Abstract
Acute renal failure (ARF) is s a major complication after cardiac surgery and its prevalence still remains high. Even minor changes in serum creatinine are related to an increase morbidity and mortality.
Recently two consensus conferences have suggested new diagnostic criteria to define acute kidney injury and risk scores to better identify patients who will probably develop ARF after cardiac surgery. In fact a prompt recognition of high risk patients could allow a more aggressive therapy at a reversible stage of an incoming ARF. To date prophylactic strategies of renal function preservation during surgery include the avoidance of nephrotoxic insult and the prevention or correction of renal hypoperfusion. Although there are still no pharmacological agents able to prevent the perioperative ARF, several trials are investigating new pharmacological approaches.
When prophylactic strategies fail and severe ARF occurs, renal replacement therapy becomes mandatory. The timing and the kind of renal replacement therapy remain an open issue. Further randomized case-control studies with adequate statistical power are needed to have more conclusive data. Aim of this paper is to start from the acute renal injury physiopathology to analyze the most common prophylactic and pharmacological strategies.
Keywords: acute renal failure, acute kidney injury, renal replacement therapy, cardiac surgery
Acute renal failure (ARF) still remains a major complication after cardiac surgery. Even minor changes in serum creatinine are related to an increase morbidity and mortality. As recently shown by Chertow et al. [1], ARF “per se” is an independent determinant of mortality as much as cardiac arrest.
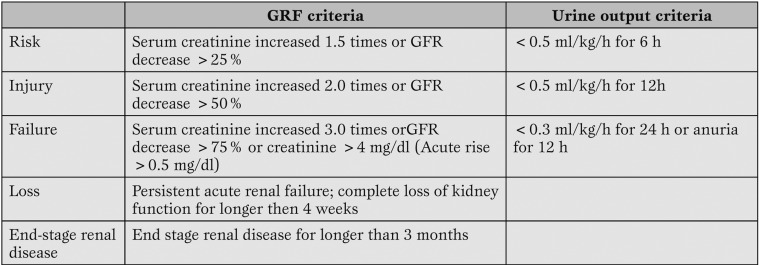
The true incidence of acute renal failure in patients undergoing cardiac surgery is still unknown because different authors have used different nomenclature to define ARF. To sort out this lack of uniformity in 2004 the Acute Dialysis Quality Initiative Group (ADQI) published the Rifle criteria [2]. RIFLE is the acronym of 3 severity grades (Risk, Injury and Failure) identified on the basis of the creatinine serum variation or urine output (the worst between them is considered), and 2 outcomes (Loss and End-Stage Kidney Disease) related to the length of loss of kidney function (Table 1).
Table 1.
The RIFLE scale, modified by Bellomo et al. (2).
The 3 classes of severity have a higher sensitivity than specificity, on the other hand the 2 categories related to the lengh of the kidney function loss have a low rate of false negative.
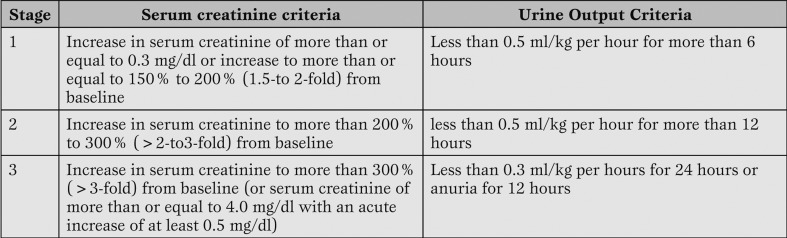
In September 2004 the ADQI group, the representatives of three nephrology societies, and the European Society of Intensive Care Medicine in Vicenza, Italy have introduced the concept of acute kidney injury (AKI) including the entire spectrum of ARF. In the same meeting they created the Acute Kidney Injury Network (AKIN) as independent multidisciplinary collaborative network formed by experts selected by the participating societies [3] (Table 2).
Table 2.
Classification/staging system for acute kidney injury (AKI). Modified from Metha et al. (3).
