Abstract
Introduction
We investigated fluid responsiveness in a population of patients undergoing coronary artery revascularization, with respect to their right ventricular ejection fraction.
Materials and Methods
This was a multicenter trial involving 11 cardiac surgical Institutions and 65 patients undergoing elective coronary artery revascularization. Hemodynamic parameters were measured before and after volume expansion using a modified pulmonary artery catheter and transesophageal echocardiographic monitoring. Patients demonstrating an increase of stroke volume >20% after volume expansion were considered as responders. Volume expansion with 7 ml/kg of plasma expander was performed when required on a clinical basis.
Results
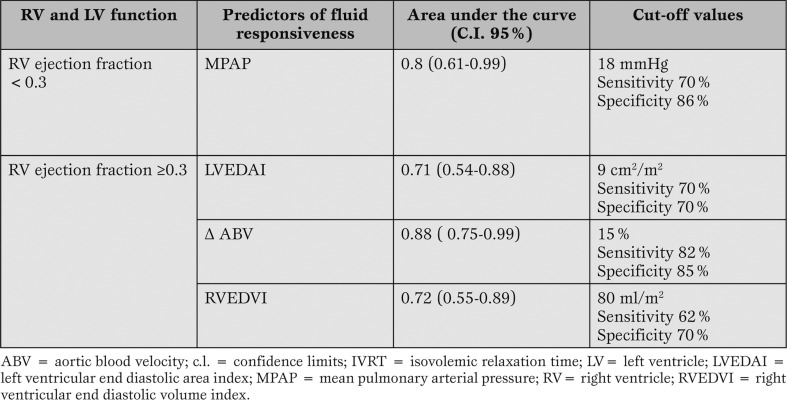
In the overall population, only the change in aortic blood velocity (cut-off 13%) was a predictor of fluid responsiveness. In patients with a reduced (<0.3) right ventricular ejection fraction only the value of mean pulmonary arterial pressure was predictive of fluid responsiveness (cut-off 18 mmHg). Patients with right ventricular ejection fraction ≥0.3 demonstrated three predictors: changes in aortic blood velocity (cut-off 15%), right ventricular end diastolic volume index (cut-off 80 ml/m2), and left ventricular end diastolic area index (cut-off 9 cm2/m2).
Conclusions
When right ventricular systolic function is depressed, the right ventricle inability to fill the left chambers results in a lack of the left-sided responsiveness predictors. When the right ventricular systolic function is preserved, all the classical fluid responsiveness predictors are confirmed. Right ventricular function is therefore to be always considered when addressing the problem of fluid responsiveness.
Keywords: Intensive care, cardiac surgery, fluid responsiveness, Mechanical ventilation, preload
Introduction
Preload assessment and fluid responsiveness of patients with a critical or sub-critical hemodynamic status have been addressed in various research studies, both in Intensive Care Unit patients [1,2,3,4,5,6,7] and during major operations [8,9,10,11,12]. Different predictors of fluid responsiveness have been identified, basically belonging to four different categories:
a) pressure-based parameters like right atrial pressure (RAP) [2], mean pulmonary arterial pressure (MPAP) and pulmonary artery occlusion pressure (PAOP) [2,5,13];
b) areas and volumes like left ventricular end diastolic area (LVEDA) [1], right ventricular end diastolic volume (RVEDV) [13], and intra-thoracic blood volume (ITBV) [14];
c) transmitral valve flow doppler parameters [12];
d) dynamic parameters like inspiratory decrease in RAP, expiratory decrease in arterial systolic pressure, respiratory changes in pulse pressure, and respiratory changes in aortic blood velocity (ABV) [1,3,4,6,7,8,9,11].
All the predictors of fluid responsiveness have potential advantages or disadvantages, and may be more or less useful in different clinical conditions; however, whenever addressing the problem of fluid responsiveness, it should be always considered that this concept depends not only on the objective preload conditions, but even on myocardial contractility. A decrease in ventricular contractility decreases the slope of the relationship between end-diastolic volume and stroke volume; moreover, in case of right ventricular dysfunction, the beneficial effects of volume expansion are unlikely to occur, even in case of low left ventricular preload [15]. Despite the potentially important role of right ventricular function in determining the individual fluid responsiveness, the majority of the studies failed to address this point. The present study is a fluid responsiveness analysis using different potential predictors in the setting of a population of patients undergone cardiac surgery, with respect to the quality of right ventricular function.
