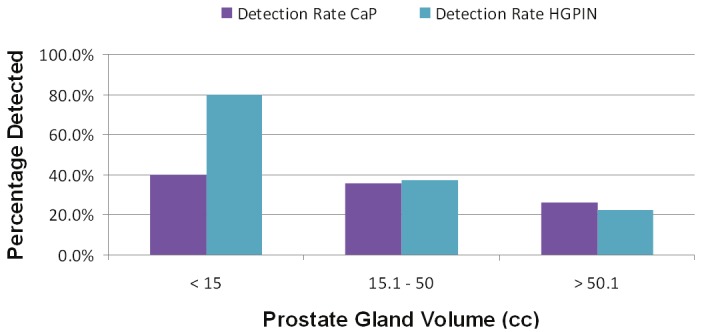
Prostate cancer and high-grade prostatic intraepithelial neoplasia (HG-PIN) diagnosed according to the proposed biopsy scheme.
Next we evaluated the effect of PSA level on PCa detection rate. Figure 4 shows that as PSA increase the PCa detection rate also increase. At a PSA level of <3 the PCa detection rate was 15% and increased to 58% with a PSA level >9. Secondly, the detection rate of HG-PIN remains stable or slightly decreased as the PSA level increased. The HG-PIN detection rate for a PSA of <3.0 was 45%, which decreased to 33% for a PSA of >15.
Figure 4.
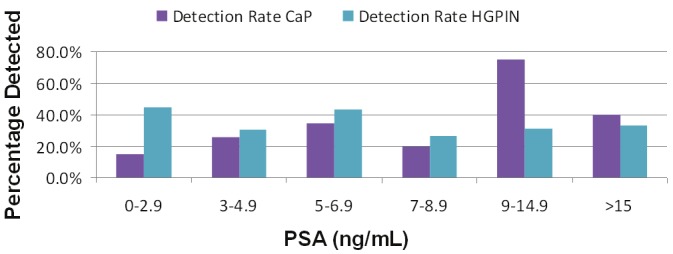
Prostate cancer and high-grade prostatic intraepithelial neoplasia (HG-PIN) diagnosed according to serum PSA.
Table 3 evaluates age, PSA, prostate volume, and GS for participants with PCa by biopsy method. PSA was the same for men with positive biopsies in both the MFSB and the MB. PSA and mean GS were much higher for men with positive TZB. Men who did not have PCa had a mean PSA of 6.8. Mean PSA for men with a positive biopsy were 11.4 for MFSB technique; 10.5 for MB technique; and 15.3 for TZB. By univariate analysis, PSA significantly predicted PCa detected by MFSB and TZB (p=0.044 and p=0.01, respectively). By multivariate analysis, only TZB predicted for PCa diagnosed by PSA (β=7.4, p<0.01). PSA density (PSA/prostate gland volume) was another significant factor by univariate analysis for PCa diagnosed by MFSB and TZB (p=0.0496 and p=0.014, respectively) but PSA density did not retain significance by multivariate analysis.
Table 3.
Demographics by age, serum PSA, prostate volume
| Prostate Cancer Diagnosed by Method | Age | PSA | Volume | GS | |
|---|---|---|---|---|---|
| MFSB (N=25) | Median | 63.0 | 7.3 | 35.6 | 6.0 |
| Mean | 63.0 | 11.4 | 46.7 | 6.5 | |
| MB (N=29) | Median | 63.0 | 7.3 | 33.9 | 6.0 |
| Mean | 62.8 | 10.5 | 43.8 | 6.5 | |
| TZB (N=15) | Median | 63.0 | 11.2 | 38.6 | 6.0 |
| Mean | 61.9 | 15.3 | 45.3 | 6.7 | |
| No Cancer (N=76) | Median | 61.0 | 5.4 | 37.9 | NA |
| Mean | 61.3 | 6.8 | 49.8 | NA | |
Discussion
The overall PCa detection rate by MB is 20-30% [7-10]. Sextant biopsy scheme using the Hodge et al. technique are estimated to miss 20-30% of PCa [16]. Multiple studies have found that laterally directed prostate needle biopsies increase PCa detection rate by 20 to 25% [10,17] for an overall detection rate of 35-44% [8-10,18]. In our study, the midline biopsy scheme, similar to the sextant pattern, had a PCa detection rate of 25%. Addition of the laterally directed fan-shaped biopsies increased the detection rate to 33%, a 24% increase. This increase is consistent with other studies noted above. If just the lateral biopsies were performed the PCa detection rate would have been 21%, therefore, based on our data, it is still important to perform the more midline biopsies. Presti et al. found that midline biopsies were necessary to achieve a PCa detection rate of 44% [18]. That study was a retrospective review of prostate biopsy results of 2,299 patients from 167 urologists in 36 states. The study ranked the detection rates in a 12-core biopsy from highest to lowest: lateral mid prostate (54%), lateral apex (52%), apex (50%), lateral base (50%), mid gland (48%), base (42%). Multiple studies, including ours, have shown that laterally directed biopsies increase PCa detection rates. However, midline biopsies also contribute to PCa detection and cannot be eliminated from the biopsy schemes.
