Abstract
Background
Lanreotide Autogel® is supplied in prefilled syringes. Therefore, it is possible for patients with neuroendocrine tumors to use self-/partner-administered injections. The primary objective of this study was to assess the proportion of patients preferring self/partner injections over injections administered by health care professionals, and to describe the impact of self/partner administration on efficacy, safety, and costs.
Methods
Of 62 eligible patients, 26 (42%) patients with neuroendocrine tumors treated with a stable dose of lanreotide Autogel 90 mg or 120 mg every 4 weeks agreed to participate in this Phase IV, international, open-label, crossover study, conducted at hospitals in Sweden, Norway, and Denmark. Patients were randomized to two blocks, starting with administration of lanreotide Autogel by either self/partner or a health care professional. Preference for injections administered by self/partner or health care professionals was measured, as well as efficacy, safety, and health care resource utilization (both direct and indirect costs).
Results
Of 25 evaluable patients, 22 (88%) preferred self/partner injections, mainly because they experienced increased independence. Based on all patients asked to participate (n = 62), 35% preferred self/partner injections on a regular basis. There was no difference in efficacy or safety between the two administration blocks.
Conclusion
Many patients with neuroendocrine tumors prefer self/partner injection of lanreotide Autogel, and are able to self/partner inject without any impact on efficacy or safety. This administration method seems to provide a good alternative for suitable patients to increase patient independence and reduce the number of clinic visits.
Keywords: neuroendocrine tumors, carcinoid syndrome, self administration, somatostatin analogs, lanreotide
Introduction
Neuroendocrine tumors are a rare and heterogeneous group of malignant tumors with an increasing incidence.1,2 Many neuroendocrine tumors secrete hormones that can give rise to endocrine syndromes. The classical carcinoid syndrome, with flushing, diarrhea, and right-sided valvular heart disease, is caused by secretion of serotonin from a metastatic ileal carcinoid tumor. Other neuroendocrine tumors (eg, of pancreatic origin) may secrete different hormones, giving rise to corresponding endocrine syndromes.
Lanreotide Autogel® (Ipsen Pharma Biotech, Signes, France) is a somatostatin analog approved for the treatment of carcinoid syndrome in over 50 countries worldwide. Clinical studies have also shown that somatostatin analogs can stabilize tumor size.3–6 The availability of lanreotide Autogel in a prefilled syringe makes it possible for the patient or his/her partner to administer the injection, rather than a health care professional. Because neuroendocrine tumors are slow-growing tumors that often require chronic treatment over a relatively long period of time, self/partner administration could be an attractive alternative for patients considered suitable by the treating physician. It is anticipated that self/partner administration could benefit patients by reducing the impact of the disease on their daily lives, as well as reducing health care resource utilization. A study in 30 patients with acromegaly showed that 14 of 15 patients in the self-/partner-administration group fulfilled all the criteria for successful unsupervised self/partner administration of injections, and all patients in this group continued with unsupervised injections after the study. The authors concluded that patients with acromegaly or their partners were able to administer lanreotide Autogel injections without a negative impact upon efficacy or safety.7 Health care professionals in another study involving 59 patients with acromegaly who self-/partner-administered lanreotide Autogel reported that 100% of patients were able to administer the injections correctly throughout the study.8
The psychosocial profiles of patients with acromegaly and neuroendocrine tumors may differ due to factors such as endocrine-related symptoms (eg, flushing and/or diarrhea of carcinoid syndrome), stress caused by the knowledge that the disease is malignant, and the need for psychosocial support from health care staff.9 The primary aim of the present study was to assess whether patients with neuroendocrine tumors prefer injections of lanreotide Autogel administered by self/partner or a health care professional. The study also assessed the impact of self/partner administration on efficacy, safety, and costs. In addition, based on their experience with the two administration methods during the study, the health care professionals’ observation of safety and their anticipated future usage of self/partner injection were assessed.
