Abstract
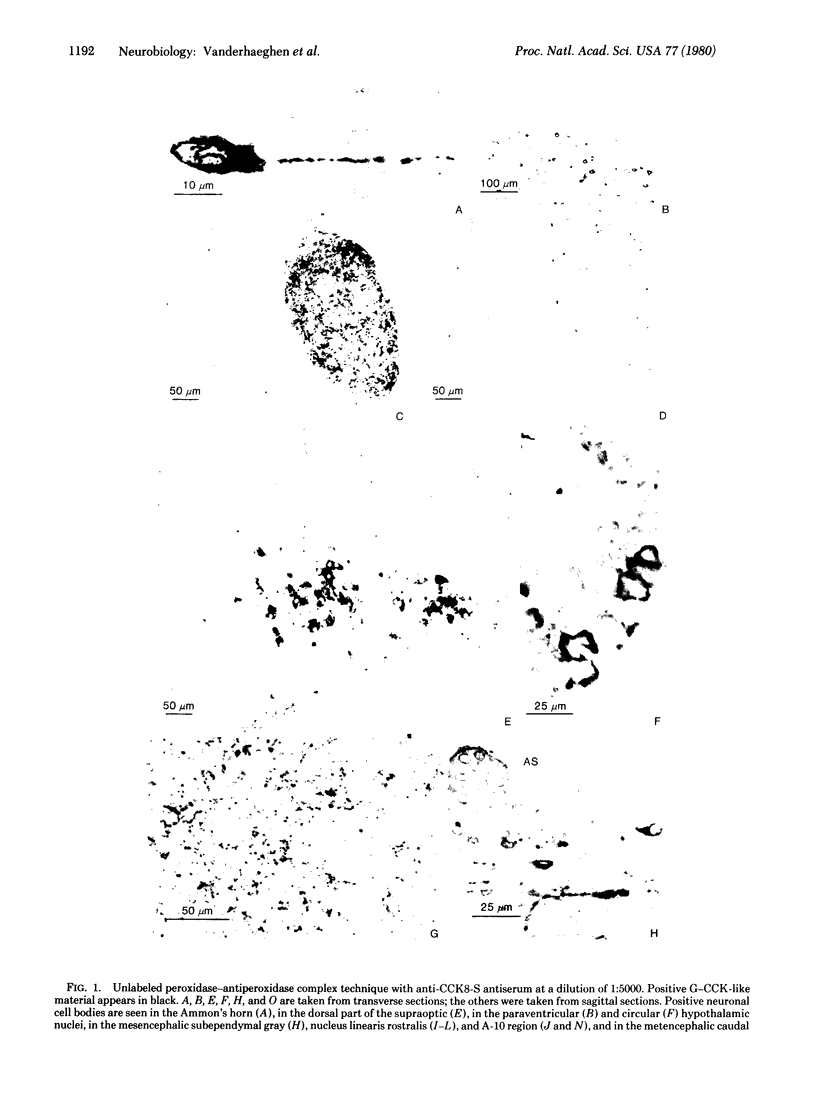
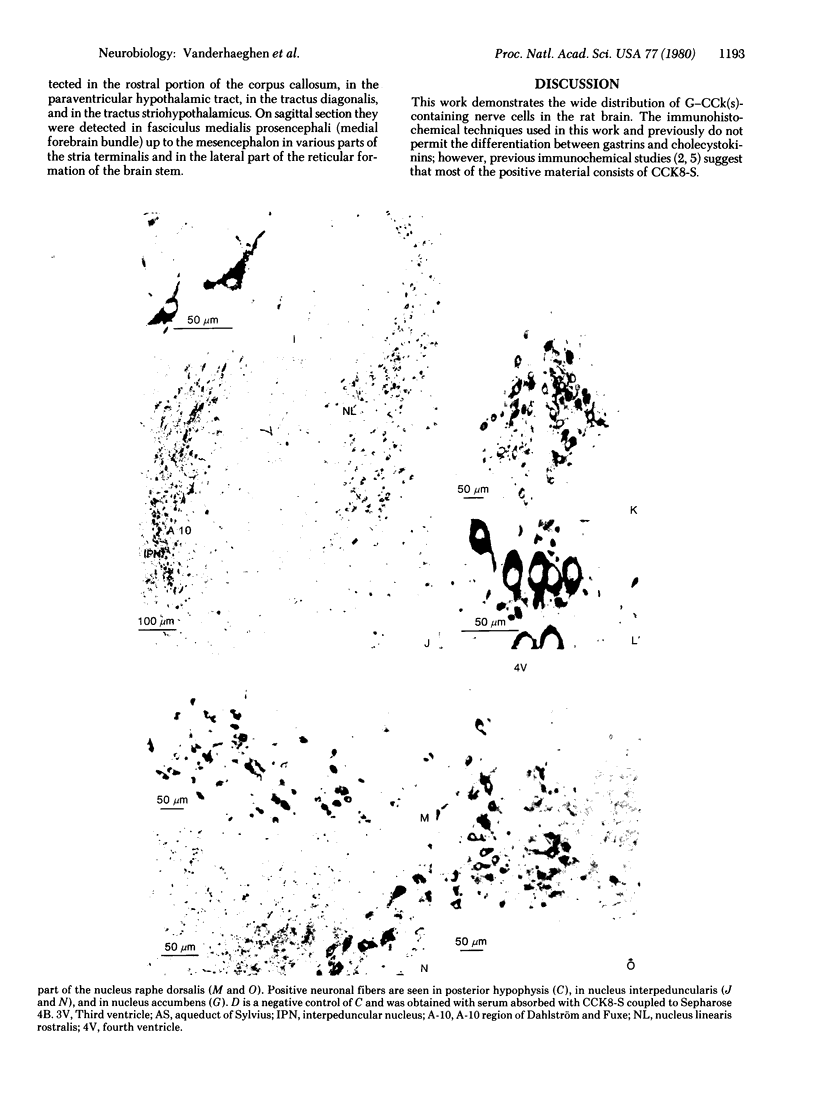
The distribution of gastrin-cholecystokinin-like peptide(s) is reported in brain and hypophysis of the rat. The unlabeled peroxidase-antiperoxidase complex immunohistochemical technique was used. Controls of specificity for various peptides were studied with solid-phase absorption. Colchicine treatment was necessary to obtain positivity in many neuronal cell bodies. In addition to their already known distribution, gastrin-cholecystokinins containing neural cell bodies and fibers were present in olfactory structures, in various preoptic and hypothalamic nuclei (except in mamillary bodies), in mesencephalic nucleus linearis rostralis, and in A-10, A-9, and A-8 regions of Dahlström and Fuxe, which include substantia nigra. From previous investigations and the present distribution study, it can be inferred that, although most of the brain material consists of cholecystokinin, gastrins may also be present in hypothalamo-posthypophyseal magnocellular cells, in nucleus tractus solitarii, and in the dorsal horn of the spinal cord. The distribution of positive cell bodies in the peripheral part of the paraventricular nucleus and in the dorsal part of the supraoptic nuclei in the hypothalamus is similar to that of oxytocin neurons. The localization of positive cell bodies in A-10, A-9, and A-8 regions of Dahlström and Fuxe is similar to that of dopaminergic neurons. The mesencephalic concentration of cell bodies and the wide distribution of fibers in striatal, hypothalamic, septal, and other hemispheric structures together with thick positive fibers in the medial forebrain bundle is consistent with the existence of ascending mesencephalic pathways, including the nigrostriate pathway.
Keywords: neurodigestive peptides, limbic system, substantia nigra, dopamine, oxytocin
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan-Palay V., Jonsson G., Palay S. L. Serotonin and substance P coexist i, neurons of the rat's central nervous system. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1582–1586. doi: 10.1073/pnas.75.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Fuxe K., Johansson O., Ljungdahl A., Goldstein M., Luft R., Efendic S., Nilsson G., Terenius L. Aminergic and peptidergic pathways in the nervous system with special reference to the hypothalamus. Res Publ Assoc Res Nerv Ment Dis. 1978;56:69–135. [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A., Steinbusch H., Verhofstad A., Nilsson G., Brodin E., Pernow B., Goldstein M. Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience. 1978;3(6):517–538. doi: 10.1016/0306-4522(78)90017-9. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Corrêa F. M., Uhl G. R., Schneider B., Snyder S. H. Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):521–525. doi: 10.1073/pnas.76.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg G. W. Neuronal dynamics and axonal flow. IV. Blockage of intra-axonal enzyme transport by colchicine. Proc Natl Acad Sci U S A. 1969 Mar;62(3):722–728. doi: 10.1073/pnas.62.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 1979 Apr 13;165(2):201–218. doi: 10.1016/0006-8993(79)90554-7. [DOI] [PubMed] [Google Scholar]
