Abstract
Background: Primary and metastatic cardiac sarcomas represent a heterogeneous group of rare neoplasms with varying clinical course and diverse histogenetic line of differentiation. To date, there exist no uniform guidelines for their surgical and/or oncological treatment. Methods: We evaluated retrospectively all patients undergoing cardiac surgery for primary or secondary cardiac sarcoma in the period 1999-2011 at the Erlangen Heart Centre to analyze their clinicopathological spectrum, treatment and outcome. Results: Five patients (3 women & 2 men; mean age: 46 years; range: 28-81 years) had primary cardiac sarcomas (6.7% of primary cardiac tumors) and 4 had cardiac metastasis from soft tissue sarcoma (1 case each of osteosarcoma, myxoid liposarcoma, alveolar soft part sarcoma and pleomorphic spindle cell sarcoma). Primary sarcomas were located in the left atrium (n=3), left ventricle (n=1) and right atrium (n=1). Histological types were myxosarcoma (3), pleomorphic undifferentiated sarcoma (1) and angiosarcoma (1). Four patients died at 2-64 months (mean, 24.5 months). Sarcoma metastasis to the heart developed at a mean of 109.5 months from initial diagnosis (range, 5-240 months). Three of them died of disease at a mean of 14 months after cardiac surgery and one is disease free 34 months after heart transplantation for metastasis. Conclusions: Primary and metastatic cardiac sarcomas are very heterogeneous in their histological appearance, clinical presentation and course of the disease. Radical surgery combined with chemoradiation is promising in patients with resectable disease and may significantly prolong survival. Cardiac transplantation represents an emerging strategy for patients with isolated unresectable cardiac involvement.
Keywords: Cardiac tumors, cardiac sarcoma, pathology, cardiac surgery, myxosarcoma, angiosarcoma
Introduction
Primary cardiac tumors are quite rare. Their incidence in early autopsy series ranged from 0.001 to 0.3% [1,2]. A majority of primary cardiac tumors (~75%) are benign; atrial myxoma being the most common tumor encountered. On the other hand, primary cardiac sarcomas are exceedingly rare. Most have been published as single case reports or small case series. However, larger collaborative series have been reported more recently from large specialized cardiac centers but most have included referral cases. Accordingly, reporting additional cases potentially contribute to the delineation of their clinicopathological and biological characterization and would enhance improvement of their diagnosis and treatment strategies. Cardiac sarcomas usually present with insidious symptoms in young and middle age patients. They generally possess a poor prognosis with overall survival ranging from 6 to 12 months [3,4]. Symptoms related to cardiac sarcomas are variable and may vary from specific cardiac symptoms (pericardial effusions with tamponade, arrhythmias, valvular dysfunction, intracardiac blood flow abnormalities, congestive heart failure, peripheral embolization with systemic deficits, dyspnea, chest pain, syncope, hemoptysis, sudden cardiac death) to general symptoms of neoplastic diseases like fever, malaise and weight loss [5-7].
As an initial modality in radiologic evaluation of cardiac sarcomas, chest radiography may reveal abnormal findings (abnormalities of cardiac contour, signs of heart failure or pleural effusions). However, diagnosis and management of these tumors has been greatly improved by the development of more informative non-invasive cardiac imaging including transthoracic (TTE) and transesophageal (TEE) echocardiography as well as computed tomography (CT) scans and cardiac-gated magnetic resonance imaging (MRI) [8,9]. The treatment of choice for these rare malignant tumors is a combination of surgical resection and radio- and/or chemotherapy. However, in most instances, and in contrast with benign cardiac tumors, surgical resection still represents a palliative strategy for many patients with cardiac sarcomas [10,11]. Therefore, a rapid and precise diagnosis is mandatory and may confer some survival advantage for individual patients. The aim of the present study was to evaluate our experience with primary and metastatic cardiac sarcomas seen at our hospital during the last 12 years and to characterize their clinicopathological spectrum and their outcome after different therapeutic modalities.
Patients and methods
All patients treated for a cardiac mass from January 1999 to December 2011 at the Center for Cardiac Surgery, University Hospital of Erlangen, Germany, have been included in this retrospective analysis. Complete patient’s follow-up was collected either from hospital records, patients themselves or their family members and/or their treating general physicians by telephone. All intra-cardiac masses were diagnosed by TTE or TEE, CT, MRT and/or cardiac catheterization. In most cases more than one diagnostic tool has been used to confirm previous findings and to plan the best operative strategy for complete tumor resection or most curative resection. Diagnosis of the different sarcoma subtypes was based on well characterized criteria for primary cardiac sarcomas or peripheral soft tissue and bone sarcomas. The term “myxosarcoma” has been increasingly used for pleomorphic predominantly myxoid cardiac sarcoma with variable resemblance to atrial myxoma. Irrespective of similarities or possible histogenetic relationship between the two entities, the term myxosarcoma is applied in this study to merely describe an “undifferentiated pleomorphic sarcoma with prominent myxoid features” and does not imply the malignant counterpart of atrial myxoma or origin from preexisting myxoma.