Materials and methods
The study was a multicenter trial conducted in 11 different Institutions. It was approved by ethical committees according to the local rules and all the patients gave a written informed consent. Each participating center was committed to guarantee not less than four and no more than 10 patients.
Patients
Eligible subjects were patients undergoing isolated, elective coronary artery bypass graft operations. Exclusion criteria were the need for preoperative inotropic support or intra-aortic balloon pumping, tricuspid regurgitation more than grade 1, atrial fibrillation or ventricular arrhythmias. All the patients received an hemodynamic monitoring with a modified pulmonary artery catheter CCO/CEDV (Edwards Lifesciences LLC, Irvine, CA) for continuous determination of cardiac output, RVEDV and right ventricular (RV) ejection fraction, and a transesophageal echocardiographic (TEE) monitoring. Hemodynamic measurements.
Hemodynamic measurements
The following hemodynamic parameters were measured: heart rate (beats/min); mean systemic arterial pressure (mmHg); MPAP (mmHg); RAP (mmHg); PAOP (mmHg); cardiac index (L/min/m2); stroke volume index (SVI, ml/m2); left ventricle (LV) ejection fraction; left ventricular end diastolic area index (LVEDAI, cm2/m2), measured using a transgastric midpapillary TEE view; right ventricular end diastolic index (RVEDVI, ml/m2); right ventricle (RV) ejection fraction; right ventricular end diastolic area index (RVEDAI), measured using a mid-esophageal TEE view; Δ aortic blood velocity (ABV, %); velocity time integral (VTI) ratio of E and A waves of transmitral flow; isovolemic relaxation time (IVRT, msec). ITBV (L) was a facultative measurement.
MPAP, RAP and PAOP were measured at end expiration. Cardiac index was continuously measured with a modified pulmonary artery catheter; SVI was assessed as cardiac index/heart rate. The modified pulmonary artery catheter is equipped with a rapid-response termistor, permitting measurement of RV ejection fraction providing that the system is interfaced to the heart rate signal. The RVEDVI is derived as SVI/RV ejection fraction. Values of cardiac index, SVI, RV ejection fraction and RVEDVI were assessed after 5 minutes of continuous monitoring, and data were considered reliable when stable after five consecutive points in time.
LV ejection fraction was estimated with bidimensional TEE and Simpson’s rules; Δ ABV was measured with a continuous doppler study of the aortic flow: the % change was calculated according to the equation: Δ ABV =2 x (Vpeak max - Vpeak min)/ (Vpeak max + Vpeak Min); a pulsatile doppler study of the transmitral flow was used to define the VTI of the E and A waves, and its ratio E/A VTI; IVRT was calculated as the time between the end of aortic flow and the beginning of mitral flow. ITBV was calculated in a subpopulation of patients using a single transpulmonary thermodilution technique.
Study protocol
The hemodynamic parameters have been measured before and after volume expansion (VE) in patients requiring, on a clinical basis, a fluid administration. The timing of VE could be:
a) intraoperatively, at the end of complete hemodynamic monitoring, with the patient being anesthetized, paralyzed, and under mechanical ventilation, with closed chest, and before the initiation of the surgical manouvers;
b) postoperatively, in the Intensive Care Unit (ICU), within the first 4 hours after the end of the operation, with the patient being anesthetized, paralyzed, and under mechanical ventilation.
In both cases the patients were ventilated at intermittent positive pressure, with zero end espiratory positive pressure, a tidal volume of 8-10 ml/kg, a respiratory rate of 10- 12 cycles/min, and a peak positive pressure <20 cm H2O. All the measurements have been done in supine position, with a zero pressure settled at the mid-axillary level. No therapeutic interventions were introduced or changed between the pre and post VE hemodynamic assessment, and the position of the patients was unchanged.
VE was represented by the intravenous infusion of 7 ml/kg of 6% hidroxyethylstarch (Voluven, Fresenius Kabi, Verona, Italy) over 20 minutes. Patients demonstrating an increase of SV >20% of baseline value after VE were defined as “responders”.