- Lorén I., Alumets J., Håkanson R., Sundler F. Distribution of gastrin and CCK-like peptides in rat brain. An immunocytochemical study. Histochemistry. 1979 Feb 21;59(4):249–257. doi: 10.1007/BF00689607. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M., Jacobowitz D. M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. II. Hindbrain (mesencephalon, rhombencephalon). J Comp Neurol. 1974 Sep 1;157(1):29–42. doi: 10.1002/cne.901570104. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rehfeld J. F. Localisation of gastrins to neuro- and adenohypophysis. Nature. 1978 Feb 23;271(5647):771–773. doi: 10.1038/271771a0. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., Vanderhaeghen J. J. Demonstration of biological activity of brain gastrin-like peptidic material in the human: its relationship with the COOH-terminal octapeptide of cholecystokinin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):524–528. doi: 10.1073/pnas.75.1.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowsky W. R., Fisher D. A. The use of thyroglobulin to induce antigenicity to small molecules. J Lab Clin Med. 1972 Jul;80(1):134–144. [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Straus E., Muller J. E., Choi H. S., Paronetto F., Yalow R. S. Immunohistochemical localization in rabbit brain of a peptide resembling the COOH-terminal octapeptide of cholecystokinin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3033–3034. doi: 10.1073/pnas.74.7.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubeau G., Desclin J., Parmentier M., Pasteels J. L. Compared localizations of prolactin-like and adrenocorticotropin immunoreactivities within the brain of the rat. Neuroendocrinology. 1979;29(6):374–384. doi: 10.1159/000122948. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Wallensten K., Rehfeld J. F., Larsson L. I., Uvnäs B. Heptadecapeptide gastrin in the vagal nerve. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5707–5710. doi: 10.1073/pnas.74.12.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Wallensten K., Uvnäs B. Release of gastrin on stimulation of the sciatic and brachial nerves of the cat. Acta Physiol Scand. 1978 Jul;103(3):349–351. doi: 10.1111/j.1748-1716.1978.tb06226.x. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K., De Mey J. The origin of the vasopressinergic and oxytocinergic fibres of the external region of the median eminence of the rat hypophysis. Cell Tissue Res. 1977 Jun 13;180(4):443–452. doi: 10.1007/BF00220167. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretroy system of the rat. Cell Tissue Res. 1975 Dec 2;164(2):153–162. doi: 10.1007/BF00218970. [DOI] [PubMed] [Google Scholar]