Results
Distribution and frequency of the different primary heart tumor types in the study period
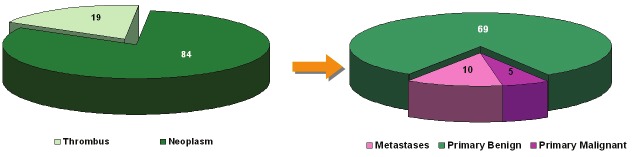
A total of 103 cardiac masses have been resected during the observation period 1999-2011 (Figure 1). The general clinicopathological features of the cohort have been published recently [12]. There were 84 cardiac neoplasms (primary and metastatic) and 19 surgically removed thrombi. The majority of the primary heart neoplasms (total: 74) were benign (n=69). Five patients had primary cardiac sarcomas (4.8% of all cardiac masses, 5.9% of all primary and metastatic neoplasms and 6.7% of all primary cardiac neoplasms resected in the study period, respectively). Cardiac metastasis from extra-cardiac neoplasms (including carcinoma, sarcoma, melanoma and lymphoma) was observed in 10 patients. Of the latter, 4 patients had sarcoma metastatic to the heart (4.8% of all primary and metastatic heart tumors and 40% of all surgically resected cardiac metastases, respectively). The clinicopathological features of primary and metastatic sarcomas are described in more details below.
Figure 1.
Distribution of surgically resected cardiac space-occupying lesions at the University of Erlangen between 1999 and 2011.
Primary cardiac sarcomas
Clinical features (Table 1)
Table 1.
Clinicopathologic features of primary cardiac sarcomas (n=5)
| No | Age/sex | Site primary tumor | Clinical symptoms | Tumor size | Histological type | treatment | Metastasis/Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 30 M | Left atrium | Constant growth of tumor mass, no symptoms | 6 cm | Myxosarcoma high-grade | Surgical resection + adjuvant chemotherapy (Adriamycin & Ifosfamide) | 3 recurrences resected at 7, 47 & 64 months. |
| Died at 64 months of post-operative complication. | |||||||
| No distant MTS at autopsy. | |||||||
| 2 | 53 F | Left ventricle+ pulmonary vessels+ pericardium | Paroxysmal dyspnea and cough | Multiple tissue fragments | Myxosarcoma high-grade | Tumor debulking | Died of cardiovascular failure at 3 months. |
| No distant MTS detected. | |||||||
| 3 | 81 F | Left atrium | Cerebral embolic phenomena | 6.5 cm | Myxosarcoma high-grade | Resection | Died of breast cancer MTS at 2 months. |
| Metastatic breast cancer as collision tumor with sarcoma. | |||||||
| 4 | 28 M | Left atrium+ pulmonary vein | Hemoptysis | 6 cm | Pleomorphic MFH | Resection | Brain MTS at 13 months (resection + RT), small bowel MTS 25 months (resection). Died at 29 months. |
| 5 | 36 F | Right atrium | Emergency intracardiac bleeding | 6 cm | Angiosarcoma | HTX after initial R1 tumor resection | Alive & well 3 months. |
M, male; F, female; MFH, malignant fibrous histiocytoma; MTS, metastasis; HTX, heart transplantation; RT, radiotherapy.
Histological diagnosis was pleomorphic myxosarcoma (high-grade undifferentiated myxoid sarcoma) in 3 patients, pleomorphic undifferentiated sarcoma/malignant fibrous histiocytoma (MFH) in one case and angiosarcoma in another case. Patients were three women and two men with a mean age at the time of primary surgical treatment of 46.6 ± 21.3 years (range 28 - 81 years). Interestingly, the mean age of the 3 women (56.7 ± 22.7 years) was significantly higher than that for men (31.5 ± 4.9 years). Symptoms upon admission varied greatly from completely asymptomatic to variable symptoms as fatigue, exercise intolerance or shortness of breath. The angiosarcoma case presented as an emergency with acute-onset hemorrhagic pericardial effusion. Two tumors were located in the left atrium, one in the left atrium + the pulmonary veins, one in the left ventricle secondarily infiltrating into all four heart chambers and one in the right atrium. Thus, all tumors but one were located in the left heart (angiosarcoma was the only tumor located in the right atrium). Representative CT scan images of one case are depicted in (Figure 2).
Figure 2.
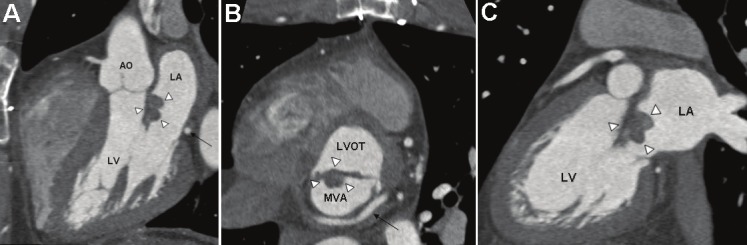
Contrast-enhanced ECG-gated spiral computed tomography. A: Three chamber view showing the left-ventricular inflow and outflow tract. Arrow-heads indicate the myxosarcoma attached to the anterior leaflet of the mitral-valve. The black arrow points to the left circumflex coronary artery. B: Short axis view of the mitral valve area (MVA) surrounded by the circumflex artery (black arrow). Arrow-heads indicate the myxosarcoma attached to the anterior leaflet of the mitral-valve. LVOT: left ventricular outflow tract. C: Long axis view showing the myxosarcoma attached to the anterior leaflet of the mitral valve (arrow-heads). LA: left atrium; LV: left ventricle.