Acute renal injury physiopathology
Acute renal injury can result from:
As in the Rifle score, in the AKI system the main criteria to classify the renal failure stage are the creatinine serum variation and/or the urine output. Major advantages of the new staging system classification for acute kidney injury are:
1) a more flexible interim classification of the renal failure (a patient on RRT is classified as stage 3 regardless of the severity class at the time of the start of the RRT) and
2) a more accurate detection of the AKI.
Using the RIFLE criteria the incidence of AKI after cardiac surgery is considerably higher than previously reported incidence of ARF (15-20%) in 2 large studies [4, 5].
1) decreased renal perfusion without cellular injury;
2) ischemic, toxic, or obstructive insult of the renal tubule;
3) a tubule-interstitial process with inflammation and edema;
4) primary reduction of the glomerulus filtering capacity.
In terms of etiology the acute renal failure is pre-renal, renal, or post-renal, unfortunately, the critically ill patients recognize a combination of multiple factors and generally a worst outcome. Anyway we have to keep in mind that the AKI is not a “yes or no” phenomenon, but a continuum process which ranges from mild changes in markers of renal function, (i.e. creatinine and urea), until the complete renal failure.
In particular elderly patients are vulnerable to prerenal azotemia because of their predisposition to hypovolemia and high prevalence of renal-artery atherosclerotic disease. In this kind of patients, who are often affected by large-vessel or small-vessel renal vascular disease, the therapy itself plays an important role, in fact, a combination of angiotensin-converting-enzyme inhibitors and diuretics can worse the renal failure increasing the hypovolemia.
The low cardiac output syndrome can lead to kidney ischemia after cardiac surgery either decreasing the renal flow or changing the renal physiology.
During the CBP the non pulse blood flow, the macroembolic and microembolic insults to the kidney (organic and inorganic debris), the release of catecholamines and inflammatory mediators such as the free hemoglobin from traumatized red blood cells increase the renal vascular resistances and decrease the glomerular filtration rate of the 25-75% compare to the perioperative period.
Often the ischemic renal injury is reversible after the correction of the underlying causes, but if the ischemia is severe the cortical necrosis is irreversible.
However the kidney can restore its structure and function also after severe ischemia by the spreading and dedifferentiation of viable cells.
From the clinical point of view the early post cardiac surgery AKI is strongly associated with two major factors: reduced functional reserve and renal ischemia. Chertow has defined the renal ischemia occult, because asymptomatic, silent, unlike myocardial and cerebral ischemia. For these reasons the development of scores able to predict the ischemic AKI becomes mandatory [6].
Predictive scores
The Continuous Improvement in Cardiac Surgery Program (CICSP) score is a good predictor of AKI in a cardiac surgery population [6]. The CICSP risk-stratification algorithms has been developed and validated in 43 Department of Veterans Affairs medical centers between the 1987 and the 1994. It includes the following risk factors: low ejection fraction ≤35% (2 points); valvular surgery (3 points); chronic obstructive pulmonary disease (2 points); NYHA functional class IV (2 points); peripheral vascular disease (2 points); preoperative use of an intra-aortic balloon pump (5 points); prior heart surgery (3 points); pulmonary rale (2 points); systolic blood pressure ≥160 mmHg and CABG surgery (3 points); systolic blood pressure ≤120 mmHg and valvular surgery (2 points); and creatinine clearance 80 to 100 mL/min (2 points), 60 to 80 mL/min (3 points), 40 to 60 mL/min (5 points), ≤40 mL/min (9 points).
