Abstract
Researchers have begun to appreciate the significant role that the microenvironment plays in tumorigenesis and are now shedding light on the role of the stroma in induction and progression of solid tumors. While the stroma of solid tumors is comprised of many cell types, including vascular and immune cells, one of the most prominent cell types in the tumor stroma is the fibroblast, called the carcinoma-associated fibroblast (CAF) or tumor-associated fibroblast (TAF). The interaction between CAFs and tumor cells is quite complex. CAFs have been implicated in tumor angiogenesis, immunosuppression, tumor cell proliferation and aggressiveness, genetic instability, and metastasis. However, their specific roles in each of these processes have only been partially elucidated. Determining the role of CAFs has been complicated by the fact that researchers have demonstrated heterogeneity in the stromal fibroblast population. This heterogeneity has brought about the concept of multiple origins for CAFs. While many origins of CAFs have been suggested, in our own laboratory we have identified a novel hematopoietic stem cell (HSC) origin of CAFs. Given the profound role of CAFs in tumor progression and prognosis, the CAF represents an exciting potential therapeutic target. The heterogeneity of the CAF population makes research directed at investigating the roles and origins of CAFs critical to development of such anti-tumor therapies.
Keywords: Hematopoietic stem cells, carcinoma associated fibroblasts, tumor associated fibroblasts, stem cell plasticity, tumor progression, metastasis
Introduction
Cancer has been likened to a perpetual wound healing process [1]. Both wound healing and cancer begin with the formation of a reactive stroma. In wound healing the reactive stroma resolves rapidly, but in cancer this inflammatory state is perpetuated as the cancer progresses. The reactive stroma is comprised of endothelial cells, immune cells, lymphocytes, macrophages, fibrocytes, fibroblasts, myofibroblasts and a milieu of growth factors, chemokines and cytokines. The fibroblast population represents one of the most prominent cell types in the reactive stroma and is therefore hypothesized to have a profound effect on surrounding epithelial cells and other stromal cells. In the tumor stroma these fibroblasts are termed carcinoma-associated fibroblasts (CAFs) or tumor-associated fibroblasts (TAFs). CAFs have been shown to play a role in a variety of tumor promoting processes including proliferation, angiogenesis, invasion and metastasis [2,3].
With the extensive role that CAFs play in tumor progression, it is not surprising that there is significant correlation between CAFs and patient prognosis. This has been shown particularly in the case of breast cancer where women with denser breast tissue have an increased tendency to develop cancer [4]. It has also been shown in non-small cell lung carcinoma where the gene signature of CAFs can be associated with patient prognosis [5], in prostate cancer where loss of the transforming growth factor beta (TGFβ) receptor II in the stromal fibroblasts is associated with tumor progression [6], and in squamous cell carcinoma where a fibrous stroma was found to be associated with poor prognosis [7]. Together, these studies suggest that CAFs are associated with increased malignant potential, suggesting that the CAF is a valuable potential therapeutic target.
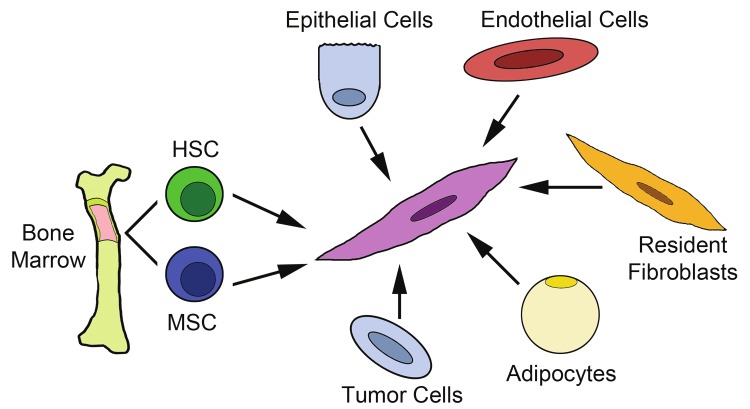
However, therapeutically targeting the CAF population may prove to be difficult. While its importance in tumor progression is becoming clear, the CAF remains loosely defined, requiring identification by multiple markers. Positive expression of a panel of fibroblast markers, and negative expression of markers of other stromal populations (e.g., macrophages, endothelial cells, pericytes and adipocytes), along with a distinctive spindle-shaped morphology are required to distinguish a CAF from other cells of the stroma. Several studies have suggested an evolving or transient collection of cell types that can transdifferentiate between one another within the stroma to generate CAFs [8,9]. Moreover, the stroma as a whole has been shown to change with tumor progression and changes in cellular response and function have also been shown to be altered with early and late events of tumor development [10]. The heterogeneity of the tumor stroma and the CAF population is suggestive of multiple origins of CAFs and/or an evolving differentiation pathway. Traditionally, fibroblasts are thought to arise from resident tissue fibroblasts [11,12]. However, recent studies have suggested alternative sources including other resident stromal cells, epithelial cells and bone marrow. Controversy exists over the cell type within the bone marrow, the mesenchymal stem cell (MSC) or the hematopoietic stem cell (HSC), that has the ability to give rise to fibroblast populations. An understanding of the differences and similarities between CAFs of each origin (Figure 1) as well as research directed at elucidating the differentiation pathway of CAFs is crucial for the ultimate goal of a stromal-based anti-tumor therapy.