Patients and methods
Study design and patients
This was a Phase IV, open-label, randomized, crossover, multicenter study conducted in Sweden, Norway, and Denmark. Of the 10 centers initially involved in the study, nine enrolled patients. Patients eligible for inclusion in the study were aged 18 years or older with a diagnosis of a neuroendocrine tumor confirmed by biopsy and radiology, treated with a stable dose of lanreotide Autogel (90 or 120 mg every 28 days) for at least 3 months and who were presumed to have clinically stable disease in the months following enrollment. Patients were excluded if they had performed self/partner administration previously or if, after three supervised training injections, they were judged by the investigator not to be competent in the injection technique.
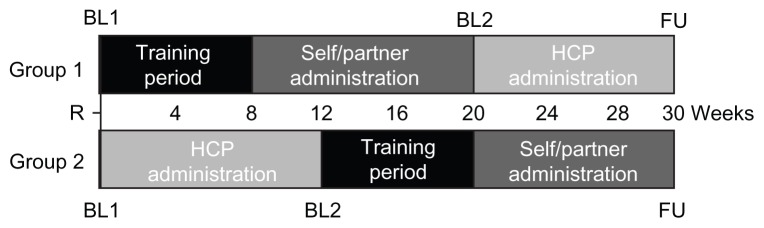
Patients were randomized to one of two sequence groups. Patients in group 1 started with two or three supervised training injections, followed by three unsupervised injections administered on every 28th day by the patient or partner (self/partner administration block), and then three injections administered according to clinical routine every 28th day by health care professionals (health care professional administration block). Patients in group 2 started with the health care professional administration block, followed by the training period and then the self/partner administration block. In both sequence groups, the patients visited the clinic for a follow-up visit 14 days after the last injection (Figure 1).
Figure 1.
Study design.
Note: Training period consisted of 2–3 supervised injections as judged necessary by the supervisor.
Abbreviations: BL, baseline; FU, follow-up; HCP, health care professional; R, randomization.
Prior to the start of the study, the protocol was reviewed and approved by regional ethics review boards in Uppsala, Sweden, in Viborg, Denmark, and in Tromsø, Norway. The study was conducted according to the Declaration of Helsinki, the International Conference on Harmonisation Consolidated Guidelines on Good Clinical Practice, and all local regulatory requirements. The study was registered on clinicaltrials.gov (NCT00681187). All patients provided their written informed consent before entering the study.
Interventions
Lanreotide Autogel 90 or 120 mg supplied as a prefilled syringe, was given as a deep subcutaneous injection, either in the upper external quadrant of the buttock (if given by a health care professional or partner) or in the upper outer thigh (if administered by the patient) every 28 days. Patients continued on the dose they were receiving at the time of study entry. Drug administrators were trained to alternate injection sites between right and left buttock/thigh (as per normal clinical practice).
Assessments and outcome measures
Patients attended three study visits, ie, at baseline, at the end of the first administration block, and at follow-up. The primary endpoint of the study was to assess the proportion of patients preferring self/partner injections over injections administered by health care professionals. Patient preference for self/partner administration versus administration by a health care professional was assessed at the last visit by asking, “If you could choose, which administration method would you like to use on a regular basis?
Health care professional-provided injection
Self-/partner-administered injection
Please give a main reason.”
Secondary endpoints comprised patient experiences with treatment administration, health care resource utilization, assessments of symptom and biochemical control, and safety.
Health care resource utilization was assessed by a questionnaire completed after each visit. This questionnaire gathered information about health care resource utilization, both direct (eg, physician visits) and indirect (total travel time and, where relevant, loss of productivity for patient and partner) for patients and partners associated with the treatment of neuroendocrine tumors, as well as costs of visits (outpatient for injections).