Twelve-core biopsy schemes are the current standard of care. Twelve cores appear to be appropriate for normal size prostates; however, prostate size inversely relates to PCa detection. What is the appropriate number of cores for different size prostates to maintain a PCa detection rate in the 35-45% range? Our study showed that as prostate size increased, in general, PCa and HG-PIN detection rates decreased. Our study initially grouped patients by prostate size: prostates that were ≤15 cc had eight biopsies, those >15 cc but ≤50 cc had fourteen biopsies and those >50 cc had twenty biopsies. For prostates <15 cc and between 15 cc and 50 cc, 8-core or 14-core biopsies provide adequate PCa detection rates (40% and 36%, respectively). However, prostates >50 cc remain problematic; even 20-core biopsies yielded a PCa detection rate of 26%, inferior to smaller prostates. Similarly, De La Taille et al. found that in prostates ≥40 cc, the PCa detection rate increased by increasing the number of biopsies; however, even with 21 biopsy cores their detection rate was still only 25% [10]. Karakiewicz et al. looked at PCa detection rate of sextant biopsy according to prostate size and found a PCa detection rate of 14% for prostates ≥50 cc [19]. Inahara et al. evaluated 8-core and 14-core biopsy schemes and found PCa detection rates of 17% and 19% respectively for prostates >40 cc [20]. This study concluded that larger prostates need at least a 14-core biopsy scheme. Based on our data and other studies we recommend that in prostates that ≤15 cc, that 8-core biopsies are sufficient; in those 15 cc to 50 cc, 12-14 biopsies are recommended; and in those >50 cc additional biopsies are needed, although even 20 biopsies in our study gave a PCa detection rate of only 26%. Additional studies of biopsy techniques for large prostates need to be conducted.
Between 20-25% of all PCa are found in the transition zone [21]. The PCa detection rate from TZB is 2-4% and adds minimally to overall PCa detection rate [10]. Our study found a 13% PCa detection rate for TZB. Our TZB also did not boost PCa detection rates much and we would not recommend TZB unless a patient has had multiple negative biopsies.
Our study also evaluated PCa detection rates according to serum PSA. As serum PSA increases, the PCa detection rate increases. However, the detection rate of HG-PIN remained stable or slightly decreased as the PSA level increased. PCa detection rate rose to 58% for a PSA > 9.0. Presti et al. also found that as PSA increased so did PCa detection [18]. Men with a PSA >10 ng/mL had a PCa detection rate of 48% which was higher than the overall 44% PCa detection rate. Also, men with positive TZB had serum PSAs that were higher than in men with positive biopsies in other locations of the prostate.
Thirty eight (38) patients diagnosed with PCa in this study chose radical prostatectomy (8 open, 8 laparoscopic), 6 had radiation, 6 had watchful-waiting, 3 had hormonal therapies, and 7 opted for focal cryotherapy [22]. Among 8 patients who had open radical prostatectomy, 3/8 had non-organ confined disease (pathologic stage pT3). Among 5 patients with organ confined disease, 2 had clinically non-threatening PCa (each had 3 tumor foci of GS 6 with total volumes of 0.091 and 0.032 cc) [14]. Seven patients who opted for focal cryotherapy had transperineal 3D mapping biopsy for additional screening [22,23]. Two patients had bilateral PCa, and hence were ineligible for focal cryotherapy.
The overall sensitivity of the extended-biopsy scheme is significantly better than the sextant biopsy or laterally directed biopsy alone and may provide additional information for therapeutic decisions. TZB should be included for men with multiple negative biopsies. Also, for prostates ≤15 cc, eight biopsies are recommended. For prostates between 15 cc and 50 cc 14 biopsies are recommended. For prostates ≥50 cc, even 20 biopsies are not enough since the detection rate dropped by 10% as compared to the 15 cc and 50 cc groups.