Surgical treatment and outcome
In all patients, surgery was performed via median sternotomy and cardiopulmonary bypass with normothermia or moderate hypothermia. Tumors were resected either through right or left atriotomy or ventriculotomy depending on the tumor location. In all cases, en bloc excision of the tumor mass was aimed; if this was not possible, the operative strategy was individualized towards the most helpful resection. Because of the extensive growth and infiltration of most sarcomas into adjacent myocardium, it was often not possible to excise the tumor mass completely with free margins. However, gross disease could be resected in 4 cases. One case (angiosarcoma) with positive margins underwent subsequent heart transplantation.
Follow-up
A follow-up of 8.4 patient years was created (mean, 1.6 years). The 30 day mortality for primary sarcoma surgery was 0/5 (0%). The one year mortality was 2/5 (40%). Both patients died during the first year postoperatively due to cancer-related progressive heart failure. All of 4 patients with extended follow-up died of progressive tumor-related heart failure at 2-64 months (mean, 24.5 months). The longest survival was 64 months (1 patient). This patient with myxosarcoma experienced three recurrences. He died of postoperative heart failure after resection of the third recurrence and unsuccessful implantation of a left ventricle assist device (LVAD) on the second postoperative day (64 months from initial diagnosis). One patient with undifferentiated pleomorphic sarcoma developed brain and small bowel metastasis at 13 and 25 months respectively. He was treated by combined surgery and radiotherapy, but he died of cardiopulmonary failure five months after intestinal metastasis. Except for this patient, no distant metastases were observed in the remaining four patients. The fifth patient (with angiosarcoma) is a recent case with a limited follow-up of 3 months (alive and well after HTX).
Pathological features of primary cardiac sarcomas
Tumors ranged in size from 6 cm to more than 8 cm (submitted as tissue pieces) with a mean size of 6.5 cm. Grossly, they varied also from pseudoencapsulated predominantly polypoid to diffusely infiltrating masses (Figure 3). The cut surface was described as fleshy with areas of necrosis and hemorrhages.
Figure 3.
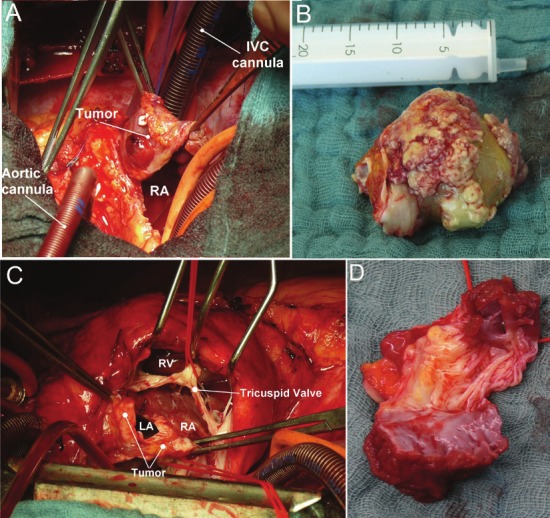
A: Intraoperative photograph showing large polypoid exophytic angiosarcoma after opening of the right atrium. RA, right atrium; IVC, inferior vena cava. B: Intraoperative gross photograph showing a giant myxosarcoma with yellowish myxoid appearance (maximal diameter of 65 x 40 x 45 mm). C: Intraoperative photograph showing a diffuse tumor recurrence in the left and right atrium attached to the tricuspid valve. D: Resected tumor mass with valve leaflets (same as C). LA, left atrium; RA, right atrium; RV, right ventricle.
Histological spectrum of primary cardiac sarcomas
Myxosarcomas showed variable but generally remarkable cellularity and significant degree of anaplasia. The tumor cells were spindled to polygonal with hyperchromatic nuclei that possess irregular nuclear membranes and smudge chromatin. The cytoplasm was pale-eosinophilic to amphophilic with ill-delineated cell borders. The tumor cells were occasionally arranged into short fascicles but most commonly formed irregular sheets within variably myxoid background. A characteristic features was the presence of numerous vascular channels in the stroma that were surrounded by hypercellular tumor cell aggregates that blended with the vascular structures (Figure 4A, B). One tumor showed areas with high-grade epithelioid morphology mimicking epithelioid angiosarcoma or melanoma (Figure 4C). Abundant myxoid matrix occasionally showed a prominent reticular microcystic pattern mimicking pulmonary oedema (Figure 4D). Other peculiar patterns seen in one case was signet ring cell pattern reminiscent of epithelioid hemangioendothelioma (Figure 4E) and multivacuolated lipoblast-like differentiation (Figure 4F). The latter was more prominent surrounding areas of necrosis. Interestingly, all tumors showed focal bland-looking areas that mimicked benign myxomas but these areas were ill-defined and blended with the highly atypical ones (Figure 4D). The most superficial parts of the tumors showed extensive necrosis surrounded by a cambium-like superficial (luminal) component. Areas of coagulative necrosis were seen in all cases but mostly were less extensive. Only one case showed unequivocal myxomatous component that blended with more atypical areas (Figure 5). This tumor also displayed focal venous invasion (Figure 5). The mitotic activity ranged from 1-4 mitoses per one HPF.
Figure 4.