Symptom control was captured by asking how the patient had perceived the symptoms with respect to episodes of flushing and/or diarrhea since the last visit. Biochemical control was assessed by measuring plasma chromogranin A levels for all patients at all visits, and urinary 5-hydroxyindoleacetic acid levels if judged relevant by the investigator. Clinical laboratory tests were performed by a central laboratory. Safety was assessed by means of a physical examination, measurement of vital signs, and adverse events, (systemic adverse events and injection site reactions) that were collected at all study visits and from patient diaries, after each injection. Each investigator was asked to complete a global evaluation at study completion. The health care professional answered two final questions, ie, one related to patient safety during self/partner administration and one asking whether the investigator would recommend suitable patients to try self/partner administration in the future.
Statistical methods
No formal statistical analyses comparing endpoints between administration blocks were performed. The endpoints are summarized using descriptive statistics. The original sample size of 42 randomized patients was based on a proportion of 75% of the patients preferring self/partner administration and a dropout rate of 10%. With 37 evaluable patients, the 95% confidence interval (CI) was calculated to ±16%. Recruitment proved much more difficult than anticipated, and after 18 months, 26 patients were randomized. Preliminary results from completed patients showed that the proportion preferring self/partner administration was in the range of 90% with a dropout rate of <5%. Based on these data, the 95% CI was recalculated and found to be still within an acceptable range. Therefore, it was decided to close inclusion despite not having met the recruitment target, because this would not significantly alter the range of the 95% CI calculated initially in the sample size justification.
The primary analysis based on the primary endpoint was performed on the intention-to-treat population (all randomized patients with at least one dose of study medication and with a preference assessment recorded). In addition, an analysis was performed on the per protocol population (all patients in the intention-to-treat population for whom no major protocol violations/deviations occurred) to study the robustness of the results. Efficacy analyses (ie, biochemical and symptom control) were performed on the intention-to-treat population. The analyses of health care resource utilization and safety data were performed on the safety population (all randomized patients with at least one dose of study medication).
The different endpoints were assessed by comparing the following patient groups (see Figure 1).
Preference was assessed in all patients, as well as in each group, to evaluate the possible impact of the sequence of administration methods on preference.
Efficacy, safety, and health care resource utilization were assessed by comparing changes in all patients during the self/partner (including training) administration block with changes in all patients during the health care professional administration block.
Safety was assessed for three different periods, ie, the training period and each administration block separately.
Experiences of health care professionals were based on a global assessment of all patients at each study center.
Results
Patients
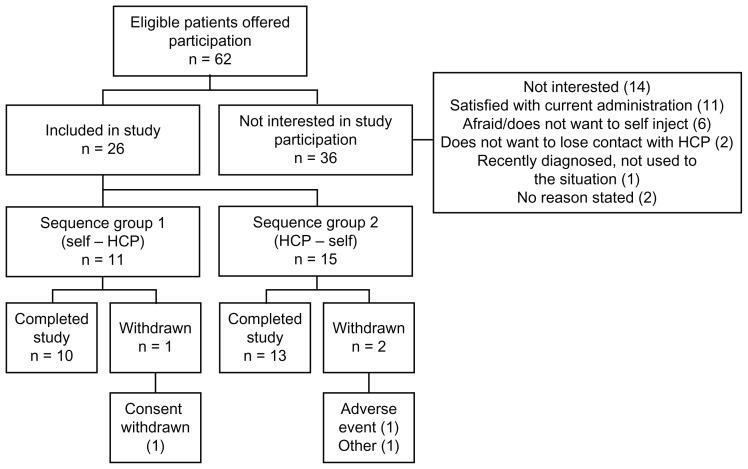
A total of 62 eligible patients were offered participation in this study. They were all registered on a patient screening log, together with the reason for nonparticipation (ICH-GCP 8.3.20). Of these, 58% (36/62) chose to continue with their current form of administration at the hospital or with their local health care professional and did not participate in the study. Reasons for nonparticipation are provided in Figure 2.
Figure 2.
Disposition of patients.
Abbreviation: HCP, health care professional.