Figure 1.
Multiple origins of carcinoma associated fibroblasts. This drawing depicts the proposed origins of CAFs including the bone marrow (HSC and MSC), epithelial and tumor cells via EMT, endothelial cells via EndMT, resident fibroblasts and adipocytes. HSC= hematopoietic stem cell; MSC= mesenchymal stem cell; EMT= epithelial-mesenchymal transition; EndMT= endothelial-mesenchymal transition.
Origins of CAFs
Epithelial-Mesenchymal Transition (EMT)
Epithelial-mesenchymal transition (EMT) refers to a process in which cells convert from an epithelial phenotype to a mesenchymal phenotype [13-15]. During this process, epithelial cells lose adherence and expression of epithelial markers and gain a more mesenchymal phenotype associated with the expression of fibroblastic markers. In vitro evidence suggests that epithelial cells can give rise to myofibroblasts [16]. In vivo, EMT has been described as a source for CAFs in various tumor types including breast cancer [17,18] and squamous skin carcinoma [19]. However, it has been suggested that, at least in breast cancer [20], only a small percentage of the CAFs share similar epithelial origins with the tumor cells.
Resident fibroblasts
It was generally held that CAFs arose from activation of resident tissue fibroblasts via signals from tumor cells [11,12,21]. Tumor cells secrete a broad spectrum of growth factors, chemokines and cytokines, allowing for signaling through various tumor-promoting pathways. Through specific cell surface receptors, fibroblasts respond to these soluble ligands in the local environment by increasing production of extracellular matrix molecules, angiogenic factors, chemokines and interleukins. This phenotype is associated with the “activation” of the fibroblast. One example of the effect of tumor cells on fibroblasts is through TGFβ signaling. TGFβ is a growth factor commonly produced by tumor cells and has been shown to exert significant effects on the stromal fibroblast population. TGFβ plays a significant role in tumor progression by upregulating α-smooth muscle actin (αSMA) and promoting collagen synthesis in fibroblasts [22] and cultured fibrocytes [23,24]. The most potent influence of TGFβ on the stromal component is thought to be the promotion of the differentiation of fibroblasts to activated fibroblasts. It has been shown that activated fibroblasts have a significantly pronounced ability to promote tumorigenesis over their non-activated counterparts with non-activated fibroblasts potentially playing a tumor inhibiting function in early stages of tumorigenesis while CAFS aid in tumor progression [25].
Other tumor microenvironment cells
Studies have suggested that adipose-derived stem cells (ASCs) and carcinoma-associated adipocytes (CAAs) contribute to the CAF population to promote tumorigenesis. Human adipose tissue derived stem cells (hASCs) were found to give rise to CAF-like cells when cultured with conditioned media from MDA-MB-231 or MCF-7 breast cancer cell lines [8]. This process was found to be dependent upon TGFβ signaling in the hASCs. Recently, CAAs have been shown to promote cancer progression in several models including prostate [26,27] and breast cancer [28,29], demonstrating the functional similarities between CAFs and CAAs. In addition to their direct effects on tumor cells, it has been suggested that one mechanism by which CAAs promote tumor progression is through CAFs that originate from the local CAA population [28].