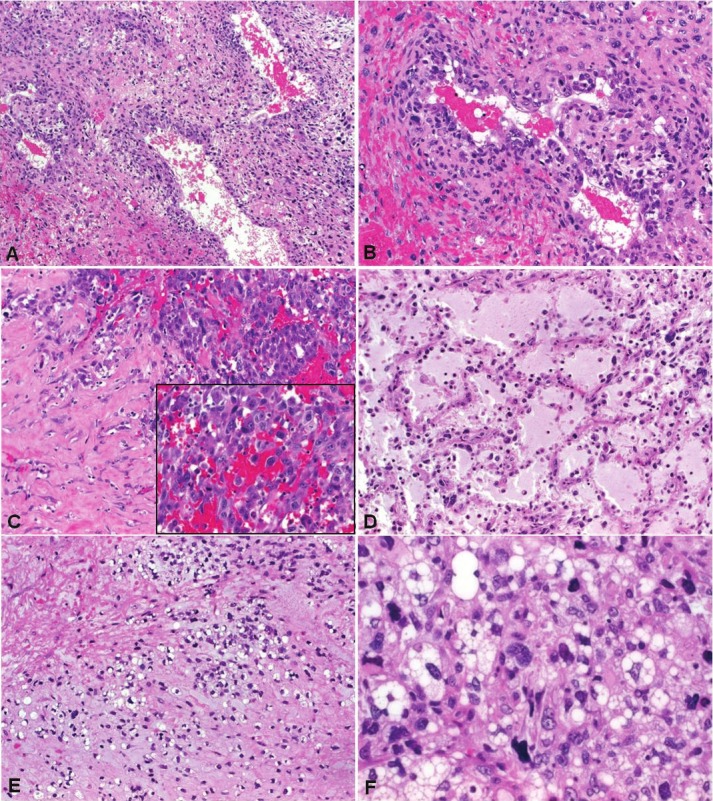
Representative histological images of primary cardiac myxosarcomas. A: prominent vascular pattern of myxosarcoma with hypercellular perivascular cuffing (B: higher magnification). C: abrupt transition to a high-grade epithelioid pattern surrounded by stromal sclerosis. D: pulmonary edema-like myxoid pattern, note clear-cut nuclear atypia. E: chondromyxoid signet ring-like areas mimicked epithelioid hemangioendothelioma. F: prominent cytoplasmic vacuoles in areas of necrosis mimicked fatty differentiation (protein S100 negative).
Figure 5.
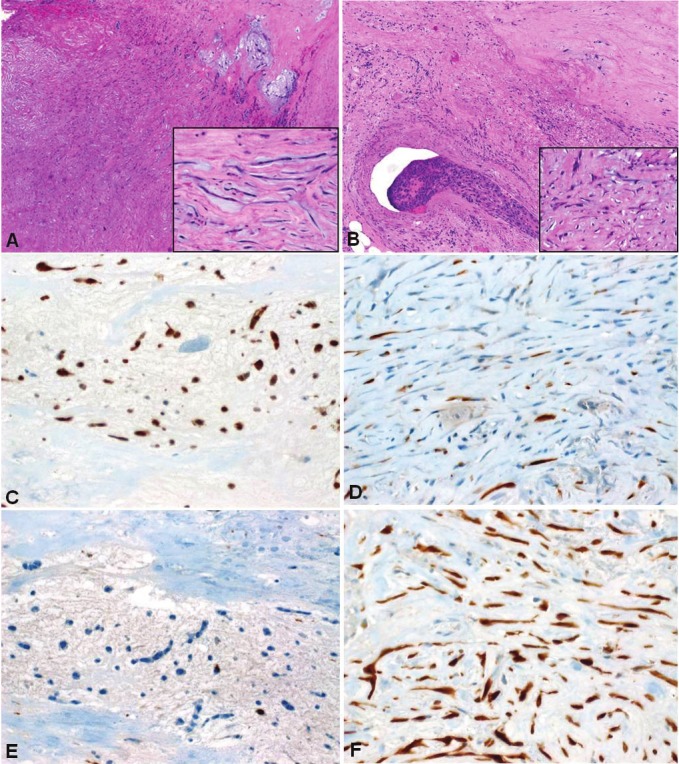
Cardiac myxosarcoma probably originating within myxoma. A: residual myxoma-like areas (upper right)blended with sclerosing atypical spindle cell sarcoma (lower left). Inset: myxoma-like cells lacked significant atypia.B: focus of venous invasion was seen within adjacent myocardium (Inset: note atypical hyperchromatic nuclei). C:Myxoma-like areas strongly expressed calretinin, as opposed to isolated positive cells in the sarcomatous area (D).On the contrary, p16 was nearly absent in the myxoma-like component (E) but diffusely ands strongly expressed inthe atypical component (F).
The case diagnosed as undifferentiated pleomorphic sarcoma/MFH showed a higher degree of cellular and nuclear pleomorphism, more solid compact growth pattern, presence of numerous multinucleated tumor giant cells and more prominent fascicular growth pattern (Figure 6A, B). The angiosarcoma showed a predominance of (Kaposiform) spindle cell morphology with fascicular growth pattern and brisk mitotic activity (Figure 6C). All tumors, irrespective of their line of differentiation, showed unequivocal infiltrative growth into the surrounding myocardial tissue.
Figure 6.