A total of 26 patients (12 female, 14 male) were included in the study (Table 1). The median age was 63 (range 38–77) years and median time since diagnosis was 4 (range 0.5–20) years. Twenty-two of the 26 patients had ileal neuroendocrine tumors, two had pancreatic neuroendocrine tumors, one had a lung neuroendocrine tumor, and one had a neuroendocrine tumor of unknown origin. All patients except one had disseminated disease; metastases were present in the liver (85%), lymph nodes (42%), bone (8%), and/or ovaries (4%). Eleven patients were randomized to group 1 and 15 patients to group 2. Seventeen patients received 90 mg (eight in group 1, nine in group 2) and nine patients received 120 mg (three in group 1, six in group 2). During self/partner administration, 22 patients chose to administer the injections themselves and four patients were given the injections by a partner.
Table 1.
Patient demographics and baseline data
| Group 1 (self–HCP) | Group 2 (HCP–self) | Total | |
|---|---|---|---|
| Patients (n) | |||
| Total | 11 | 15 | 26 |
| Number (%) of patients per country | |||
| Denmark | 3 (27) | 4 (27) | 7 (27) |
| Norway | 1 (9) | 3 (20) | 4 (15) |
| Sweden | 7 (64) | 8 (53) | 15 (58) |
| Gender, n (%) | |||
| Female | 4 (36) | 8 (53) | 12 (46) |
| Male | 7 (64) | 7 (47) | 14 (54) |
| Age, years | |||
| Median | 62.6 | 63.2 | 62.9 |
| Minimum, maximum | 52, 77 | 38, 75 | 38, 77 |
| Time since diagnosis, years | |||
| Median | 7.5 | 3 | 4 |
| Minimum, maximum | 0.5, 15 | 0.5, 20 | 0.5, 20 |
| Number (%) of patients per primary site | |||
| Small intestine | 9 (82) | 13 (87) | 22 (85) |
| Pancreas | 1 (9) | 1 (7) | 2 (8) |
| Lung | 0 | 1 (7) | 1 (4) |
| Unknown | 1 (9) | 0 | 1 (4) |
Abbreviation: HCP, health care professional.
All 26 patients were included in the safety population. A total of 25 patients were included in the intention-to-treat analysis; one patient was excluded because there was no preference assessment. A total of 22 patients were included in the per protocol analysis; two patients were excluded due to early withdrawal (one patient withdrew consent and one patient was withdrawn by the investigator due to disease progression) and one due to a major protocol deviation, where the patient received only one of the three protocol-mandated self/partner injections in his home.
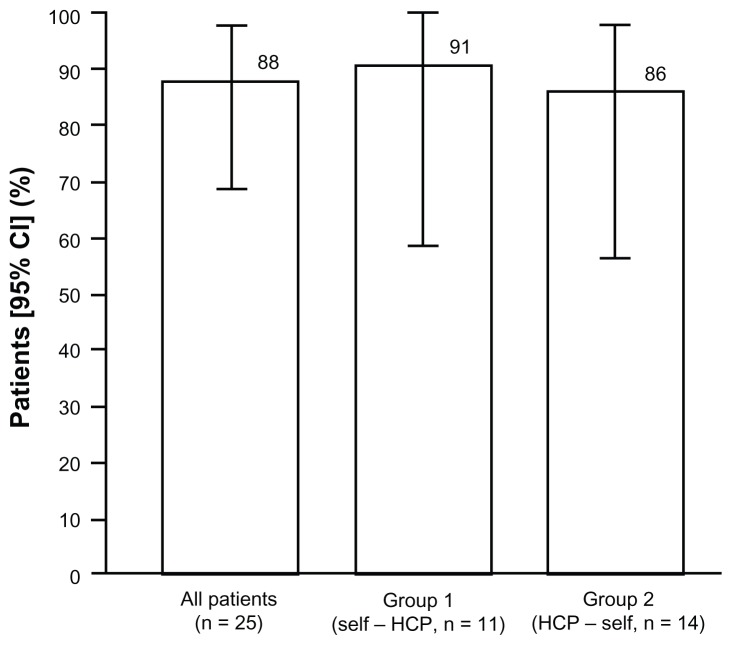
Primary endpoint: patient preference
In the intention-to-treat population, 22 (88%; 95% CI 68.8–97.5) preferred self-/partner-administered injections and three (12%) preferred injections administered by health care professionals (Figure 3). In the per protocol population, 20 (91%; 95% CI 70.8–98.9) patients preferred self-/partner-administered injections and two (9%) patients preferred injections administered by health care professionals. Self/partner administration was preferred by 10/11 (91%) patients in sequence group 1 (self/partner followed by administration from a health care professional), and 12/14 (86%) patients in sequence group 2 (health care professional followed by self/partner administration, Figure 3). All three patients who preferred injections administered by a health care professional were younger than 65 years. There were no differences in preferences noted with regard to employment status (5/6 [83%] of employed and 17/19 [89%] of unemployed/retired patients preferring self/partner administration), gender (11/12 [92%] female and 11/13 [85%] male preferring self/partner administration) or drug administrator (19/21 [90%] self and 3/4 [75%] self or partner preferring self/partner administration).