Acknowledgements
This study was supported by United States Public Health Service Grant CA66161.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Djavan B, Milani S, Remzi M. Prostate biopsy: who, how and when. An update. Can J Urol. 2005;12(Suppl 1):44–48. [PubMed] [Google Scholar]
- 3.Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, Parnes HL, Coltman CA Jr. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 5.Montironi R, Mazzuccheli R, Scarpelli M, Lopez-Beltran A, Fellegara G, Algaba F. Gleason grading of prostate cancer in needle biopsies or radical prostatectomy specimens: contemporary approach, current clinical significance and sources of pathology discrepancies. BJU Int. 2005;95:1146–1152. doi: 10.1111/j.1464-410X.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 6.Raja J, Ramachandran N, Munneke G, Patel U. Current status of transrectal ultrasound-guided prostate biopsy in the diagnosis of prostate cancer. Clin Radiol. 2006;61:142–153. doi: 10.1016/j.crad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–74. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 8.Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, Evans R, Johnston D, Chen M. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152–157. [PubMed] [Google Scholar]
- 9.Presti JC Jr, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163:163–166. [PubMed] [Google Scholar]
- 10.de laTaille A, Antiphon P, Salomon L, Cherfan M, Porcher R, Hoznek A, Saint F, Vordos D, Cicco A, Yiou R, Zafrani ES, Chopin D, Abbou CC. Prospective evaluation of a 21-sample needle biopsy procedure designed to improve the prostate cancer detection rate. Urology. 2003;61:1181–1186. doi: 10.1016/s0090-4295(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 11.Crawford ED, Hirano D, Werahera PN, Lucia MS, DeAntoni EP, Daneshgari F, Brawn PN, Speights VO, Stewart JS, Miller GJ. Computer modeling of prostate biopsy: tumor size and location--not clinical significance--determine cancer detection. J Urol. 1998;159:1260–1264. doi: 10.1016/s0022-5347(01)63576-6. [DOI] [PubMed] [Google Scholar]
- 12.Stamey TA. Making the most out of six systematic sextant biopsies. Urology. 1995;45:2–12. doi: 10.1016/s0090-4295(95)96168-2. [DOI] [PubMed] [Google Scholar]
- 13.Ochiai A, Babaian RJ. Update on prostate biopsy technique. Curr Opin Urol. 2004;14:157–162. doi: 10.1097/00042307-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kawata N, Miller GJ, Crawford ED, Torkko KC, Stewart JS, Lucia MS, Miller HL, Hirano D, Werahera PN. Laterally directed biopsies detect more clinically threatening prostate cancer: computer simulated results. Prostate. 2003;57:118–128. doi: 10.1002/pros.10285. [DOI] [PubMed] [Google Scholar]
- 15.Nash PA, Bruce JE, Indudhara R, Shinohara K. Transrectal ultrasound guided prostatic nerve blockade eases systematic needle biopsy of the prostate. J Urol. 1996;155:607–609. [PubMed] [Google Scholar]
- 16.Presti JC Jr. Prostate biopsy: how many cores are enough? Urol Oncol. 2003;21:135–140. doi: 10.1016/s1078-1439(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 17.Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol. 2007;52:1309–1322. doi: 10.1016/j.eururo.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Presti JC Jr, O’Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003;169:125–129. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- 19.Karakiewicz PI, Bazinet M, Aprikian AG, Trudel C, Aronson S, Nachabe M, Peloquint F, Dessureault J, Goyal MS, Begin LR, Elhilali MM. Outcome of sextant biopsy according to gland volume. Urology. 1997;49:55–59. doi: 10.1016/S0090-4295(96)00360-3. [DOI] [PubMed] [Google Scholar]
- 20.Inahara M, Suzuki H, Kojima S, Komiya A, Fukasawa S, Imamoto T, Naya Y, Ichikawa T. Improved prostate cancer detection using systematic 14-core biopsy for large prostate glands with normal digital rectal examination findings. Urology. 2006;68:815–819. doi: 10.1016/j.urology.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 21.McNeal J. Origin and development of carcinoma of the prostate. Cancer. 1969;23:24. doi: 10.1002/1097-0142(196901)23:1<24::aid-cncr2820230103>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Barqawi AB, Crawford ED. The current use and future trends of focal surgical therapy in the management of localized prostate cancer. Cancer J. 2007;13:313–317. doi: 10.1097/PPO.0b013e318156eb99. [DOI] [PubMed] [Google Scholar]
- 23.Crawford ED, Wilson SS, Torkko KC, Hirano D, Stewart JS, Brammell C, Wilson RS, Lucia MS, Werahera PN. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int. 2005;96:999–1004. doi: 10.1111/j.1464-410X.2005.05801.x. [DOI] [PubMed] [Google Scholar]