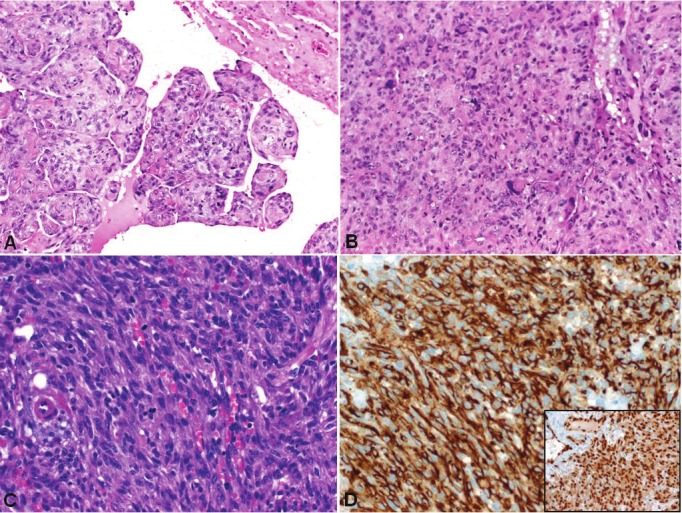
Example of non-myxoid cardiac sarcomas. A: This undifferentiated sarcoma/MFH showed a papillary polypoid component that was probably responsible for the brain and intestinal metastasis detected later in this patient. B: typical solid growth pattern with pleomorphic multinucleated tumor cells and significant mitotic activity. C: the angiosarcoma displayed deceptive spindle cell morphology. D: strong cytoplasmic expression of CD31 (main image) and nuclear reactivity with ERG (subimage) in the angiosarcoma.
Immunohistochemistry in primary cardiac sarcomas
Immunohistochemical analysis revealed diffuse and strong expression for vimentin in all cases. Alpha-smooth muscle actin was variably expressed in myxosarcomas, occasionally showing a submembranous myofibroblast-like pattern. None of the tumors, except the angiosarcoma, expressed CD34 or other endothelial markers. Furthermore, calretinin was strongly expressed in the bland-looking myxoma-like areas of one myxosarcoma (Figure 5). The angiosarcoma strongly expressed CD31, ERG, factor VIII and FLI-1 (all represent markers of endothelial differentiation) (Figure 6D) but not HHV8 and D2-40, thus ruling out the possibility of anaplastic (so-called sarcomatous) Kaposi sarcoma.
Heart metastasis from extra-cardiac sarcoma (Table 2)
Table 2.
Clinicopathologic features of sarcomas metastatic to the heart (n=4)
| No | Age primary nsarcoma/sex | Age cardiac MTS | Site primary tumor | Histological type | Site of cardiac MTS | Time to cardiac MTS | Clinical symptoms | Other organ MTS | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 F | 49 | Left upper arm | Conventional osteosarcoma | Left atrium | 13 years | Functional mitral stenosis | Lung MTS 4 years before cardiac MTS | Died of lung MTS at 10 months. |
| 2 | 31 F | 51 | Right gluteus maximus | Alveolar soft part sacroma | Interventricular septum, left ventricle | 20 years | Paroxysmal cardiac pain. | Brain MTS 16 years ago and concurrent to heart MTS | Alive & well 34 months after HTX. |
| 3 | 38 F | 38 | Thoracic wall | Spindle cell sarcoma | Left atrium, mitral valve | 5 months | Bronchus tumor infiltration, no symptoms | None | Died of cardiopulmonary failure at 30 months. |
| 4 | 62 M | 65 | Left thigh | Myxoid liposarcoma | Right atrium+ inferior vena cava | 3 years | Slight dyspnea | None | Died of cardiopulmonary failure at 2 months. |
F, female; M, male; MTS, metastasis; A&W, alive and well; HTX, heart transplantation.
There was one case each of osteosarcoma, myxoid liposarcoma, spindle cell sarcoma and alveolar soft part sarcoma that gave rise to intra-cardiac metastasis. Patient’s mean age was 41.7 and 50.7 years at the time of primary sarcoma diagnosis and cardiac metastasis surgery, respectively. The mean age of sarcoma metastasis patients was higher than that of primary cardiac sarcoma patients (50.7 years vs. 45.6 years). Three of the 4 primary sarcomas were localized in the extremities (left upper arm, right gluteus maximus and left thigh) and one was localized in the thoracic wall. The time interval between diagnosis of the extra-cardiac sarcoma and the heart metastasis surgery was 5, 36, 156 and 240 months (mean, 109 months). Three of the 4 cardiac metastases were located in the left heart (2 in the left atrium and 1 in the left ventricle) and one involved the right atrium and the inferior vena cava (Figure 7A). The osteosarcoma patient developed multiple lung metastasis 4 years prior to cardiac metastasis. The patient with the alveolar soft part sarcoma had a history of treated brain metastasis 15 years prior to cardiac metastasis and a second brain metastasis synchronous to cardiac metastasis (also resected). The detailed clinicopathological features of this case have been published previously [13]. However, more extended follow-up was obtained for this patient who had undergone cardiac transplantation as a result of unresectable sarcoma metastasis. She is currently (during writing of this manuscript) alive and well without evidence of disease 34 months after cardiac transplantation. The remaining two patients had no evidence of other organ involvement. Three patients died of tumor-related cardiopulmonary failure or lung metastasis at 2, 10 and 30 months (mean, 14 months) after cardiac metastasis surgery. The histology of cardiac sarcoma metastasis corresponded to that of the primary tumor. However, particular growth pattern seemed to be unique to cardiac metastasis. Extensive necrosis affecting the most superficial part of the intracavitary cardiac metastasis was a characteristic feature with prominent organisation and increased angioma-like vasculature. One patient’s metastasis was initially judged at frozen section as a benign angiomatous lesion. Representative images of cardiac metastasis from soft tissue sarcoma are depicted in Figure 7B-D.