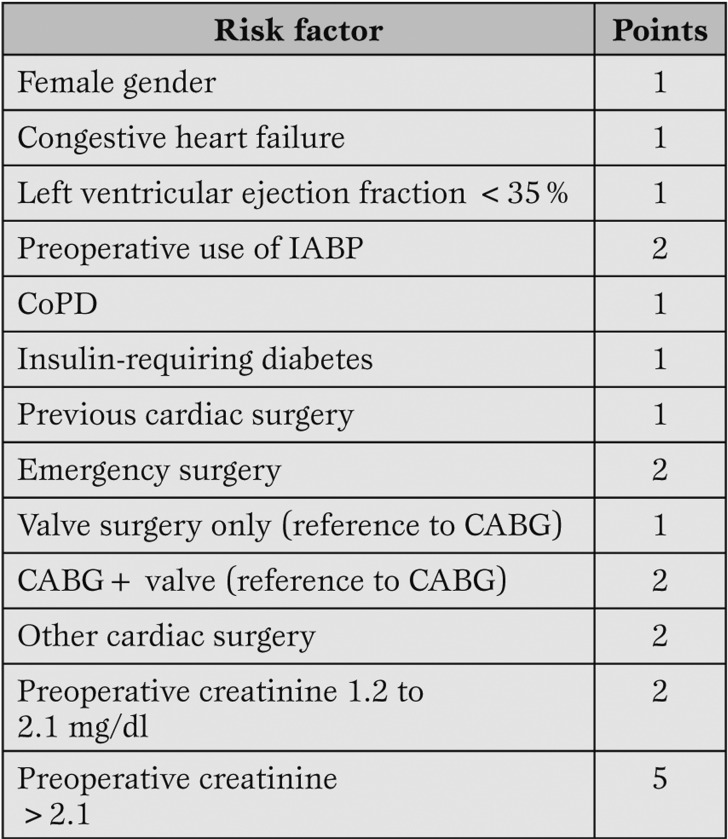
More recently Thakar et al have developed another clinical score to predict postoperative ARF “weighing” the effect of ARF’s major risk factors [7].
A total of 33,217 patients who underwent open-heart surgery at the Cleveland Clinic Foundation (1993 to 2002) have been studied.
The primary outcome was ARF requiring dialysis. The score was a a good predictor of ARF across all risk categories (Table 3).
Table 3.
The Thakar score (Minimum score 0; maximum score 17). Modified from Thakar et al. (7).
These results allowed to identify high-risk subgroups who could be involved in future randomized trials with the aim to identify strategies able to reduce the incidence of AKI after cardiac surgery.
Prophylactic strategies
The prophylactic strategies of the renal function preservation during the surgery traditionally emphasized two goals:
1) avoidance of nephrotoxic insult;
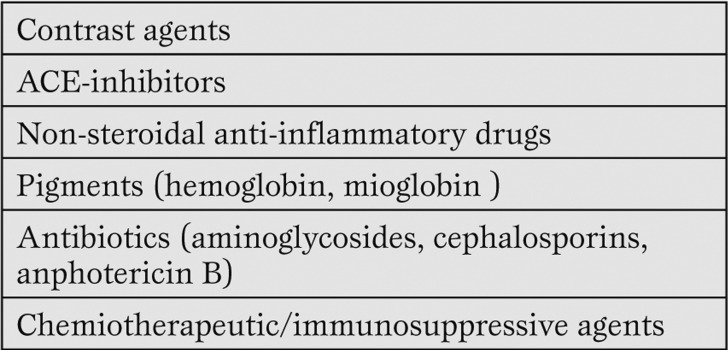
2) prevention or reversibility of the renal hypoperfusion supporting the renal perfusion pressure by the cardiac outcome, the arterial pressure and an adequate intra-vascular volume state [8]. Renal injury in critically ill patients is worsened by contrast agents and antibiotics among others (Table 4).
Table 4.
Nephrotoxins
Minimizing and avoiding the use of contrast agents perioperatively, especially in patients at high-risk for contrast nephropathy, helps to reduce renal injury. When possible, plasmatic nephrotoxic drugs concentrations must be measured daily.
The kidney performance is strictly bridged to the cardiac performance in fact the kidneys, although their combined weight is less than 1% of total body weigh, normally receive the 20-25% of the cardiac output (CO).
A low CO, during the surgery, decrease dramatically the renal perfusion pressure and activates a number of renal vasoconstrictor systems (sympathetic nervous system, renin angiotensyn system, and vasopressin secretion) which damage indirectly the kidneys. Marathias et al. have shown that preoperative intravenous hydration has decreased the risk of irreversible renal damage in patients with moderate-to-severe renal insufficiency undergoing cardiac surgery regardless of the kind of fluid adopted (crystalloids or colloids) [9]. Obviously, an excessive perioperative fluid load should not be administrated in order to avoid several complications such as pulmonary oedema.