Patients grouping
The patients population was divided into two groups, according to the RV ejection fraction measured before VE. A cut-off value of 0.3 was chosen as the grouping determinant.
Statistical analysis
The effect of VE on hemodynamic parameters was assessed using a Student’s t test for paired data. Between groups differences have been assessed using a Student’s t test for unpaired data. Differences in baseline hemodynamic parameters between responders and non responders were assessed using a Student’s t test for unpaired data in each group. Hemodynamic parameters associated with the status of responder were further analyzed with a receiver operating characteristic (ROC) curve, in order to detect the area under the curve and the cut-off points with the respective sensitivity and specificity values. The analysis was conducted for the overall population and for the two groups. All data in tables are presented as mean ± standard deviation of the mean. A p value <0.05 was considered statistically significant.
Results
Sixty-five patients were enrolled in the 11 participating Institutions. 56 were males; the age was 64±8 years, the body surface area 1.8±0.15. Forty-eight patients received volume expansion intraoperatively, and 17 in the ICU. The hemodynamic profile of patients before and after VE was not significantly different between patients receiving VE intraoperatively or in the ICU, and the rate of responders was not significantly different.
Therefore, all the patients were included in the overall population, without distinction based on the timing of VE.
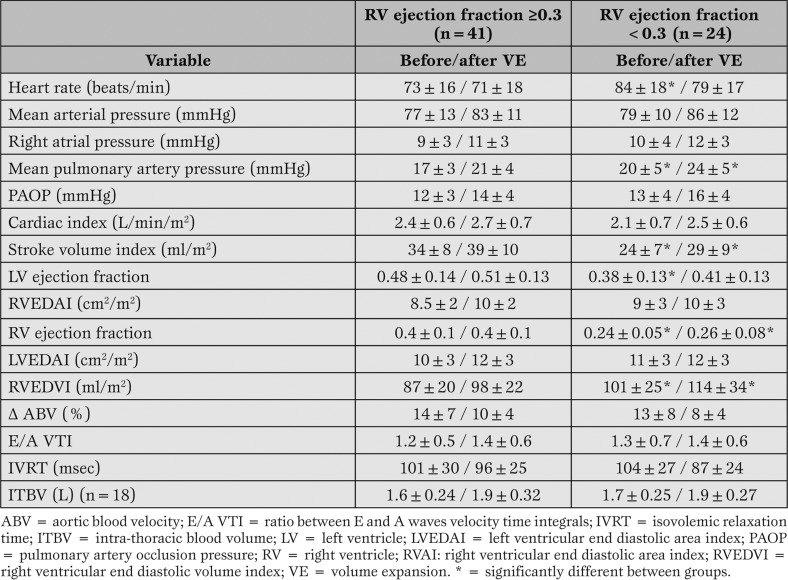
Forty-one patients had a RV ejection fraction ≥0.3, and 24 <0.3. On this basis, the two groups were created. The hemodynamic parameters before VE and the effects of VE in the two groups are reported in Table 1.
Table 1.
Effects of volume expansion on hemodynamic parameters in the two groups.
Significant differences between groups before VE were observed in heart rate, MPAP, SVI, RVEDVI and both LV and RV ejection fractions. These differences remained confirmed after VE, excepted heart rate. VE induces significant within groups differences in almost all the hemodynamic parameters (excepted RV ejection fraction and E/A VTI in both groups). After VE, 24 patients (14 in the group with RV ejection fraction ≥0.3 and 10 in the other) demonstrated a SV increase >20% and were considered as responders.
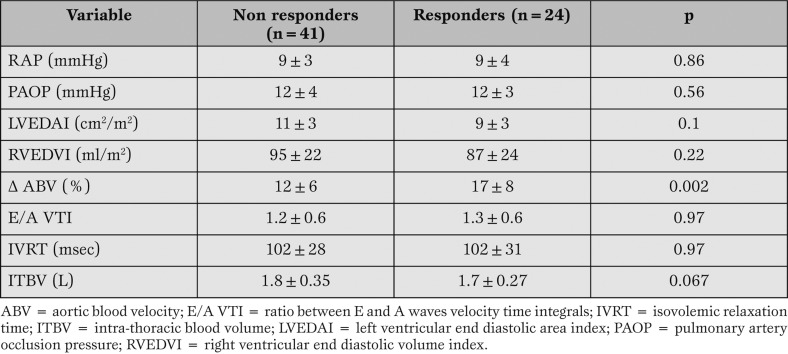
Differences between responders and non responders with respect to the possible predictors are reported in Table 2 for the overall population.