Most recently, studies from Kalluri’s group have suggested that endothelial-mesenchymal transition (EndMT) is a source of CAFs [9,30]. Treatment of primary mouse endothelial cells with TGFβ resulted in a spindle shaped morphology and was associated with declining CD31 expression and increased expression of the fibroblast marker fibroblast-specific protein-1 (FSP-1) [9]. Cells co-expressing CD31 and αSMA or CD31 and FSP-1 were also identified in B16F10 melanoma tumors, indicating a cell transitioning from an endothelial to a fibroblastic or myofibroblastic state [9]. Furthermore, a transgenic mouse model was used which permanently labels endothelial cells with LacZ under the control of the ROSA promoter [9]. Using this mouse model and subcutaneous injection of melanoma cells, the authors reported that approximately 30% of the FSP-1 positive cells expressed β-galactosidase and 12% of αSMA positive cells also expressed β-galactosidase, suggesting an endothelial origin for CAFs [9].
Bone marrow: MSCs and HSCs
Several studies have also suggested a bone marrow origin for myofibroblasts and CAFs [31] (and reviewed in [32,33]). It is commonly held that the bone marrow contains two types of stem cells, the mesenchymal stem cell (MSC) and the hematopoietic stem cell (HSC). MSCs are defined by their adherence to plastic and potential to differentiate into mesenchymal tissue cells such as bone, fat, muscle, cartilage and fibroblasts [32,34,35]. HSCs are defined by their capability of hematopoietic reconstitution in vivoand have the ability to give rise to a few tissue cell types including mast cells and osteoclasts. It is unclear, however, which stem cell within the bone marrow is the progenitor cell that gives rise to the CAF.
MSC-origin of CAFs
Given the potential of MSCs to give rise to a variety of mesenchymal cells including fibroblasts, there has been a growing interest in elucidating the role of the MSC in tumorigenesis (reviewed in [36]). These studies have been complicated by the fact that, unlike the HSC, the MSC is not as clearly defined [12,36-38]. Despite the challenges associated with identification and isolation of MSC, several studies have demonstrated a role for these cells in tumor progression. Studies from Weinberg’s group showed that human MSCs are specifically recruited to the tumor stroma in a xenograft model of breast cancer [39]. Recruited MSCs were also found to enhance breast cancer metastasis to lung. In vivo studies demonstrated localization of murine MSCs to the tumor microenvironment in a 4T1 breast cancer model [40]. In a humanized model, studies examining tumor-exposed human MSCs showed that prolonged exposure to MDA-MB-231 human breast cancer cells resulted in activation of the MSCs to resemble CAFs [41]. Similar studies using breast, pancreatic and ovarian adenocarcinoma xenograft models showed that MSCs exposed to tumor acquire markers of CAFs and express a CAF-like phenotype with the ability to promote tumor vascularization and growth [42]. This idea was further supported by Quante et. al., who demonstrated that 20% of CAFs in a model of inflammation-induced gastric carcinoma originate from the MSC in the bone marrow based on the expression of αSMA [31]. Together, these studies suggest that the bone marrow stromal population, or MSC, may contribute to the CAF population.
HSC-origin of CAFs
The idea of a blood-borne matrix-producing cell has been described in the literature for over a century [12,43-45] (and reviewed in [21]). These studies describe “blood-borne fibroblasts”, which may represent the earliest description of cells that would later be named “fibrocytes”. Fibrocytes were first described by Bucala in 1994 as a unique circulating CD34+ collagen-producing cell [46]. Together, these studies have brought to light the possibility of fibroblast populations arising from hematopoietic origin. Fibrocytes can give rise to mature fibroblasts in culture and are an important component of the normal and reactive stroma. Fibrocytes are defined as a mesenchymal cell type in peripheral blood that rapidly enters sites of tissue injury [46] and are considered to be precursors of activated fibroblasts [47]. They express both fibroblastic features, such as expression of collagen-1, and hematopoietic phenotypes, such as positive expression of CD11b, CD13, CD34, CD45RO, MHC class II and CD86 [24,46-48]. Once in tissues, fibrocyte-derived cells may express markers of matrix production including collagen, vimentin and prolyl-4-hydroxylase (PHD4). Fibrocytes have been shown to play a role in numerous pathologies including wounding [24,49] and fibrosis [39,47].