Figure 7.
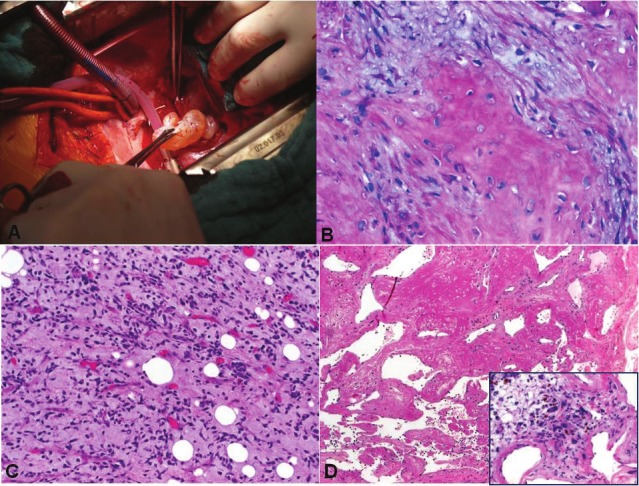
Representative gross and histological images of secondary cardiac sarcomas. A: Intra-operative gross photograph of intracavitary sarcoma metastasis. B: histological image of osteosarcoma metastasis showed prominent osteoid with myxoid pattern that would be mistaken for a myxomatous primary neoplasm. C: metastatic myxoid liposarcoma retained features of the primary tumor with hypercellular areas. D: this metastasis from pleomorphic sarcoma resected after chemoradiation deceptively mimicked thrombosed benign angiomatous lesion on frozen section, but numerous atypical degenerating pleomorphic tumor cells were seen between the vascular channels (inset).
Discussion
Primary cardiac sarcomas represented 6.7% of primary cardiac tumors in our series. Most were high-grade myxoid or undifferentiated pleomorphic sarcomas that qualified as myxosarcomas or undifferentiated sarcoma/MFH, respectively. One patient suffered from angiosarcoma. We encountered no cases of primary liposarcoma, leiomyosarcoma, synovial sarcoma, rhabdomyosarcoma, or other rare sarcoma subtypes included in previous series [14,15]. It is remarkable, that some of these rare histological subtypes are not represented, even in the series of 75 cases published by Burke et al [7]. This remarkable variation reflects the rarity of the individual sarcoma subtypes in the heart and underscores the importance of reporting even small complementary case series. In the series published by Burke et al (n=75), angiosarcoma, undifferentiated sarcoma and osteosarcoma were the most common subtypes encountered. However when lumped together, tumors of probable fibro-/myofibroblastic line of differentiation and undifferentiated tumors (undifferentiated sarcoma/MFH/myxosarcoma) outnumbered all other subtypes [7].
The development of non-invasive cardiac imaging like TTE, TEE, CT and MRT are of utmost value in the preoperative assessment of cardiac tumors [9]. Although TTE is useful in the initial evaluation of suspected tumors, TEE is frequently required for a more comprehensive and accurate assessment [16,17]. CT can adequately demonstrate the morphology, location, and extent of a cardiac mass and its main advantage over the echocardiography is in allowing for assessment of extra-cardiac disease or metastases [18,19]. In addition, cardiac-gated MRI has the advantages of a wide field of view, high contrast and spatial resolution, and multiplanar imaging capabilities which allow precise demonstration and location of a mass, including its anatomic relationship to the cardiac chambers and any involvement of myocardium, pericardium, or contiguous structures [20,21]. The accuracy of cardiac imaging diagnosis depends on the image quality grade and a comprehensive imaging protocol [22].
Cardiac sarcomas are often not resectable entirely due to their large size, anatomic location and infiltrative growth, and only tumor debulking may be possible in such cases [23,24]. Accordingly, surgery represents only a palliative strategy in several cases. However, surgery should be recommended to prevent potentially life-threatening complications. Adjuvant chemo- and/or radiotherapy can prolong survival in a few patients, but is rarely curative [25]. The mean survival for 40 patients with follow-up in the series of Burke et al was 11 months (median, 6 months) [7]. Most patients died of tumor-related cardiopulmonary complications or metastatic disease. Zhang et al showed tumor grade to have a potential prognostic significance in cardiac sarcoma [26]. However, most primary cardiac sarcomas, as in our study, are high-grade tumors. The potential value of cardiac sarcoma grading needs to be validated in larger future studies. Distant metastasis from cardiac sarcoma was seen in 31/75 cases [7], mostly in the lungs, but none metastasized to the gastrointestinal tract in that series. In our cases, only one tumor has metastasized (brain and intestine).
