Abstract
Inter-individual pharmacokinetic variation of H2-receptor antagonist is related to genetic polymorphism of CYP2C19. We investigated the frequency of CYP2C19 genetic polymorphism and the treatment duration of cimetidine by CYP2C19 genotypes in functional dyspeptic patients without definite causes who were treated with cimetidine in Korea. One hundred subjects with functional dyspepsia participated in this study from March 1, 2010 to June 30, 2011. They were tested by upper gastrointestinal endoscopy and treated for their dyspepsia with cimetidine. The single nucleotide polymorphisms (SNPs) of CYP2C19 were genotyped using the Seeplex CYP2C19 ACE Genotyping system. There were no significant differences in the demographic, clinical, or laboratory findings among the CYP2C19 subgroups which are wild type homozygote (W/W), heterozygote (W/V), and variant homozygote (V/V). The frequencies of CYP2C19 subgroups were 33 (33%) in W/W, 49 (49%) in W/V, and 18 (18%) in V/V, respectively. The mean duration of cimetidine treatment (in weeks) was the shortest in the V/V among the CYP2C19 genotypes (W/W: 5.1±1.5, W/V: 4.0±1.7, V/V: 2.1±0.7; p<0.001). This study can also act as a basis for further investigation to identify the underlying genetic, epigenetic, or environmental factors in CYP2C19 enzyme activity.
Keywords: Cimetidine, CYP2C19, Dyspepsia
INTRODUCTION
Dyspepsia is a symptom of the upper abdomen in which patients experience sensations of fullness, bloating, or early satiety [1]. Functional dyspepsia is diagnosed when a specific cause is not found, although the disease may be multifactorial. The treatment of functional dyspepsia must be empiric since a definitive diagnosis is rare. H2-receptor antagonists (H2RAs) is commonly prescribed for the treatment of functional dyspepsia in Korea because those are cheaper than proton pump inhibitors (PPIs).
Although many drugs are primarily metabolized by the cytochrome P450s (CYPs), drug metabolism by the CYPs is difficult to study because of many CYPs members and their diverse substrate array [2]. Cimetidine (N-cyano-N'-methyI-N"-[2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]ethyl] guanidine), a H2RA, is metabolized mainly by cytochrome P450 2C19 (CYP2C19) [3]. The CYP2C19 genetic polymorphism can influence metabolic activity of the subsequent enzymes [4]. Consequently, it increases the level of exposure in the poor metabolizers (PMs) group enhancing drug effect. The prevalence of PMs with CYP2C19 enzyme polymorphisms is 2~6% of Caucasians, 15~20% of Japanese, and 10~20% of Africans [5].
This study's goal was to investigate the frequency of CYP2C19 genetic polymorphism and the treatment duration of cimetidine by CYP2C19 genotypes in Korean functional dyspeptic patients who were treated by cimetidine and where there was no definite cause.
METHODS
Subjects and study protocol
This study (IRB No. 10-04) was approved by the Institutional Review Board of the Kosin University Gospel Hospital, Busan, Korea, and was performed in accordance with the Helsinki Declaration.
One hundred subjects gave informed written consent and participated in this study from March 1, 2010 to June 30, 2011. Patients did not have any 'alarm' symptoms, including progressive unintentional weight loss, chronic gastrointestinal bleeding, epigastric mass, unexplained iron-deficiency anemia, progressive dysphagia, or persistent vomiting. The participants were screened with clinical laboratory tests and interviewed with regard to their medical histories. They all were tested by upper gastrointestinal endoscopy and were treated with cimetidine for their dyspepsia. A total of 100 patients received 450 mg cimetidine daily until the dyspepsia resolved, then were tested for CYP2C19 polymorphism.
CYP2C19 genotyping
Blood samples were collected into tubes containing 5.4 mg EDTA. Genomic DNA was extracted from leukocytes using AccuPrep® Genomic DNA Extraction kits (Bioneer Corporation, Daejeon, Korea). The CYP2C19 polymorphism was determined using the Seeplex CYP2C19 ACE Genotyping system (Seegene, Seoul, Korea), which is a simple, innovative dual priming oligonucleotide (DPO) primer-based multiplex polymerase chain reaction system which has high specificity and sensitivity for detecting two single nucleotide polymorphisms: the CYP2C19*2 and CYP2C19*3 alleles [6]. The allele specific DPO primers have an SNP in the middle of the 30-segment which maximizes disturbance of the 30-segment annealing. Multiplex PCR analysis of the genomic DNA was performed to detect alleles, together with a general primer to detect CYP2C19 (492 bp) using 2X Mastermix (Solgent). After a preheating step at 94℃ for 5 min, 35 amplification cycles were carried out in the thermal cycler under the following conditions: denaturation at 94℃ for 30 s, annealing at 63℃ for 30 s and extension at 72℃ for 30 s. Amplification was completed with a final extension step at 72℃ for 5 min.