Figure 3.
Proportion of patients preferring self/partner administration (intention-to-treat population).
Abbreviations: CI, confidence interval; HCP, health care professional.
The main reasons provided by 15/22 of the patients choosing self/partner administration were “time saving”, “more independence”, “simpler”, “practical”, and “don’t have to travel to the hospital”. One patient found it “less painful”, one patient stated “comfort” as the reason, and four patients did not provide a reason for preferring self/partner administration. The three patients preferring injections administered by a health care professional provided the following reasons: “needle too thick, afraid of injections”, “psychological discomfort and irritation at injection site”, and “stressful for my wife to inject, however [it is] good that [my] partner can handle the injections when travelling”.
Secondary endpoints
Health care resource utilization
Table 2 summarizes the resource utilization and costs anticipated for each administration method, and indicates that cost savings may be possible if patients self/partner inject rather than have injections administered by a health care professional. From a health care payer perspective, direct health care costs may be reduced as self/partner injection obviates the need for primary care nurse visits in an outpatient clinic. From a societal perspective, indirect costs may be reduced because patients/partners do not spend time travelling for injections. The data from the study also indicate that there were no increases in costs relating to self/partner administration compared with administration by a health care professional due to adverse drug reactions (no patients during either administration block), visits to a health care professional due to carcinoid symptoms (eight patients during each administration block), or non-study-related telephone contact with a study health care professional (18 contact instances during each administration block). Thus, potential cost savings associated with the use of injections by self/partners would result mainly from fewer clinic visits for injections.
Table 2.
Health economic data
| Resource utilization and costs differing by injector | Cost per patient per injection | |
|---|---|---|
|
| ||
| Injections by HCP | Injections by patient/partner | |
| Direct costs | ||
| Nursing visits | €65 | None |
| 1 visit/injection | ||
| €65 per primary care nurse visit in Sweden (2009 price from Swedish south-eastern health care region) | ||
| Indirect costs | ||
| Patient/partner time for travel and injection | €7.95 | €0.10 |