Cardiac sarcomas tend to show a site-specific histology. In particular, angiosarcomas were almost invariably located in the right atrium [14,27] contrasting with a left atrial localization of osteosarcomas and a majority of myxosarcomas [7,28]. Histologically, myxosarcomas differ from atrial myxomas by their higher cellularity, significant atypia, brisk mitotic activity, presence of coagulative necrosis and infiltrative growth. None of the three left atrial myxosarcomas in the series by Burke et al showed transition from benign areas or typical myxoma component making it unlikely that these cases represent malignant myxoma or transformed myxomas [7]. Our 3 myxosarcomas blended with less atypical areas reminiscent of myxoma. However, the myxoma-like component in two cases showed unequivocal atypia and expressed p16 but lacked calretinin (marker of atrial myxoma) expression, thus being identical to the high-grade component of the tumor. However, one of our cases showed focal areas typical of myxoma with calretinin+/p16- profile that was the opposite of the high-grade component in the same neoplasm suggesting an origin from preexisting myxoma. Thus, it is likely that myxosarcoma and myxoma represent distinct entities, but more studies on their expression profiles are needed to address the issue of histogenetic relationship if any. The expression of smooth muscle actin was common as vimentin in most tumors in the series of Burke et al and probably reflects the ubiquity of this marker in a variety of undifferentiated and pleomorphic neoplasms.
In this study, we described 4 patients with cardiac metastasis from soft tissue sarcomas. Two larger series of sarcoma metastases to the heart has been reported recently [29,30]. Considering these two series and ours, most patients had other organ metastasis either concurrent to or preceding cardiac metastasis. Leiomyosarcoma and clear cell sarcoma dominated the Takenaka et al series. Metastasis affected mostly the left atrium. However the duration between primary sarcoma diagnosis and cardiac metastasis varied greatly among the Takenaka et al´ series (range, 8-108 months, mean 44.5 months) and our cases (range, 5-240 months; mean, 109 months). Based on overlapping age at presentation, tumor site and histology, a thorough search for an extra-cardiac primary tumor seems warranted upon diagnosis of a cardiac sarcoma. The remote clinical history is of great relevance, given that 2 of our 4 cases developed cardiac metastasis after ≥ 13 years. Cardiac transplantation might represent an option for patients with unresectable sarcoma metastasis confined to the heart.
In conclusion, primary and metastatic cardiac sarcomas can present with highly variable and non-specific symptoms with overlapping features. The most useful diagnostic tools are echocardiography, CT and MRT. Nevertheless, complete surgical tumor resection often proves to be challenging if not absolutely unfeasibly. Combined modern treatment modalities including heart transplantation for selected cases may improve survival.
Conflict of interest statement
We declare that we have no conflicts of interest.
References
- 1.Burke AP, Virmani R. Tumors of the heart and great vessels. In: Rosai J, Sobin LH, editors. Atlas of Tumor Pathology. Third series, fascicle 16. Washington, DC: Armed Forces Institute of Pathology; 1996. [Google Scholar]
- 2.Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol. 2010;19:343–52. doi: 10.1016/j.carpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Kajihara N, Tanoue Y, Eto M, Tomita Y, Masuda M, Morita S. Surgical experience of cardiac tumors: early and late results. Surg Today. 2006;36:602–7. doi: 10.1007/s00595-006-3217-6. [DOI] [PubMed] [Google Scholar]
- 4.Buresly KM, Shukkur AM, Uthaman B. Unusual survival time of primary cardiac sarcoma of the right ventricle. Heart Views. 2011;12:35–8. doi: 10.4103/1995-705X.81552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossert T, Gummert JF, Battellini R, Richter M, Barten M, Walther T, Falk V, Mohr FW. Surgical experience with 77 primary cardiac tumors. Interact Cardiovasc Thorac Surg. 2005;4:311–5. doi: 10.1510/icvts.2004.103044. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugam G. Primary cardiac sarcoma. Eur J Cardiothorac Surg. 2006;29:925–32. doi: 10.1016/j.ejcts.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–95. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073–103. doi: 10.1148/radiographics.20.4.g00jl081073. [DOI] [PubMed] [Google Scholar]
- 9.Auger D, Pressacco J, Marcotte F, Tremblay A, Dore A, Ducharme A. Cardiac masses: an integrative approach using echocardiography and other imaging modalities. Heart. 2011;97:1101–9. doi: 10.1136/hrt.2010.196006. [DOI] [PubMed] [Google Scholar]
- 10.Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, Barbato L, Verzini A, Fortunato G, di Summa M. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg. 1999;68:1236–41. doi: 10.1016/s0003-4975(99)00700-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim MP, Correa AM, Blackmon S, Quiroga-Garza G, Weilbaecher D, Bruckner B, Ramlawi B, Rice DC, Vaporciyan AA, Reardon MJ. Outcomes after right-side heart sarcoma resection. Ann Thorac Surg. 2011;91:770–6. doi: 10.1016/j.athoracsur.2010.09.079. [DOI] [PubMed] [Google Scholar]