The CYP2C19 polymorphisms were expressed as wild type homozygote (W/W: *1/*1 allele), heterozygote (W/V: *1/*2, *1/*3 alleles), and variant homozygote (V/V: *2/*2, *2/*3, *3/*3 alleles) [7,8].
Statistical analysis
Statistical analysis was performed using PASW Statistics 18 (IBM, Chicago, IL, USA). Continuous variables are reported as the mean and standard deviation, and categorical variables are reported as frequency and percentage. ANOVA was used to compare demographic variables and the mean durations of cimetidine treatment among the CYP2C19 subgroups. Pearson's Chi-squared test was used to compare endoscopic diagnosis with CYP2C19 genotypes. A two-tailed value of p<0.05 was considered statistically significant.
RESULTS
Genetic polymorphism of CYP2C19 genotypes
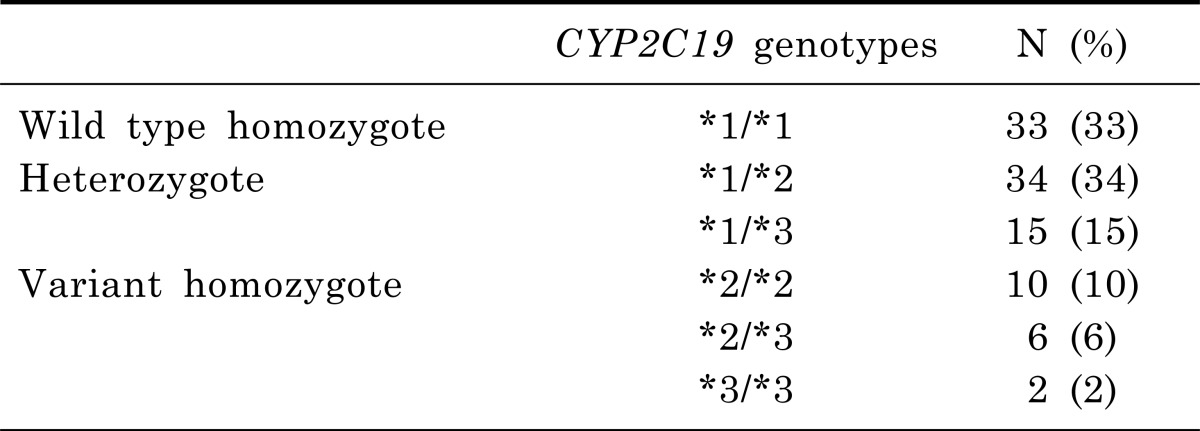
The number of CYP2C19 subgroups was 33 (33%) in W/W, 49 (49%) in W/V, and 18 (18%) in V/V, respectively. The frequencies of the CYP2C19 *1/*1, *1/*2, *1/*3, *2/*2, *2/*3, and *3/*3 genotype were 33 (33%), 34 (34%), 15 (15%), 10 (10%), 6 (6%) and 2 (2%), respectively (Table 1).
Table 1.
Genetic polymorphism by CYP2C19 genotypes of subjects
Clinical characteristics of subjects
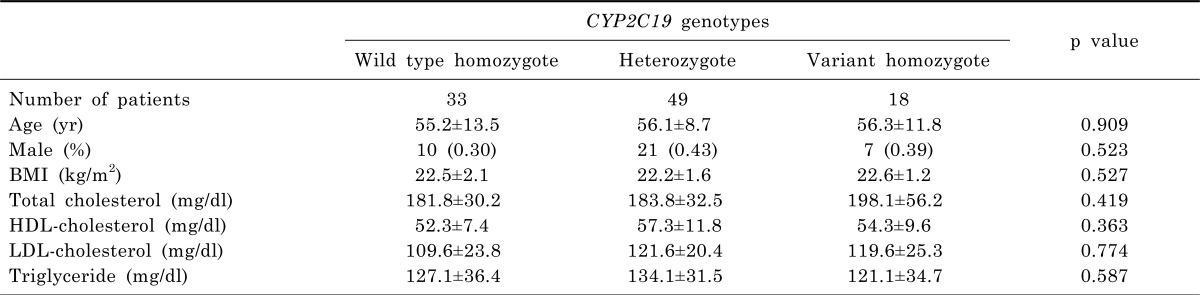
Subject demographics, as well as clinical and laboratory findings, are shown in Table 2. There were no significant differences in the demographic, clinical and laboratory findings among the CYP2C19 subgroups.
Table 2.
Demographic and clinical characteristics by CYP2C19 genotypes of subjects
BMI, body mass index.
Continuous data are shown as mean±SD. Categorical data are shown as number (%).
p<0.05 by ANOVA.