Data from our laboratory supports these early studies and demonstrates an HSC origin for fibroblasts. We have developed a method for transplantation of a clonal population from a single sorted HSC defined as an EGFP+Lin-Sca-1+CD34-SP cell. Using this single cell transplantation model, we have demonstrated that HSCs can give rise to a variety of mesenchymal cell types including adipocytes [50], cardiac valve interstitial cells [51], circulating fibroblast precursors [52], fibroblasts and myofibroblasts [53] and CAFs [54-56] (reviewed in [56]). Using this model, we conducted an in vitro examination of fibrocytes derived from the peripheral blood cells of clonally engrafted mice [55]. In these studies, nucleated blood cells were cultured and the appearance of EGFP+ spindle-shaped or polygonal cells were detected by the seventh day of cultivation. Flow cytometric time course analysis of expression of the hematopoietic marker CD45 and the fibroblast marker, (DDR2; discoidin domain receptor 2, a collagen receptor), showed that as these cells matured, they lost expression of CD45 and gained expression of DDR2. These findings were supported in our tumor studies using clonally engrafted mice which identified a circulating population of fibroblast precursors, termed circulating fibroblast precursors (CFPs), that expressed markers of both hematopoietic cells (CD34, CD45) and fibroblasts (collagen I (Col I), DDR2) [52]. We have also shown that CFPs differentiate along the monocyte/macrophage lineage based on co-expression of CD11b (Mac-1) and lack of expression of F4/80, and by rapid differentiation of CD45+DDR2+Mac1hi to Col1+αSMA+ cells with fibroblastic morphology [54]. This circulating population, defined as CD45+DDR2+cells, was shown to increase in circulation with tumor burden, incorporate into the tumor stroma and contribute to the CAF population. Analysis of solid tumor sections from Lewis lung carcinoma (LLC) and melanoma (K1735-M2) harvested from clonally engrafted animals showed the presence of HSC-derived CFPs [52] and CAFs [52,54]. These EGFP-expressing cells had a fibroblastic morphology and made up 8-28% of the tumor stromal cells [52,54]. Characterization of these HSC-derived cells showed that they were activated fibroblasts that expressed αSMA and produced collagen. Also prevalent in the specimens were EGFP+ pericyte-like perivascular cells, suggesting that HSCs also contribute to tumor vasculature [54].
HSC-derived CAFs contribute to the tumor microenvironment
Together, these studies indicate multiple origins for CAFs and suggest that the CAF is a transitory cell type that both responds to and affects the tumor microenvironment, resulting in promotion of primary tumor growth and vascularization, tumor cell invasion and metastasis and preparation of the metastatic niche (reviewed in [2,3,32]). Potential roles for HSC-derived CAFs and their precursors will be discussed herein.
Recruitment of HSC-derived fibroblasts and generation of an activated stroma
Early communication between the tumor cells and fibroblasts is involved in the development of a reactive stroma. This process involves not only the recruitment of inflammatory cells, but the recruitment and activation of fibroblast precursors/fibroblasts to the tumor site. Our laboratory has shown that the number of HSC-derived CFPs increases with tumor burden [52]. As further evidence towards the importance of CFPs in the reactive stroma, these studies demonstrated that when this population is inhibited, tumor burden is decreased. Findings from these studies also support a role for chemokines in the recruitment of fibroblast precursors to the tumor microenvironment. Specifically, we have shown that monocyte chemoattractant protein-1 (MCP-1 or CCL2)/CCR2 is involved in the recruitment of CFPs by multiple tumor types in vitro and recruitment to the tumor site in an in vivo Lewis lung carcinoma model [52]. The role of MCP-1 in fibroblast precursor recruitment is supported by studies investigating the chemokine’s involvement in fibrocyte recruitment in wound healing [57] and fibrosis [58] models. Stromal cell-derived factor-1 (SDF-1 or CXCL12) and its receptor CXCR4 have also been implicated in regulating chemotaxis of fibrocytes in multiple models [24,39]. Once recruited to the tumor microenvironment, subpopulations of the fibroblast precursors/fibroblasts then become activated. Our studies have shown that CFPs may be stimulated to express markers of activated fibroblasts including collagen, vimentin and αSMA by exposure to tumor conditioned media [52]. These findings are supported by our in vivo data demonstrating the activated fibroblast phenotype of HSC-derived cells recruited from the bone marrow to the tumor stroma [54].