Table 2.
Hemodynamic variables before volume expansion and fluid responsiveness in the overall population.
Only the Δ ABV was significantly different between responders and non responders at the statistical analysis (Student’s t test for unpaired data). A ROC curve was used to define the area under the curve for Δ ABV as predictor of fluid responsiveness. The value was 0.71 (c.l. 95% 0.56-0.87); a cut-off value of 13% Δ ABV was identified, with a sensitivity of 70 % and a specificity of 72%.
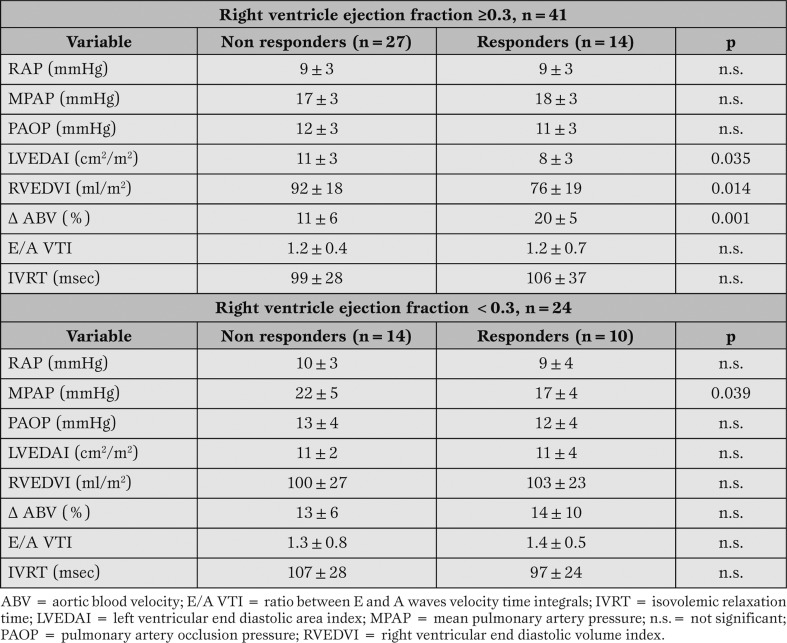
In Table 3 the differences between responders and non responders have been analyzed in the two groups. ITBV values have been omitted, due to the small amount of data in each class.
Table 3.
Hemodynamic variables before volume expansion and fluid responsiveness in the two groups.
<span style="font-size: x-small;">In the group of patients with a RV ejection fraction ≥0.3 we could identify three fluid responsiveness predictors: LVEDAI, RVEDVI, and ? ABV. In the group of patients with a RV ejection fraction <0.3, only the MPAP was significantly associated to fluid responsiveness.</span>
