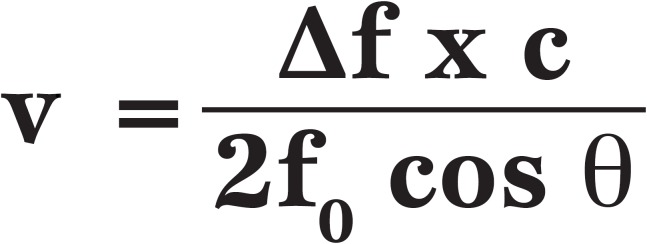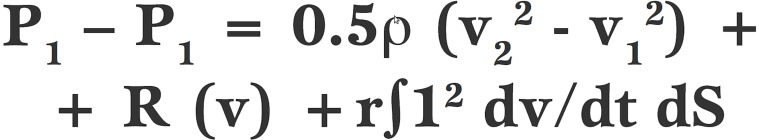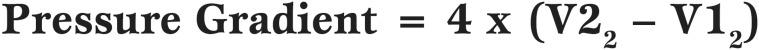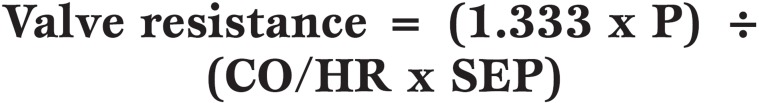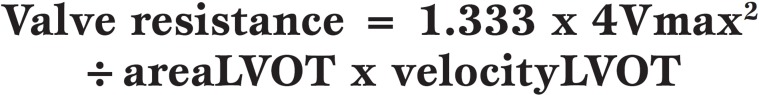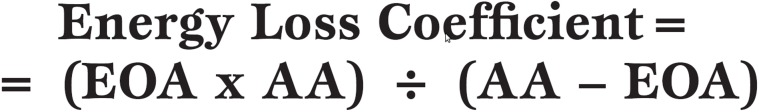Abstract
Intraoperative transesophageal echocardiography is now used routinely during aortic valve replacement, allowing immediate evaluation of replaced or repaired valves. It is well recognised that high transvalvular pressure gradients can be detected immediately after implantation of a prosthetic aortic valve which may be due to multifactorial confounding variables, including functional phenomena, pressure recovery and prosthesis-patient mismatch. This review article explores the variety of methods available for assessing prosthetic aortic valve function and considers causative factors which may contribute to high transvalvular gradients in an attempt to determine whether a physical problem with the valve exists, or whether the valve is functioning normally.
Keywords: transesophageal echocardiography, cardiac surgery, aortic valve replacement, prosthetic aortic valve
Introduction
Intraoperative transesophageal echocardiography (TEE) is now used routinely during aortic valve replacement (AVR). This allows immediate evaluation of a replaced or repaired valve and may give vital information on surgical and non-surgical complications. Any abnormalities detected may require immediate surgical re-intervention. It is well recognised that high transvalvular pressure gradients can be detected immediately after implantation of a prosthetic aortic valve (AV) which may be due to multifactorial confounding variables, including functional phenomena, pressure recovery and prosthesis-patient mismatch. This review article aims to explore in greater depth the variety of methods available for assessing and evaluating prosthetic AV function.
We then consider the causative factors which may contribute to high transvalvular gradients immediately after AVR in an attempt to determine whether a physical problem with the valve exists, or whether the valve is functioning normally.
Value of intraoperative TEE in aortic valve surgery
The routine use of intraoperative TEE in patients undergoing valve replacement for aortic stenosis (AS) has been validated in several studies. A retrospective study of 383 patients with severe AS undergoing AVR showed the impact of intraoperative TEE [1]. In six patients a mitral valve replacement or repair was performed on the evidence of the intraoperative examination, although not originally planned. In 25 patients the mitral procedure was cancelled because of the intraoperative findings, while the surgical plan was changed in another 18 patients.