Several factors have been implicated in the transition of fibroblasts to activated fibroblasts. As described above, TGFβ is the most thoroughly investigated of these factors having been shown both in vivo and in vitro to upregulate α-smooth muscle actin and promote collagen synthesis in fibroblast populations [22-24]. In addition, this factor acts as a chemotactic agent for fibroblasts [59] and acts to upregulate production of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and MCP-1 [60-62], all of which contribute to the reactive stroma during tumorigenesis. Analysis of HSC-derived CFPs shows that a subpopulation of these cells express the TGFβ type II receptor, suggesting that they are also influenced by this cytokine (unpublished data). Given the demonstrated HSC origin for CFPs, specifically via the monocyte/macrophage lineage, we also investigated the role of cytokines known to play a critical role in HSC differentiation. These studies revealed a role of macrophage colony stimulating factor (M-CSF) in the differentiation of HSC-derived CFPs to the activated phenotype [52]. These studies are supported by the finding that M-CSF promotes proliferation of fibroblastic stromal cells in culture [63,64].
Potential role of HSC-derived fibroblasts in immune suppression
Another component to the successful establishment of the primary tumor is the ability of the tumor to evade immune surveillance. The inflammatory and immune response directed at eradicating the tumor has been shown in many cases to be unsuccessful, instead ultimately acting to promote tumor progression through production of inflammatory cytokines [65]. This is due in part to the presence of a rich environment of growth factors, cytokines and chemokines that continually recruit macrophages and other immune cells to the tumor site. Fibrocytes were so named because of their similarities to both fibroblasts and the immune cells, the leukocytes [46]. Fibrocytes play an important role in immune function through their ability to induce T-lymphocyte proliferation [66], activate CD8+ T-lymphocytes [67] and produce inflammatory factors in wound chambers [48]. They also possess cell surface receptors necessary for antigen presentation and interaction with T-cells (MHC class II antigen) [68]. Fibrocytes produce extracellular matrix components such as collagen, angiogenic factors (e.g., VEGF and PDGF-A), as well as other cytokines including M-CSF, hepatocyte growth factor (HGF) and others [21]. It is likely that as fibrocytes and CFPs mature into fibroblasts in tumor some of these qualities would be maintained. While the interactions between CAFs and immune cells in the tumor immune response have not been studied, it has been reported that CAFs produce factors such as TGFβ, and interleukin-6 (IL-6) which are known to promote differentiation and activation of T-cells, cells on the frontline of the immune response to tumor.
HSC-derived CAFs in tumor cell migration and invasion
A cardinal indicator of tumor progression is the appearance of tumor metastases. One of the initial steps in metastasis is the migration and invasion of the tumor cell through the extraceullular matrix and through the basement membrane. This is followed by intravasation of tumor cells into a local blood vessel and the extravasation of the tumor cell to colonize and proliferate at a distant site. Signals for the initiation of migration and invasion may include chemotactic signals in the local environment (from stromal cells) or through a concentration gradient set up across tissues and the blood serum from circulating cells including HSC-derived CFPs, as was shown in our previous studies with MCP-1 [52]. It is also suggested that CAFs promote migration and invasion of tumor cells. The concept of CAF driven tumor cell migration and invasion is predicated on the idea that in normal wound healing, fibrocytes/fibroblasts are known to produce extracellular matrix (ECM) degrading compounds such as matrix-metalloproteinases (MMPs) in order to move into a wound site and affect repair. MMPs are enzymes which degrade matrix collagens, fibronectins and proteoglycans to allow tumor cells to cross the structural barrier of the basement membrane, a key step in metastasis [69]. CAFs have been shown to produce a variety of MMPs including MMP1, MMP2, MMP3, MMP9, MMP11, MMP13, and MMP14 [69]. Degradation of the ECM allows movement of cells through the dense matrix and releases factors that are stored within the scaffold of the ECM. Degradation of the ECM and subsequent release of stored growth factors is expected to have significant effects on the ability of tumor cells to migrate and invade through the ECM, critical steps in the metastatic process.