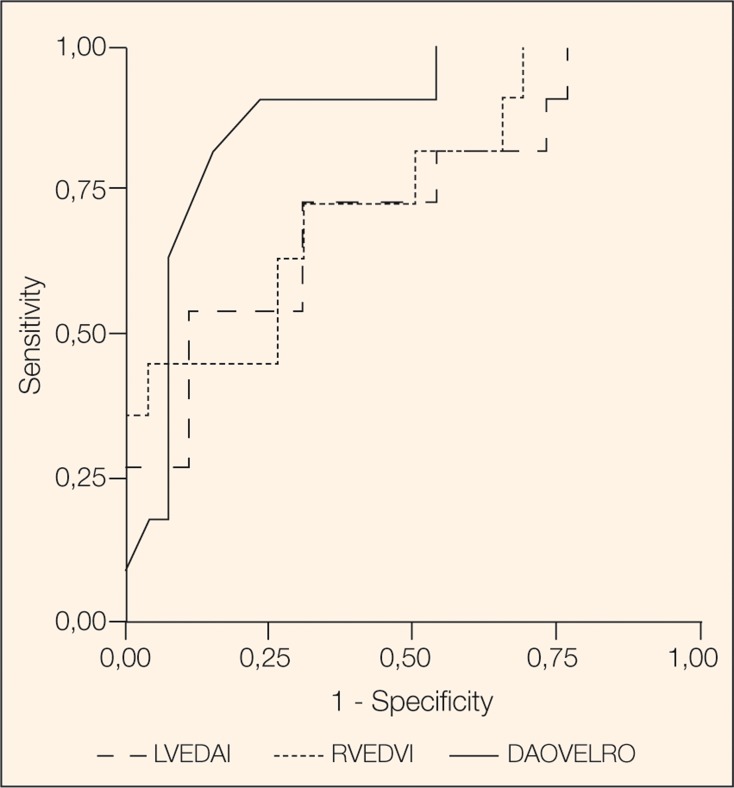
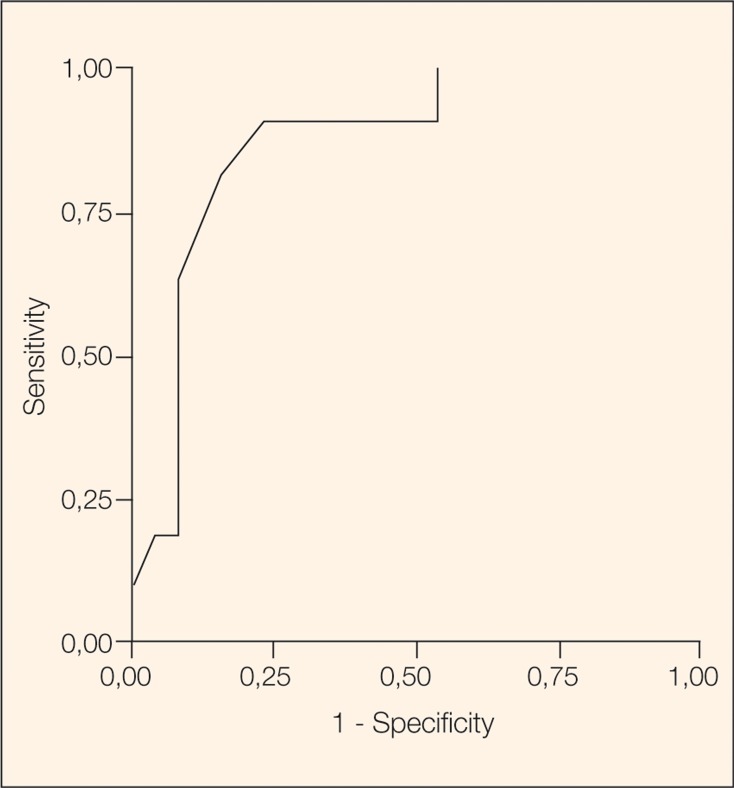
The variables being significantly different between responders and non responders in the two groups were analyzed with a receiver operating characteristic (ROC) curve analysis (Figure 1 and Figure 2) to detect the area under the curve and adequate cut-off values for sensitivity and specificity.
Figure 1.
Receiver Operating Characteristics (ROC) analysis for patients with a RV ejection fraction ≥ 0.3. Predictors: RVEDVI, Δ ABV, LVEDAI.
Figure 2.
Receiver Operating Characteristics (ROC) analysis for patients with a RV ejection fraction < 0.3. Predictor: MPAP.
The final model including the predictors of fluid responsiveness at different right ventricular ejection fractions is reported in Table 4.
Table 4.
Predictors for fluid responsiveness in the two groups.