| 1.4 hours per patient/partner for travel and injection | 0.0183 hour (66 seconds)a per patient/partner for injection | |
| 23% (6/26) of patients or accompanying partners are employed, on average | 23% (6/26) of patients or accompanying partners are employed, on average | |
| €25/h mean income (Statistics Sweden, 2009: 165 hours working month with an average monthly income of €3100 plus 31.42% payroll tax) | €25/hour mean income (Statistics Sweden, 2009: 165 hours working month with an average monthly income of €3100 plus 31.42% payroll tax) | |
Efficacy
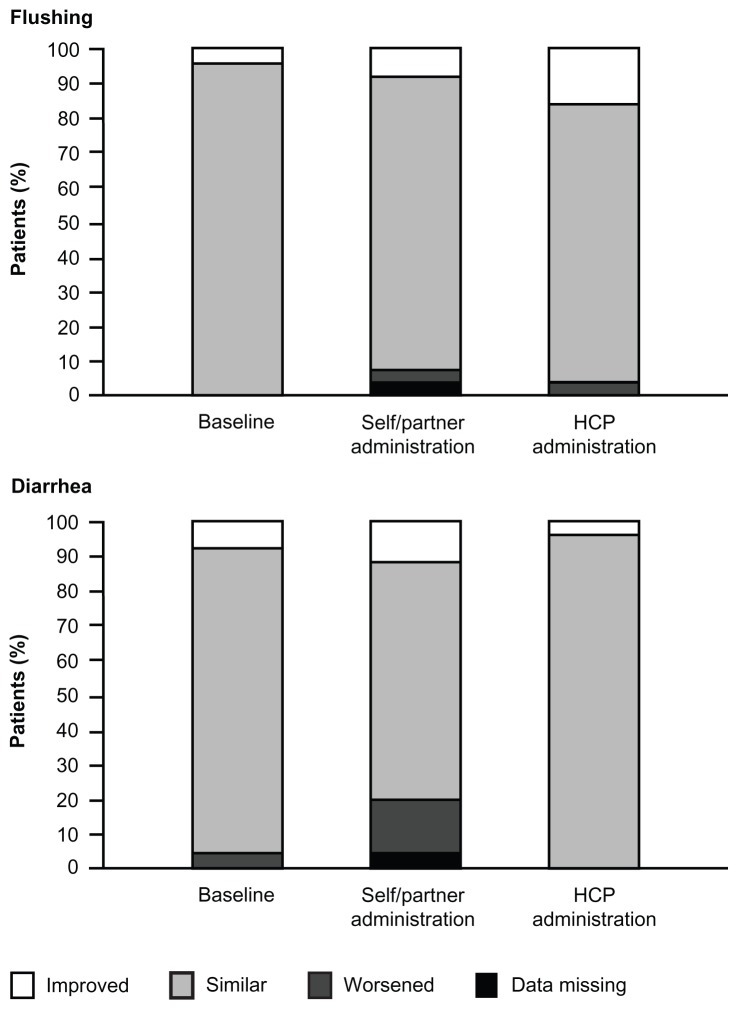
Patient experiences after each injection, symptom control (flushing and diarrhea), and biochemical control data are presented in Table 3 and Figure 4. There were no notable differences in flushing symptoms between the administration methods; one patient (4%) worsened after each type of administration. However, four patients (16%) reported that their diarrhea worsened after self-/partner-administered injections, whereas no patients reported worsened diarrhea after injections administered by a health care professional. On the other hand, three (12%) patients reported decreased diarrhea after the self-/partner-administered injections, compared with one (4%) after the injections administered by a health care professional. Biochemical control was similar after both administration methods.
Table 3.
Summary of efficacy endpoints by administration period
| Biochemical control | n | Self/partner administration | HCP administration | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before | After | Before | After | ||
| Mean (SD) Cg A, nmol/L |
22 | 37.5 (58.4) | 47.9 (74.9) | 42.1 (70.7) | 37.4 (58.0) |
| Mean (SD) 5-HIAA, μmol/day |
12 | 220.2 (238.3) | 219.2 (227.6) | 217.1 (244.3) | 219.6 (218.5) |
|
| |||||
| Patient experiences | Training | Self/partner administration | HCP administration | ||
|
| |||||
| Interference of at least one injection with: n (%) | |||||
| Activities of daily living | 6 (24%) | 2 (8%) | 6 (24%) | ||
| Psychological well being | 4 (16%) | 2 (8%) | 1 (4%) | ||
Notes: Data are from the intention-to-treat population (n = 25) except for urinary 5-HIAA levels, which were measured as judged necessary by the investigator. Data are presented only for patients with values for all three visits in an administration block. Reference values for Cg A and urinary 5-HIAA were <4.0 nmol/L and >50 μmol/day, respectively.
Abbreviations: Cg A, chromogranin A; 5-HIAA, 5-hydroxyindoleacetic acid; HCP, health care professional; SD, standard deviation.
Figure 4.
Change in patient-perceived symptom control from last visit.