Our laboratory has shown that this process is bi-directional wherein HSC-derived CFPs promote migration and invasion of Lewis lung carcinoma (LLC) cells, while LLC cells also promote the migration of HSC-derived CFPs and CAFs ([52] and unpublished data). This corresponds with the finding of fibroblast-led invasion of tumor cells [70]. It is known that fibroblasts play a significant role in matrix remodeling and in this study, Gaggiol et al. showed that fibroblasts are able to form tracks through the ECM allowing invading tumor cells to efficiently follow behind [70]. This connection was supported by evidence that tumor cells break off from the primary tumor with stromal cells attached and that these stromal cells support the viability of the tumor cells through the circulation to the metastatic site [71].
HSC contribution to angiogenesis
Crosstalk between the tumor cells and CAFs has profound effects on tumor angiogenesis, without which tumors cannot maintain growth beyond 1 mm3 [72]. CAFs have been shown to produce angiogenic factors including SDF-1 [73], TGFβ [38] and VEGF [74] which support endothelial cell proliferation and tumor vascularization. In addition to their production of angiogenic cytokines, CAFs may play a more direct role in tumor vascularization through their ability to serve as vascular support cells. CAF and myofibroblast expression of αSMA closely links this cell type with the pericyte, a fibroblast-like cell which plays a supportive role for endothelial cells in both normal and tumor systems. Pericytes, also referred to as perivascular mural cells, act to stabilize vessel walls, influence vascular permeability, inhibit endothelial cell proliferation, and promote endothelial cell survival and maturation. Several studies have described a bone marrow origin for tumor vascular pericytes [35,75]. Studies from our group using our single cell transplantation strategy have shown that both CAFs [52,54] and pericytes [54,76,77] arise from a common progenitor, the hematopoietic stem cell.
Conclusions and perspectives
The varying fibroblast populations identified in tumor stroma illustrate the transient nature of fibroblasts and perhaps other cells of the stroma. Elucidating the contribution of CAFs to tumor progression is essential for developing a comprehensive understanding of the tumor microenvironment. It is clear that CAFs play a critical role in tumor progression as well as patient prognosis and survival. It has been suggested that not only do CAFs define a widely heterogeneous population of the stroma, but that this population varies with tumor progression, tumor location, and tumor type. While multiple CAF origins have been shown by many laboratories, little is known about the specific contribution of each uniquely originating CAF. Further evaluation and profiling of CAFs of a particular origin may lead to an appropriate and critical target for the arrest of tumor development and the prevention of tumor metastasis.
The transient nature of CAFs, as well as the variation in CAFs from one tumor type to another, suggests science has a long way to go in terms of targeting the CAF population therapeutically. However, targeting CAFs at their origin in a tumor-stage specific manner may lead to significant advances in treatment and even prevention of metastases. Our lab has shown that CFPs are derived from HSCs and that these cells act as an intermediate between the HSC and the CAF. The identification of differentiation and maturation pathways from the HSC to the CAF provide a potential opportunity to target CAFs in multiple ways depending on tumor stage. For example, early in tumor development it may be possible to target CAFs at their origin in the bone marrow (HSC), or in the circulation as CFPs in order to prevent their incorporation into the tumor and inhibit their tumor promoting effects. In later stages of cancer, it may be possible to target the HSC-derived CAF directly at the tumor site to reduce tumor volume and inhibit further promotion of tumor progression by CAF-produced factors. Moreover, the intrinsic ability of fibrocytes and CFPs to migrate to the tumor site offers potential for drug delivery vehicles for chemotherapy. This potential to serve as a novel target to inhibit cancer progression and metastasis will continue to drive the investigation into the origins and roles of CAFs in the tumor microenvironment.
Acknowledgements
The research presented in this article was supported in part by the Flow Cytometry and Cell Sorting Shared Resource, funded by a Cancer Center Support Grant P30 CA138313, to the Hollings Cancer Center at the Medical University of South Carolina. The authors would like to specifically thank Dr. Haiqun Zeng for assistance in FACS sorting reviewed in this manuscript. We also thank Ms. Dayvia A. Laws for her assistance in manuscript preparation. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
This work is supported in part by the NIH/NCI (R01 CA148772, ACL), the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs (Merit Award, ACL), and the Hollings Cancer Center (Translational Research Pilot Project, P30 CA138313, ACL).