Beyond an adequate CO and an intravascular volume status an optimal arterial pressure is mandatory to ensuring an adequate renal perfusion pressure. In the normal mammalian kidney, loss of autoregulation of RBF generally occurs at a mean arterial pressure (MAP) of 75-80 mmHg. There are no absolute MAP values to sustain the renal perfusion pressure in fact, a MAP of 65 mmHg could be inadequate for renal resuscitation in elderly or diabetics patients, while it could be high enough for patients without co-morbidities. A blood pressure of 60 mmHg is likely to be inadequate in every patient [10].
When the volume expansion is not sufficient to achieve these goals in ICU patients, the vasoactive drugs (many of which have inotropic and vasopressor properties) could be used. Anyway, to our knowledge no randomized controlled studies have investigated if the perfusion pressure affects the renal outcome.
Recently Di Giantomasso et al. [11] in a study on animals showed that 0.4 mcg/kg/minute of norepinephrine (NE) in the normal mammalian circulation increased renal blood flow, urine output and creatinine clearance. Notably the effect of NE on the renal function was safe in patients with post-bypass hypotensive vasodilatation [12]. Although further studies are needed to evaluate the effect of NE, in subject with normal cardiac function or septic shock there are no reasons to avoid NE administration in patient with poor renal function. To date, NE remains the vasopressor of choice in hypotensive states with preserved or increased cardiac output for its efficacy in restoring the MAP.
Pharmacological strategies
Diuretics
The administration of loop diuretics in ARF patients is common practice in ICUs.
Loop diuretics:
1) improve the tubular flow and the hydraulic pressure;
2) increase the production of vasodilating prostaglandins increasing the cyclo-oxygenases’s activity;
3) reduce the efficacy of the Na-K-2Cl co-transporter (NKCC2) decreasing the sodium transport and the O2 consumption;
4) preserve the vulnerable medullary tubulus segments from the ischaemic damage.
Mehta et al. showed that the use of diuretics in critically ill patients with ARF is associated with an increased risk of death and non recovery of renal function [13]. The following consideration should be done:
1) the use of diuretics by converting an oliguric in a nonoliguric form could delay the recognition of ARF, the severity of the ARF and the institution of dialysis;
2) the successful conversion of oliguria to diuresis does not mean a milder form of ARF;
3) the diuretics have no impact on the patient outcome.
In a double-blind randomized controlled trial continuous infusion of furosemide has been associated with the highest rate of renal impairment in cardiac surgery patients [14]. The same authors have suggested the use of mannitol to protect the renal function. In fact the mannitol:
1) induces an osmotic diuresis which prevents the tubular obstruction;
2) decrease the epithelial and endothelial cell swelling limiting the vascular congestion and tubular obstruction;
3) is a free radicals scavenger;
4) increases the synthesis of intra-renal prostaglandin generating an efficacious renal vasodilation. Sides effects are volume depletion, and an increased medullary consumption of O2. Despite of these features, a small prospective randomized clinical trial in cardiac surgery patients without previous renal impairment failed to show any significant differences in renal outcome when mannitol was administrated with prophylaxis purpose [15].
Steroids
In a randomized clinical trial, anti-inflammatory agents such as dexamethasone, administrated before CPB, showed no protective effect on perioperative renal dysfunction in low-risk cardiac surgical patients [16].
Dopamine receptors agonists
When infused in so-called “renal doses,” between 0.5 to 2 μg/kg body weight/minute, dopamine increases renal plasma flow, GFR, and sodium excretion. In a double-blind, randomized, controlled trial, 126 patients with preoperatively normal renal function undergoing elective cardiac surgery received a continuous infusion “renal dose” dopamine (2 μg/kg/minute), furosemide (0.5 mg/kg/minute), or isotonic sodium chloride as placebo, at the beginning of surgery for 48 hours or until the discharge from the intensive care unit [14]. The continuous infusion of dopamine was ineffective for renal protection and not superior to isotonic saline in preventing postoperative dysfunction. Also a larger randomized trial in early ARF has failed to show any benefit of the dopamine in preventing renal injury, renal replacement therapy, or death [17].