Note: Baseline refers to change from last prestudy visit to the clinic.
Abbreviation: HCP, health care professional.
Safety
During the trial, 14 patients reported 45 adverse events that were considered related to the study drug, and none of these were considered to be serious. Twenty-eight of the related adverse events were injection site reactions captured from the patient diaries. Ten patients reported 14 nonrelated serious adverse events, of which one was fatal. The fatal serious adverse event was reported as “death not otherwise specified” for a 57-year-old male who was found deceased in his home. One patient was withdrawn due to disease progression (nonserious, moderate in intensity). The most frequently reported adverse event was injection site pain (n = 6, 23%) followed by headache and abdominal pain (both n = 4, 15%), and disease progression, nausea, flushing, and diarrhea (all n = 3, 12%, Table 4).
Table 4.
Summary of adverse events
| AE category | Training period | Self administration block | HCP administration block | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| n | m | n | m | n | m | n | m | |
| All related AEs | ||||||||
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 2 | 2 | 6 | 12 | 9 | 14 | 4 | 5 |
| Mild | 7 | 17 | 5 | 11 | 7 | 12 | 11 | 40 |
| Injection site reactions | 6 | 8 | 3 | 8 | 7 | 12 | 11 | 28 |
| AEs reported by at least three patients | ||||||||
| Injection site pain | 4 | 4 | 1 | 3 | 3 | 6 | 6 | 13 |
| Headache | 3 | 4 | 1 | 1 | 0 | 0 | 4 | 5 |
| Abdominal pain | 1 | 1 | 2 | 2 | 1 | 1 | 4 | 4 |
| Disease progression | 0 | 0 | 2 | 3 | 2 | 2 | 3 | 5 |
| Nausea | 3 | 4 | 1 | 1 | 0 | 0 | 3 | 5 |
| Flushing | 0 | 0 | 1 | 2 | 2 | 2 | 3 | 4 |
| Diarrhea | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 |
Note: Data are number of patients in the safety population (n = 26).
Abbreviations: AE, adverse event; HCP, health care professional; m, number of events; n, number of patients.
There were no notable differences in the frequency of serious adverse events between the administration blocks or training period. The number of patients reporting injection site reactions during the training, self/partner, and health care professional administration blocks was six (23%), three (12%), and seven (27%), respectively, whereas flushing and diarrhea were reported by one (4%), two (8%), and three patients (12%), respectively. There were no notable differences in the intensity of serious adverse events between the administration blocks or training period. Measurements of vital signs and physical examinations performed during the study did not raise any safety concerns.
Experience of health care professionals
All nine investigators stated that they felt confident in the safety of self/partner administration and that they would recommend suitable patients to try self/partner administration in the future.
Discussion
This Phase IV study assessed preference for self/partner administration with lanreotide Autogel compared with injections given by health care professionals in patients with neuroendocrine tumors. Fifty-eight percent of the patients who were offered participation in the study chose to continue with their current form of administration at the hospital or local health care professional because they were “content with current situation” or “afraid to self-inject”. Of the patients willing to try self/partner injections, 88% preferred this method of administration. Hence, a total of 35% of the 62 patients asked to participate in the study preferred self/partner administration.
The main benefit when handling the injections at home was increased independence and time saved travelling to the clinic, resulting in a decreased impact of treatment on the patients’ daily lives. It was anticipated that younger patients of working age would be more inclined to self/partner administer to gain increased independence from medical care, as shown previously in patients with acromegaly.6 Given the limited number of patients, this could not be assessed in this study. However, it was noted that the three patients who preferred injections administered by a health care professional were aged under 65 years. No difference in preference was noted with regard to employment status.
As in all clinical studies, patients have the choice of participating or not. This will inevitably introduce bias to the results because only those agreeable to self/partner administration agreed to participate. The results from this study are in line with previous studies in patients with acromegaly where a vast majority of those who tried self/partner administration chose to continue this means of administration after study completion.7,8 Our study also reports the proportion of patients willing to test this method of administration among all patients asked for participation in the study. Because this information has not been reported in the acromegaly studies, it is not possible to assess how potential differences in psychosocial profiles between patients with neuroendocrine tumors affect the willingness to try self/partner administration. However, these differences do not seem to affect the preferred method of administration in patients motivated to try self/partner administration.