References
- 1.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. The New England journal of medicine. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 5.Navab R, Strumpf D, Bandarchi B, Zhu CQ, Pintilie M, Ramnarine VR, Ibrahimov E, Radulovich N, Leung L, Barczyk M, Panchal D, To C, Yun JJ, Der S, Shepherd FA, Jurisica I, Tsao MS. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7160–7165. doi: 10.1073/pnas.1014506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS 2nd, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer research. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi Y, Ishii G, Taira T, Fujii S, Yanagi S, Hishida T, Yoshida J, Nishimura M, Nomori H, Nagai K, Ochiai A. Fibrous stroma is associated with poorer prognosis in lung squamous cell carcinoma patients. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:1460–1467. doi: 10.1097/JTO.0b013e318229189d. [DOI] [PubMed] [Google Scholar]
- 8.Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song YH. Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Analytical cellular pathology. 2010;33:61–79. doi: 10.3233/ACP-CLO-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer research. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 10.Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Seminars in cancer biology. 2005;15:329–341. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Clark RA. Extracellular matrix alters PDGF regulation of fibroblast integrins. The Journal of cell biology. 1996;132:239–249. doi: 10.1083/jcb.132.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunphy JE. The Fibroblast - A Ubiquitous Ally for the Surgeon. New England Journal of Medicine. 1963;268:1367–1377. [Google Scholar]
- 13.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Hay ED. An overview of epithelio-mesenchymal transformation. Acta anatomica. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 16.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) The Journal of clinical investigation. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen OW, Lind Nielsen H, Gudjonsson T, Villadsen R, Ronnov-Jessen L, Bissell MJ. The plasticity of human breast carcinoma cells is more than epithelial to mesenchymal conversion. Breast cancer research: BCR. 2001;3:213–217. doi: 10.1186/bcr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Ronnov-Jessen L. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. The American journal of pathology. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nature cell biology. 2002;4:487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 20.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nature genetics. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 21.Bucala R. Fibrocytes: Discovery of a Circulating Connective Tissue Cell Progenitor. In: Bucala R, editor. Fibrocytes: New INsights into Tissue Repair and Systemic Fibroses. Hackensack: World Scientific Publishing Co. Pte. Ltd.; 2007. pp. 1–18. [Google Scholar]
- 22.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Laboratory investigation; a journal of technical methods and pathology. 1993;68:696–707. [PubMed] [Google Scholar]
- 23.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. The Journal of biological chemistry. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 24.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. Journal of immunology. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 25.Paland N, Kamer I, Kogan-Sakin I, Madar S, Goldfinger N, Rotter V. Differential influence of normal and cancer-associated fibroblasts on the growth of human epithelial cells in an in vitro cocultivation model of prostate cancer. Molecular cancer research: MCR. 2009;7:1212–1223. doi: 10.1158/1541-7786.MCR-09-0073. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko A, Satoh Y, Tokuda Y, Fujiyama C, Udo K, Uozumi J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. International journal of urology: official journal of the Japanese Urological Association. 2010;17:369–376. doi: 10.1111/j.1442-2042.2010.02472.x. [DOI] [PubMed] [Google Scholar]
- 27.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. The Journal of biological chemistry. 2003;278:42660–42667. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 28.Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. The International journal of developmental biology. 2011;55:851–859. doi: 10.1387/ijdb.113365jt. [DOI] [PubMed] [Google Scholar]
- 29.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer research. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 30.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. British journal of cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Frontiers in bioscience: a journal and virtual library. 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonda TA, Varro A, Wang TC, Tycko B. Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Seminars in cell & developmental biology. 2010;21:2–10. doi: 10.1016/j.semcdb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 35.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circulation research. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 36.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews. Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 37.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Experimental hematology. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, Tanasie G, Bunu C, Crisnic D, Gherghiceanu M, Tatu FR, Tatu CS, Vermesan S. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. Journal of cellular and molecular medicine. 2011;15:635–646. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. The Journal of clinical investigation. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, Wu JC, Chen X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem cells. 2009;27:1548–1558. doi: 10.1002/stem.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer research. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PloS one. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stirling GA, Kakkar VV. Cells in the circulating blood capable of producing connective tissue. British journal of experimental pathology. 1969;50:51–55. [PMC free article] [PubMed] [Google Scholar]