Discussion
The experimental design of this study, which includes the measurement of many hemodynamic parameters, is time and expertise demanding; moreover, the need for enrolling an adequate number of patients for each class of right and left ejection fraction, induced us to establish a multicenter trial. As a result, the final patients population (n=65) is the wider series until now investigated in this kind of studies.<span style="font-size: x-small;">
Looking at the overall population, only the dynamic indicator ? ABV is a predictor of fluid responsiveness, with a cut-off value of 13%.</span> This indicator was already identified as a predictor for fluid responsiveness in a recent study [6] dealing with patients in septic shock, with a cut-off value of 12%. Our results seem to confirm this observation, and the role of dynamic preload indicators as major determinants of fluid responsiveness. We did not measure other similar dynamic variables (pulse pressure variations, systolic pressure varia-tions) but there is a general agreement about their high sensitivity and specificity [1,3,7]. The most relevant finding, in our study, is the failure of many traditional fluid responsiveness indicators whenever the RV ejection fraction is <0.3. There is little information in literature about RV function and fluid responsiveness; however, since the VE is exerted on the right side of the circulation, at least two right-sided factors should be considered: the venous bed compliance and the RV contractility. The fluids infused may become part of the venous blood (unrecruitable volume) or directly contribute to the right and left heart chambers filling (recruitable volume) depending on the right ventricular contractility.
We are aware that RV ejection fraction, being dependent on the afterload, is not a reliable measure of contractility, but can be considered, in clinical terms, an index of RV systolic function. Our results demonstrate that whenever the RV ejection fraction is severely depressed, the only predictor of fluid responsiveness is the MPAP, while the traditional left-sided indicators (LVEDAI and Δ ABV) loose clinical utility. In other terms, RV dysfunction splits the concept of fluid responsiveness from the concept of LV preload: even if the second is low, the patient could be fluid unresponsive [15]. This clinical condition is acutely present in right ventricular infarction, when the RV appears dilated and hypocontractile, the LV empty and hyperkinetic, and the dynamic indicators of preload are suggestive for an hypovolemic state.
Patients with a RV ejection fraction ≥0.3 demonstrated three predictors of fluid responsiveness: Δ ABV, RVEDVI, and LVEDAI. The cut-off forΔ ABV was settled at 15%, again consistently with the already available information.
RVEDVI is a significant predictor of fluid responsiveness with a cut-off value settled at 80 ml/m2. There is not a general agreement about the role of RVEDVI in predicting fluid responsiveness. Some concerns have been raised about the precision of the algorythm for RV ejection fraction assessment in case of tricuspid regurgitation [16]: thermodilution underestimates actual ejection fraction in a direct linear relationship to the degree of tricuspid valve regurgitation. Due to these concerns, tricuspid regurgitation was carefully estimated with the TEE color doppler, and no patient was enrolled in case of tricuspid regurgitation more than trivial during the experimental procedure.
Diebel and associates [13] found a correlation between fluid responders and RVEDVI, identifying a predictive cut-off value for being a responder of less than 90 ml/m2. Conversely, Wagner and Leatherman [2] failed to confirm the predictive role of RVEDVI in terms of fluid responsiveness. It should however be considered that the series of Wagner and Leatherman had a RV ejection fraction of 0.31±0.1, therefore including many patients below the critical level of 0.30, which in our study too excludes RVEDVI as a fluid responsiveness predictor. LVEDAI is confirmed as fluid responsiveness predictors in patients with acceptable RV function, with a cut-off of 9 cm2/m2 was identified. This value is consistent with the data from Tousignant and associates [5] and Swenson and associates [17].
We are aware of some limitations of our study: the grouping variable (RV ejection fraction) is not a direct index of RV contractility; the choice of a RV ejection fraction of 0.3 for splitting the groups is based on the clinical practice and that can be matter of discussion; finally the two groups are different not only with respect to the grouping variable, but even in terms of LV ejection fraction. This is almost inevitable, since RV dysfunction is often linked to LV dysfunction, and finding out a consistent group of “pure” RV dysfunction would require a cohort of patients larger than the one presently studied.
In a recent review article [7] Michard and Teboul emphasized the lack of value of ventricular preload indicators (RAP, PAOP, RVEDVI, LVEDAI) as predictors of fluid responsiveness in critically ill patients, while confirmed the importance of dynamic indices. In our series, we can confirm that in unselected patients the dynamic index (Δ ABV) is the most reliable predictor of fluid responsiveness.