- 12.Strecker T, Rösch J, Weyand M, Agaimy A. Primary and metastatic cardiac tumors: imaging characteristics, surgical treatment and histopathological spectrum: a 10-year-experience at a German Heart Center. Cardiovasc Pathol. 2012 Jan 31;21:436–43. doi: 10.1016/j.carpath.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Strecker T, Schmid A, Agaimy A, Hugo C, Weyand M, Zielezinski T. Giant metastatic alveolar soft part sarcoma in the left ventricle: appearance in echocardiography, magnetic resonance imaging, and histopathology. Clin Cardiol. 2011;34:E6–8. doi: 10.1002/clc.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CH, Dancer JY, Coffey D, Zhai QJ, Reardon M, Ayala AG, Ro JY. Clinicopathologic study of 24 patients with primary cardiac sarcomas: a 10-year single institution experience. Hum Pathol. 2008;39:933–8. doi: 10.1016/j.humpath.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Kumar N, Agarwal S, Ahuja A, Das P, Airon B, Ray R. Spectrum of cardiac tumors excluding myxoma: Experience of a tertiary center with review of the literature. Pathol Res Pract. 2011;207:769–74. doi: 10.1016/j.prp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Alam M, Rosman HS, Grullon C. Transesophageal echocardiography in evaluation of atrial masses. Angiology. 1995;46:123–8. doi: 10.1177/000331979504600205. [DOI] [PubMed] [Google Scholar]
- 17.Lee EY, Jeon ES, Choi JO, Lee SC, Park SW, Park PW. Primary cardiac sarcoma mimicking mural thrombus. Eur J Echocardiogr. 2011;12:713. doi: 10.1093/ejechocard/jer104. [DOI] [PubMed] [Google Scholar]
- 18.Dawson WB, Mayo JR, Müller NL. Computed tomography of cardiac and pericardial tumors. Can Assoc Radiol J. 1990;41:270–5. [PubMed] [Google Scholar]
- 19.Hoey E, Ganeshan A, Nader K, Randhawa K, Watkin R. Cardiac neoplasms and pseudotumors: imaging findings on multidetector CT angiography. Diagn Interv Radiol. 2012;18:67–77. doi: 10.4261/1305-3825.DIR.4215-11.2. [DOI] [PubMed] [Google Scholar]
- 20.Freedberg RS, Kronzon I, Rumancik WM, Liebeskind D. The contribution of magnetic resonance imaging to the evaluation of intracardiac tumors diagnosed by echocardiography. Circulation. 1988;77:96–103. doi: 10.1161/01.cir.77.1.96. [DOI] [PubMed] [Google Scholar]
- 21.Randhawa K, Ganeshan A, Hoey ET. Magnetic resonance imaging of cardiac tumors: part 2, malignant tumors and tumor-like conditions. Curr Probl Diagn Radiol. 2011;40:169–79. doi: 10.1067/j.cpradiol.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Beroukhim RS, Prakash A, Buechel ER, Cava JR, Dorfman AL, Festa P, Hlavacek AM, Johnson TR, Keller MS, Krishnamurthy R, Misra N, Moniotte S, Parks WJ, Powell AJ, Soriano BD, Srichai MB, Yoo SJ, Zhou J, Geva T. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. 2011;58:1044–54. doi: 10.1016/j.jacc.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Park KS, Song BG, Ok KS, Park DW, Jung HJ, Kwak MO, Cho WH, Choi SK. Primary cardiac angiosarcoma treated by complete tumor resection with cardiac reconstruction. Heart Lung. 2011;40:e41–3. doi: 10.1016/j.hrtlng.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Hsieh T, Salehi A. Recurrent Cardiac Intimal (Spindle Cell) Sarcoma of the Left Atrium. J Cardiothorac Vasc Anesth. 2011 Sep 14; doi: 10.1053/j.jvca.2011.07.027. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Yokouchi Y, Hiruta N, Oharaseki T, Ihara F, Oda Y, Ito S, Yamashita H, Ozaki S, Gomi T, Takahashi K. Primary cardiac synovial sarcoma: a case report and literature review. Pathol Int. 2011;61:150–5. doi: 10.1111/j.1440-1827.2010.02631.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang PJ, Brooks JS, Goldblum JR, Yoder B, Seethala R, Pawel B, Gorman JH, Gorman RC, Huang JH, Acker M, Narula N. Primary cardiac sarcomas: a clinicopathologic analysis of a series with follow-up information in 17 patients and emphasis on long-term survival. Hum Pathol. 2008;39:1385–95. doi: 10.1016/j.humpath.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge Y, Ro JY, Kim D, Kim CH, Reardon MJ, Blackmon S, Zhai J, Coffey D, Benjamin RS, Ayala AG. Clinicopathologic and immunohistochemical characteristics of adult primary cardiac angiosarcomas: analysis of 10 cases. Ann Diagn Pathol. 2011;15:262–7. doi: 10.1016/j.anndiagpath.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Burke AP, Virmani R. Osteosarcomas of the heart. Am J Surg Pathol. 1991;15:289–95. doi: 10.1097/00000478-199103000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Takenaka S, Hashimoto N, Araki N, Hamada K, Naka N, Joyama S, Kakunaga S, Ueda T, Myoui A, Yoshikawa H. Eleven cases of cardiac metastases from soft-tissue sarcomas. Jpn J Clin Oncol. 2011;41:514–8. doi: 10.1093/jjco/hyq246. [DOI] [PubMed] [Google Scholar]
- 30.Cuadrado M, García-Camarero T, Expósito V, Val-Bernal JF, Gómez-Román JJ, Garijo MF. Cardiac intracavitary metastasis of a malignant solitary fibrous tumor: case report and review of the literature on sarcomas with left intracavitary extension. Cardiovasc Pathol. 2007;16:241–7. doi: 10.1016/j.carpath.2007.02.006. [DOI] [PubMed] [Google Scholar]