Fenoldopam stimulates dopamine 1 (and not dopamine 2) receptors, thus inducing, theoretically, a greater vasodilation in the renal medulla than in the cortex. Furthermore, fenoldopam has no alpha or beta adrenergic activity and as evidenced by Aravidan et al., in a rat model, fenoldopam is able to reduce the ischemic-reperfusion injury-induced inflammation involving NF-kB pathway [18]. In patients at high risk of postoperative acute renal failure undergoing cardiac surgery with cardiopulmonary bypass, fenoldopam prophylaxis was an independent protective factor for postoperative renal failure within the subgroup of patients who suffered a postoperative low output syndrome [19].
On the other hand Bove et al. in a prospective single-center, randomized, double-blind trial in a cardiac surgery setting evidenced no significant difference in peak postoperative serum creatinine level, need of renal replacement therapy and intensive care unit, hospital stay, and mortality between fenoldopam and placebo group [20]. Recently two interesting meta-analysis showed fenoldopam efficacy in preventing renal damage [21, 22] in critically ill patients or in those undergoing major surgery. Anyway further large multicentric randomized clinical trials are needed to justify a widespread use of fenoldopam to avoid renal impairment.
Radical scavengers
Because cardiopulmonary bypass and cardioplegic arrest are associated with formation of free radicals, which damage various organs particullary the kidneys, radical scavengers were hypothesized to protect the renal function.
Oxidative stress could be attenuated by N-acetylcysteine (NAC), which directly scavenges reactive oxygen species, regenerates the glutathione pool, and reduces oxidative stress during CPB. Nevertheless in a phase II, randomized, controlled trial, Haase et al. have shown that N-acetylcysteine has been no more effective than placebo in attenuating cardiopulmonary bypass-related acute renal failure in high-risk cardiac surgery patients [23].
Arteriolar vasodilator
Natriuretic peptides showed to cause afferent arteriolar vasodilation and efferent arteriolar vasoconstriction, thereby increasing GFR. They also block tubular re-absorption of sodium chloride, re-distribute renal medullary blood flow, disrupt TGF and reverse endothelin-induced vasoconstriction. In the NAPA trial, a prospective double-blind clinical trial, the administration of nesiritide, a recombinant human B-type natriuretic peptide, in patients undergoing CABG with CPB was associated with a better renal function and survival outcome [24]. Sackner-Berneistein et al. in a recent metanalysis on the use of the nesiritide in acutely decompensated heart failure (ADHF) showed that nesiritide significantly increases the risk of renal function impairment although the worsening renal function could reflect only hemodynamic effects and no a true renal injury [25].
Calcium channel blockers cause afferent arteriole vasodilation and natriuresis. They also reduce intracellular calcium influx and act as a free radical scavenger. In a placebo-controlled study led in patients undergoing CABG, diltiazem has increased urine output and creatinine clearances [26]. However two more recent placebo controlled clinical trials showed no effect on renal function [27, 28].
Prostaglandins are involved in the afferent arteriole vasodilatation such as the inhibition of pro-inflammatory cytockines during cardiac surgery. A pilot study indicated that low-dose of prostacyclin could preserve the renal function in high-risk patients after coronary bypass surgery [29].
On the hypothesis that the adenosine receptors are involved in modulating intrarenal haemodynamics during ischaemia theophylline was studied with a double-blind, placebo-controlled trial in patients with normal preoperative renal function who underwent elective CABG [30]. No difference in the incidence of acute renal failure has been found between cases and controls.
Clonidine has been associated with a higher creatinine clearance in two randomized double blind trial conducted by Myles et al. and Kulka et al. [31, 32].
Optimization of renal function prior to the surgery could reduce the risk of perioperative ARF.
Durmaz et al. have performed an interesting study to assess whether correcting fluid and electrolyte abnormalities with dialysis in patients with chronic renal insufficiency prior to surgery would have reduced the incidence of post-operative ARF and mortality [33].