- 44.Paget J. Lectures on Surgical Pathology. Philadelphia: Lindsay & Blakinston; 1860. [Google Scholar]
- 45.Algower M, Hulliger L. Origin of fibroblasts from mononuclear blood cells: A study on in vitro formation of the collagen precursor, hydroxyproline, in buffy coat cultures. Surgery. 1960;47:603–609. [Google Scholar]
- 46.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Molecular medicine. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. Journal of immunology. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 48.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. Journal of immunology. 1998;160:419–425. [PubMed] [Google Scholar]
- 49.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Experimental cell research. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Sera Y, LaRue AC, Moussa O, Mehrotra M, Duncan JD, Williams CR, Nishimoto E, Schulte BA, Watson PM, Watson DK, Ogawa M. Hematopoietic stem cell origin of adipocytes. Experimental hematology. 2009;37:1108–1120. 1120.e1101–1104. doi: 10.1016/j.exphem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circulation research. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 52.Abangan RS Jr, Williams CR, Mehrotra M, Duncan JD, Larue AC. MCP1 directs trafficking of hematopoietic stem cell-derived fibroblast precursors in solid tumor. The American journal of pathology. 2010;176:1914–1926. doi: 10.2353/ajpath.2010.080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, Drake CJ, Ogawa M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Experimental hematology. 2006;34:219–229. doi: 10.1016/j.exphem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 54.LaRue AC, Masuya M, Ebihara Y, Fleming PA, Visconti RP, Minamiguchi H, Ogawa M, Drake CJ. Hematopoietic origins of fibroblasts: I. In vivo studies of fibroblasts associated with solid tumors. Experimental hematology. 2006;34:208–218. doi: 10.1016/j.exphem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 56.Ogawa M, Larue AC, Watson PM, Watson DK. Hematopoietic stem cell origin of connective tissues. Experimental hematology. 2010;38:540–547. doi: 10.1016/j.exphem.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, reepithelialization, contraction, and angiogenesis. Annals of surgery. 2011;254:1066–1074. doi: 10.1097/SLA.0b013e3182251559. [DOI] [PubMed] [Google Scholar]
- 58.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. American journal of physiology. Lung cellular and molecular physiology. 2011;300:L341–353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. The Journal of experimental medicine. 1987;165:251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90:649–652. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- 61.Ma J, Wang Q, Fei T, Han JD, Chen YG. MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007;109:987–994. doi: 10.1182/blood-2006-07-036400. [DOI] [PubMed] [Google Scholar]
- 62.Taylor LM, Khachigian LM. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-beta involves transactivation by Smads. The Journal of biological chemistry. 2000;275:16709–16716. doi: 10.1074/jbc.275.22.16709. [DOI] [PubMed] [Google Scholar]
- 63.Deryugina EI, Ratnikov BI, Bourdon MA, Gilmore GL, Shadduck RK, Muller-Sieburg CE. Identification of a growth factor for primary murine stroma as macrophage colony-stimulating factor. Blood. 1995;86:2568–2578. [PubMed] [Google Scholar]
- 64.Yamada M, Suzu S, Akaiwa E, Wakimoto N, Hatake K, Motoyoshi K, Shimamura S. Properties of primary murine stroma induced by macrophage colony-stimulating factor. Journal of cellular physiology. 1997;173:1–9. doi: 10.1002/(SICI)1097-4652(199710)173:1<1::AID-JCP1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 65.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer immunology, immunotherapy: CII. 2011;60:319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. Journal of leukocyte biology. 2005;77:923–933. doi: 10.1189/jlb.1204701. [DOI] [PubMed] [Google Scholar]
- 68.Chesney J. Fibrocytes: Immunologic Features. In: Bucala R, editor. Fibrocytes: New Insights into Tissue Repair and Systemic Fibroses. Hackensack: World Scientific Publishing Co. Pte. Ltd.; 2007. pp. 09–36. [Google Scholar]
- 69.Noel A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Seminars in cell & developmental biology. 2008;19:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Gaggioli C. Collective invasion of carcinoma cells: when the fibroblasts take the lead. Cell adhesion & migration. 2008;2:45–47. doi: 10.4161/cam.2.1.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK. Malignant cells facilitate lung metastasis by bringing their own soil. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 73.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 74.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 75.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M. Hematopoietic origin of microglial and perivascular cells in brain. Experimental neurology. 2004;186:134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Masuya M, Drake CJ, Fleming PA, Reilly CM, Zeng H, Hill WD, Martin-Studdard A, Hess DC, Ogawa M. Hematopoietic origin of glomerular mesangial cells. Blood. 2003;101:2215–2218. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]