A comparison of efficacy and safety between the administration methods indicated no difference with regard to patient-perceived control of flushing symptoms, biochemical control, and/or frequency and intensity of serious adverse events. There was a slight difference between the groups with regard to patient-perceived control of diarrhea symptoms. The pattern of changes is difficult to interpret and might be due to the small sample size.
Studies in patients with acromegaly have shown that self/partner administration does not affect the safety profile or control of growth hormone and insulin growth factor-like 1 levels.7,8 Collectively, these results indicate that there are no additional concerns related to efficacy or safety in patients who self/partner administer lanreotide Autogel compared with those who receive the injections from a health care professional. However, patients learning to self/partner administer injections need to be instructed by a health care professional to ensure proper administration. Furthermore, all nine investigators who included at least one patient in the study stated that they felt confident in the safety of self/partner administration during the self/partner administration block and that they would recommend suitable patients to try this in the future.
Health economic analyses performed were based on costs in Sweden and conditions in Scandinavia, and need to be locally adapted for other countries. With regard to health care resource utilization, we found that the reduced number of patient visits to the clinic could result in direct cost savings of about EUR 65 per injection. Furthermore, data from this study suggest that additional savings in indirect costs, through reduction of travel time for patients and partners for injections by a health care professional in an outpatient clinic setting, could be realized through patient/partner injection at home. Additional modeling could be performed to quantify further the full economic impact of patient/partner injection versus injection by health care professionals, from both health care payer and societal perspectives.
In conclusion, this study shows that self/partner administration of lanreotide Autogel is a feasible option and a potentially good alternative to injection administered by a health care professional for suitable patients with neuroendocrine tumors, mainly due to increased independence, similar efficacy and safety, and the potential to reduce both direct and indirect costs.
Footnotes
Disclosure
This study was sponsored by Ipsen Nordic. AÖ is an employee of Ipsen AB. VJ, BW, AA, CJ, JC, NW, HG, JF, and DG were compensated by Ipsen AB for work done in this study. The authors also acknowledge Watermeadow Medical (supported by Ipsen Nordic) for editing assistance. The authors have no other disclosures to report.
References
- 1.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(1):1–18. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Rindi G. The ENETS guidelines: the new TNM classification system. Tumori. 2010;96(5):806–809. doi: 10.1177/030089161009600532. [DOI] [PubMed] [Google Scholar]
- 3.Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and non-functional neuroendocrine tumors. Cancer. 1993;72(1):244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Ducreux M, Ruszniewski P, Chayvialle JA, et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol. 2000;95(11):3276–3328. doi: 10.1111/j.1572-0241.2000.03210.x. [DOI] [PubMed] [Google Scholar]
- 5.Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer. 2001;37(8):1014–1019. doi: 10.1016/s0959-8049(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 6.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 7.Bevan JS, Newell-Price J, Wass JA, et al. Home administration of lanreotide Autogel by patients with acromegaly, or their partners, is safe and effective. Clin Endocrinol. 2008;68(3):343–349. doi: 10.1111/j.1365-2265.2007.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvatori R, Nachtigall LB, Cook DM, et al. Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naïve patients with acromegaly. Pituitary. 2009;13(2):115–122. doi: 10.1007/s11102-009-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fröjd C, Larsson G, Lampic C, et al. Health related quality of life and psychosocial function among patients with carcinoid tumours. A longitudinal, prospective, and comparative study. Health Qual Life Outcomes. 2007;5:18. doi: 10.1186/1477-7525-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess A, Davies PR, Adelman D. Long-acting somatostatin analog injection devices: a quantitative time and perception study to explore nurses’ preferences. Endocr Rev. 2011;32(3):P1–P452. [Google Scholar]