Endoscopic diagnosis and CYP2C19 genotypes
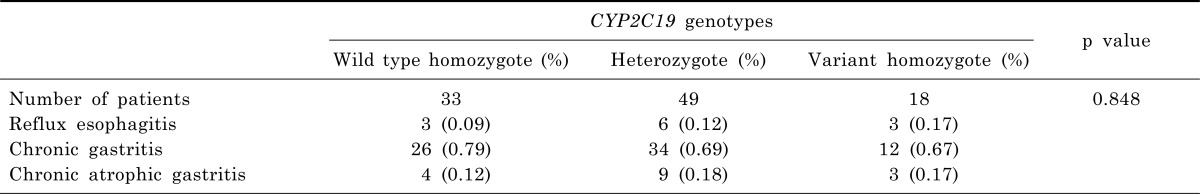
Chronic gastritis was the most endoscopic finding among subgroups. According to endoscopic finding, V/V was more frequency in reflux esophagitis than others. However, endoscopic diagnosis showed no significant differences among subgroups (Table 3).
Table 3.
Endoscopic diagnosis by CYP2C19 genotypes of subjects
p<0.05 by Pearson's Chi-squared test.
The mean duration of cimetidine treatment according to CYP2C19 genotypes
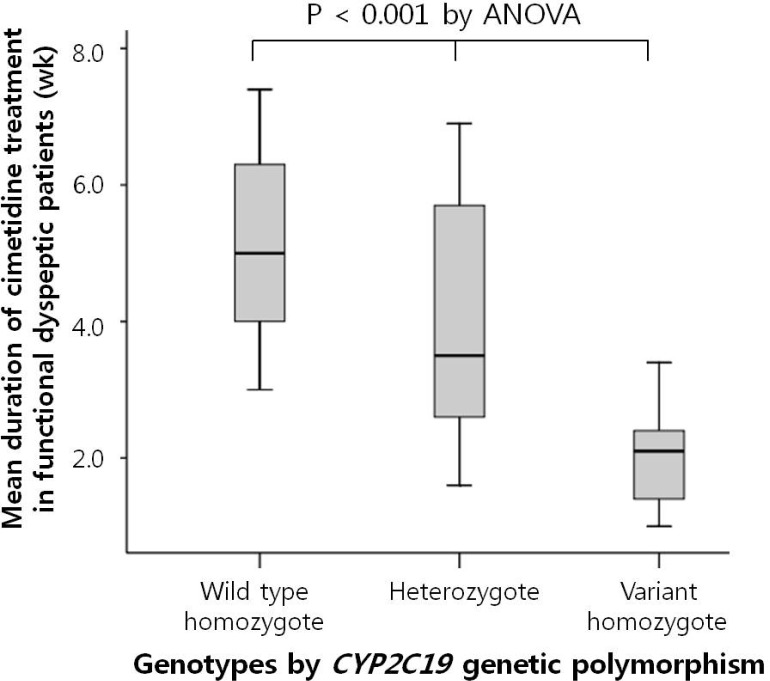
The mean duration of cimetidine treatment (in weeks) was the shortest in the V/V among the CYP2C19 genotypes (W/W: 5.1±1.5, W/V: 4.0±1.7, V/V: 2.1±0.7; p<0.001) (Fig. 1). There was no side effect due to cimetidine treatment.
Fig. 1.
Mean treatment duration (weeks) by CYP2C19 genotypes in functional dyspeptic patients who were treated with cimetidine in Korea. It showed significant differences among the different CYP2C19 subgroups. It was the shortest in the V/V among the CYP2C19 genotypes (wild type homozygote: 5.1±1.5, heterozygote: 4.0±1.7, variant homozygote: 2.1±0.7; p<0.001).
DISCUSSION
Poor CYP2C19-dependent cimetidine metabolism is associated with variant homozygote genotype [8]. The frequency of variant homozygote CYP2C19 in functional dyspeptic patients who were treated with cimetidine was 18% in this study. It was similar to that of Japanese populations (18~23%) [9]. However, it was higher than other ethnic groups; whites Americans (5.0%), African Americans (5.4%) and Mexican Americans (9.8%) [10]. It suggests that the ethnic variation acts on the CYP2C19 polymorphism. We did not analyze CYP2C19*17, which is known to be an ultra-metabolizer phenotype. The frequency of CYP2C19*17 has been reported to be very low in Asian populations [11].