The study showed a significant low incidence of acute renal damage, death and a shorter length of ICU and hospital stay between the patients who received hemodialysis twice within 72 hours before the surgery and who not. However the results can not be conclusive because of a small size of patients involved into the study.
Renal replacement therapy
There is no agreement on the timing and the kind of RRT in AKI. Bellomo et al. have suggested these criteria to start the RRT [34]:
1) anuria or oliguria (urine output <200 ml/12 h);
2) hyperkalemia (k >6.5 mmol/l);
3) severe acidemia (pH <7.1);
4) azotemia (urea >30 mmol/l);
5) clinically significant organ edema (particularly lung);
6) uremic encephalopathy, pericarditis, or neuropathy/myopathy;
7) severe dysnatremia (Na >160 or <115 mmol/l);
8) hyperthermia;
9) drug overdose with a dialyzable product.
About the kind of RRT to use in ARF there are no conclusive studies. In fact, although the intermittent hemodialysis (IHD) remains the most common treatment, continuous renal replacement therapies (CRRT) and slow, low-efficiency daily dialysis (SLEDD) are becoming widely used. Each technique carries its own set of advantages and disadvantages.
Conclusions
In conclusion AKI is one of the most serious complications of cardiac surgery associated with increased morbidity and mortality. Ischemic injury of the kidney, exotoxins (antibiotics, anesthetic agent, contrast media, diuretics), endotoxins (myoglobin), and preexisting renal impairment are risk factors associated to acute postoperative renal failure.
Maintenance of adequate intravascular volume perioperatively, optimization of pre-operative renal function, and the avoidance of nephrotoxic medications are currently the keys to prevent perioperative AKI. Several studies on the use of pharmacological agents have failed to show any effect to prevent the perioperative AKI. Further randomized case-control studies with adequate statistical power are needed to have more conclusive data.
Footnotes
Conflict of interest No conflict of interest acknowledged by the authors.
References
- Chertow G M, Levy E M, Hammermeister K E. et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Ronco C, Kellum J A. et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R L, Kellum J A, Shah S V. et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuitunen A, Vento A, Suojaranta-Ylinen R. et al. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–546. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Heringlake M, Knappe M, Vargas Hein O. et al. Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol. 2006;72:645–654. [PubMed] [Google Scholar]
- Chertow G M, Lazarus J M, Christiansen C L. et al. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- Thakar C V, Arrigain S, Worley S. et al. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- Tang I Y, Murray P T. Prevention of perioperative acute renal failure: what works? Best Practice & Research Clinical Anaesthesiology. 2004;18:91–111. doi: 10.1016/j.bpa.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Marathias K P, Vassili M, Robola A. et al. Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery. Artificial Organs. 2006;30:615–621. doi: 10.1111/j.1525-1594.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Lee R W C, Di Giantomasso D, Bellomo R. et al. Vasoactive drugs and the kidney. Best Practice & Research Clinical Anaesthesiology. 2004;18:53–74. doi: 10.1016/j.bpa.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Di Giantomasso D, May C N, Bellomo R. Norepinephrine and vital organ blood flow. Intensive Care Medicine. 2008;28:1804–1809. doi: 10.1007/s00134-002-1444-x. [DOI] [PubMed] [Google Scholar]
- Morimatsu M, Uchino S, Chung J. et al. Norepinephrine for severe vasodilatation after cardiac surgery: impact on renal function. Intensive Care Medicine. 2003;29:1106–1112. doi: 10.1007/s00134-003-1810-3. [DOI] [PubMed] [Google Scholar]
- Mehta R L, Chertow G M. Diuretics in critically ill patients with acute renal failure. JAMA. 2003;289:1379–1381. doi: 10.1001/jama.289.11.1379-c. [DOI] [PubMed] [Google Scholar]
- Lassnigg A, Donner E, Grubhofer G. et al. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;1:97–104. doi: 10.1681/ASN.V11197. [DOI] [PubMed] [Google Scholar]