However, expecially in cardiac surgical patients, the clinical scenario is more complex, involving the ability of RV to recruit the fluid volume (RV systolic function) and the ability of LV to accept that volume (LV diastolic function). On the basis of our data, we can conclude that whenever the RV systolic function is poor, no left-sided predictors of fluid responsiveness can be identified.
Acknowledgments
We are indebted to G. Isgrò, MR. Piccirillo, V. De Santis, F. Cislaghi, A. Cialfi, Al-Zubaidi Abubaker, C. Marusceac.
Footnotes
Source of support This study was supported, in part, by a Research Grant from Edwards LifeSciences Italy.
References
- Tavernier B, Makhotine O, Lebuffe G. et al. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313–1321. doi: 10.1097/00000542-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Right ventricular end-diastolic volume as a predictor of the hemodynamic response to a fluid challenge. Chest. 1998;113:1048–1054. doi: 10.1378/chest.113.4.1048. [DOI] [PubMed] [Google Scholar]
- Michard F, Boussat S, Chemla D. et al. Relation 29 between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- Michard F, Teboul JL. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care. 2000;4:282–289. doi: 10.1186/cc710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant CP, Walsh F, Mazer CD. The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg. 2000;90:351–355. doi: 10.1097/00000539-200002000-00021. [DOI] [PubMed] [Google Scholar]
- Feissel M, Michard F, Mangin I. et al. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119:867–883. doi: 10.1378/chest.119.3.867. [DOI] [PubMed] [Google Scholar]
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- Pizov R, Segal E, Kaplan L. et al. The use of systolic pressure variation in hemodynamic monitoring during deliberate hypotension in spine surgery. J Clin Anesth. 1990;2:96–100. doi: 10.1016/0952-8180(90)90061-7. [DOI] [PubMed] [Google Scholar]
- Di Corte CJ, Latham P, Greilich PE. et al. Esophageal doppler monitor determinations of cardiac output and preload during cardiac operations. Ann Thorac Surg. 2000;69:1782–1786. doi: 10.1016/s0003-4975(00)01129-2. [DOI] [PubMed] [Google Scholar]
- Burhe W, Buhre K, Kazmaier S. et al. Assessment of cardiac preload by indicator dilution and transoesophageal echocardiography. Eur J Anaesthesiol. 2001;18:662–667. doi: 10.1046/j.1365-2346.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- Berkenstadt H, Margalit N, Hadani M. et al. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg. 2001;92:984–989. doi: 10.1097/00000539-200104000-00034. [DOI] [PubMed] [Google Scholar]
- Lattik R, Couture P, Denault AY. et al. Mitral doppler indices are superior to two-dimensional echocardiographic and hemodynamic variables in predicting responsiveness of cardiac output to a rapid intravenous infusion of colloid. Anesth Analg. 2002;94:1092–1099. doi: 10.1097/00000539-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Diebel LN, Wilson RF, Tagett MG. et al. End-diastolic volume: a better indicator of preload in the critically ill. Arch. Surg. 1992;127:817–821. doi: 10.1001/archsurg.1992.01420070081015. [DOI] [PubMed] [Google Scholar]
- Wiesenack C, Prasser C, Keyl C. et al. Assessment of intrathoracic blood volume as an indicator of cardiac preload: single transpulmonary thermodilution technique versus assessment of pressure preload parameters derived from a pulmonary artery catheter. J Cardiothorac Vasc Anesth. 2001;15:584–588. doi: 10.1053/jcan.2001.26536. [DOI] [PubMed] [Google Scholar]
- Magder S. The cardiovascular management of the critically ill patients. In: Pinsky MR (ed) Applied Cardiovascular Physiology. Springer, Berlin Heidelberg New York. 1997:28–35.
- Spinale FG, Mukherjee R, Tanaka R. et al. The effects of valvular regurgitation on thermodilution ejection fraction measurements. Chest. 1992;101:723–731. doi: 10.1378/chest.101.3.723. [DOI] [PubMed] [Google Scholar]
- Swenson JD, Harkin C, Pace NL. et al. Transesophageal echocardiography: an objective tool in defining maximum ventricular response to intravenous fluid therapy. Anesth Analg. 1996;83:1149–1151. doi: 10.1097/00000539-199612000-00003. [DOI] [PubMed] [Google Scholar]