There were no differences in the demographic and endoscopic features among patients with different CYP2C19 genotypes. It was similar with the result of Sheu et al. [7]. In addition, they showed that the 1-year cumulative failure rate of on-demand therapy was significantly higher in W/W than other subgroups. However, we did not check the degree and the effect of reflux esophagitis. The variant homozygote CYP2C19 group exhibited a significantly lower mean duration of cimetidine treatment when compared with the wild type homozygote group in this study. Although we did not prove that CYP2C19 polymorphism is linked to dyspepsia recurrence, we showed a statistically significant association between CYP2C19 polymorphism and the mean duration of cimetidine treatment in the subjects with functional dyspepsia.
We excluded current smokers in this study because smoking could induce dyspeptic symptoms. A previous study reported the effects of smoking on CYP2C19 genotype in influencing H. pylori eradication success, although they did not examine the interaction between CYP2C19 polymorphisms and smoking [12]. Smoking is an important factor that decreases blood flow in the stomach and increases gastric reflux into the esophagus [12].
We suggest that the interaction between smoking or recurrence of dyspepsia and CYP2C19 polymorphisms during the cimetidine treatment be examined in the future.
A comprehensive pharmacogenomic test with genotyping of multiple genetic variations in various portions of the CYP2C19 gene would likely provide a better prediction of the combined effects of genetic mutations on drug metabolism, which could better guide the selection of appropriate treatment durations. This study can also act as a basis for further investigation to identify the underlying genetic, epigenetic, or environmental factors in CYP2C19 enzyme activity.
ABBREVIATIONS
- CYP2C19
cytochrome P450 2C19
- DPO
dual priming oligonucleotide
- H2RA
H2-receptor antagonist
- PM
poor metabolizer
- PPI
proton pump inhibitor
- V/V
variant homozygote
- W/W
wild type homozygote
References
- 1.Andersen PM. Genetic factors in the early diagnosis of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 1):S31–S42. doi: 10.1080/14660820052415899. [DOI] [PubMed] [Google Scholar]
- 2.Lee AY, Lee KH, Ko DS, Chey WY. Constitutive expression and changes of cytochrome P450 isozymes mRNAs by vehicles (petrolatum, DMSO, ethanol) in rat skin using semi-quantitative RT-PCR. Korean J Physiol Pharmacol. 2001;5:407–412. [Google Scholar]
- 3.Badyal DK, Dadhich AR. Cytochrome p450 and drug interactions. Indian J Pharmacol. 2001;33:248–259. [Google Scholar]
- 4.Wijnen PA, Op den Buijsch RA, Drent M, Kuijpers PM, Neef C, Bast A, Bekers O, Koek GH. Review article: The prevalence and clinical relevance of cytochrome P450 polymorphisms. Aliment Pharmacol Ther. 2007;26(Suppl 2):211–219. doi: 10.1111/j.1365-2036.2007.03490.x. [DOI] [PubMed] [Google Scholar]
- 5.Flockhart DA. Drug interactions and the cytochrome P450 system. The role of cytochrome P450 2C19. Clin Pharmacokinet. 1995;29(Suppl 1):45–52. doi: 10.2165/00003088-199500291-00008. [DOI] [PubMed] [Google Scholar]
- 6.Chun JY, Kim KJ, Hwang IT, Kim YJ, Lee DH, Lee IK, Kim JK. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007;35:e40. doi: 10.1093/nar/gkm051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheu BS, Cheng HC, Yeh YC, Chang WL. CYP2C19 genotypes determine the efficacy of on-demand therapy of pantoprazole for reflux esophagitis as Los-Angeles grades C and D. J Gastroenterol Hepatol. 2012;27:104–109. doi: 10.1111/j.1440-1746.2011.06848.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52:349–355. doi: 10.1046/j.0306-5251.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura M, Ieiri I, Mamiya K, Urae A, Higuchi S. Genetic polymorphism of cytochrome P450s, CYP2C19, and CYP2C9 in a Japanese population. Ther Drug Monit. 1998;20:243–247. doi: 10.1097/00007691-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Luo HR, Poland RE, Lin KM, Wan YJ. Genetic polymorphism of cytochrome P450 2C19 in Mexican Americans: a cross-ethnic comparative study. Clin Pharmacol Ther. 2006;80:33–40. doi: 10.1016/j.clpt.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Kim KA, Song WK, Kim KR, Park JY. Assessment of CYP2C19 genetic polymorphisms in a Korean population using a simultaneous multiplex pyrosequencing method to simultaneously detect the CYP2C19*2, CYP2C19*3, and CYP2C19*17 alleles. J Clin Pharm Ther. 2010;35:697–703. doi: 10.1111/j.1365-2710.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Matsuo K, Sawaki A, Wakai K, Hirose K, Ito H, Saito T, Nakamura T, Yamao K, Hamajima N, Tajima K. Influence of smoking and CYP2C19 genotypes on H. pylori eradication success. Epidemiol Infect. 2007;135:171–176. doi: 10.1017/S0950268806006613. [DOI] [PMC free article] [PubMed] [Google Scholar]