- Ip -Yam P C, Murphy S, Baines M. et al. Renal function and proteinuria after cardiopulmonary bypass: the effects of temperature and mannitol. Anesth Analg. 1994;78:842–847. doi: 10.1213/00000539-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Loef B G, Henning R H, Baines M. et al. Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass. Br J Anaest. 2004;93:793–798. doi: 10.1093/bja/aeh266. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Chapman M, Finfer S. et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–2143. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- Aravindan N, Natarajan M, Shaw A D. Fenoldopam inhibits nuclear translocation of nuclear factor kappa B in a rat model of surgical ischemic acute renal failure. JCTVA. 2006;20:179–186. doi: 10.1053/j.jvca.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Ranucci M, Soro G, Barzaghi N. et al. Fenoldopam prophylaxis of postoperative acute renal failure in high-risk cardiac surgery patients. Ann Thorac Surg. 2004;78:1332–1338. doi: 10.1016/j.athoracsur.2004.02.065. [DOI] [PubMed] [Google Scholar]
- Bove T, Landoni G, Calabrò M G. et al. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery. A prospective double blind, randomized clinical trial. Circulation. 2005;111:3230–3235. doi: 10.1161/CIRCULATIONAHA.104.509141. [DOI] [PubMed] [Google Scholar]
- Landoni G, Biondi-Zoccai G G, Tumlin J A. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007;49:56–58. doi: 10.1053/j.ajkd.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Landoni G, Biondi-Zoccai G G, Marino G. et al. Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2008;22:27–33. doi: 10.1053/j.jvca.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Haase M, Haase-Fielitz A, Bagshaw S M. et al. Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med. 2007;35:1–8. doi: 10.1097/01.CCM.0000261887.69976.12. [DOI] [PubMed] [Google Scholar]
- Mentzer R M, Oz M C, Sladen R N. et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery. JACC. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Sackner-Bernstein J D, Skopicki H A, Aaronson K D. et al. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- Amano J, Suzuki A, Sunamori M. et al. Effect of calcium antagonist diltiazem on renal function in open heart surgery. Chest. 1995;107:1260–1265. doi: 10.1378/chest.107.5.1260. [DOI] [PubMed] [Google Scholar]
- Bergman A S F, Odar-Cederlof I, Westman L. et al. Diltiazem infusion for renal protection in cardiac surgical patients with preexisting renal dysfunction. JCTVA. 2002;16:294–299. doi: 10.1053/jcan.2002.124136. [DOI] [PubMed] [Google Scholar]
- Diltiazem may preserve renal tubular integrity after cardiac surgery. Can J Anesth. 2003;30:107–112. doi: 10.1007/BF03017799. [DOI] [PubMed] [Google Scholar]
- Morgera S, Woydt R, Kern H. et al. Low-dose prostacyclin preserves renal function in high-risk patients after coronary bypass surgery. Crit Care Med. 2002;30:107–112. doi: 10.1097/00003246-200201000-00017. [DOI] [PubMed] [Google Scholar]
- Kramer B K, Preuner J, Ebenburger A. et al. Lack of renoprotective effect of theophylline during aorto-coronary bypass surgery. Nephrol Dial Transplant. 2007;17:107–112. doi: 10.1093/ndt/17.5.910. [DOI] [PubMed] [Google Scholar]
- Myles P S, Hunt J O, Holdgaard H O. et al. Clonidine and cardiac surgery:haemodinamic and metabolic effects, myocardial ischaemia and recovery. Anaesth Intensive Care. 1999;27:137–147. doi: 10.1177/0310057X9902700202. [DOI] [PubMed] [Google Scholar]
- Kulka P J, Tryba M, Zenz M. Preoperative alpha sub 2 adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery:results of a randomized, controlled trial. Crit Care Med. 1996;24:947–952. doi: 10.1097/00003246-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Durmaz I, Yagdi T, Calkaur T. et al. Prophylactic dialysis in patients with renal dysfunction undergoing onpump coronmary artery bypass surgery. Annals of Thoracic Surgery. 2003;75:859–864. doi: 10.1016/s0003-4975(02)04635-0. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Ronco C. Indications and criteria for initiating renal replacement therapy in the intensive care unit. Kidney International. 1998;66:106–110. [PubMed] [Google Scholar]