The clinical impact and cost-saving implications of routine intraoperative TEE during valve replacement operations have been confirmed in a prospective study [2]. Other authors also highly recommend postbypass intraoperative TEE as an integral diagnostic modality contributing valuable data in valve replacement surgery [3, 4]. TEE can confirm successful de-airing after any open-heart procedure [5]. Even the smallest air bubble can cause severe postoperative instability if it enters one of the coronary arteries.
Quantifying the severity of disease is usually not necessary in the patient whose diagnosis is well established preoperatively, but is included in the comprehensive examination.
This provides an up-to-date baseline for future reference and adds information regarding any acute disease progression. It must be remembered that the systemic vascular resistance (SVR) has an influence on flow across the stenotic valve and intraoperative hypotension may therefore increase the transvalvular pressure drop in the anaesthetised patient.
Likewise, systemic hypertension in the patient with AS may lead to a decrease in LV output and thus a reduction in the transvalvular pressure gradient [6,7,8]. To complicate matters, in the anesthetised, offloaded patient there is decreased venous return, with a subsequent decrease in LV preload and stroke volume. This will also affect the flow velocity and pressure drop measured across a diseased valve.
Methods of assessment of aortic prosthetic valve function: 2D Assessment
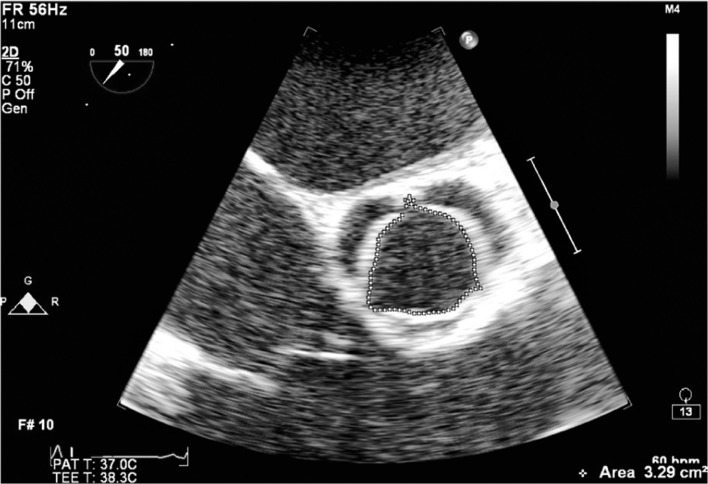
The general principles for evaluating prosthetic valve function are similar to those of native valve stenosis [9]. Two-dimensional views of an AV prosthesis after separation from bypass are helpful in assessing the valve but in reality are often limited by the associated echo drop-out and shadowing caused by oedema, hematoma and metallic scattering of the ultrasound beam. The easiest replacement valves to assess with 2D echo are the stentless aortic bioprosthesis and the homograft, which typically have a long leaflet coaptation line in the mid-esophageal AV long axis view (ME AV LAX). There are no metal components in the stentless valve or homograft. In the mid-esophageal AV short axis view (ME AV SAX), the three leaflets can usually be seen opening and an assessment of aortic valve area (AVA) by planimetry is possible, but should not be relied upon as a single method of evaluation [10] The stentless AV prosthesis allows superior hemodynamics to a stented or mechanical prosthesis [11] (Figures 1a and 1b).
Figure 1a.
Planimetry of a normal aortic valve area in the ME AV SAX view. The AVA in this patient is 3.3 cm2 at the leaflet tips.
Figure 1b.
Planimetry of a stenotic aortic valve area in the ME AV SAX view. The AVA in this patient is 0.5 cm2 at the leaflet tips. When severely calcified it is impossible to use this technique reliably.
In a stented bioprosthesis visualisation of the three leaflets opening can be difficult depending on the degree of artefact from the metallic stent. Likewise, a metallic bileaflet prosthesis causes echo dropout at the level of and below the valve.
However, with most prostheses it is usually possible to see whether there is any movement or rocking of the valve, or if it is well-seated throughout the cardiac cycle. Echo-dropout and shadowing can be minimised when the AV is viewed from the deep transgastric (TG) view, which is often also the best view for interrogation of Doppler velocities across the valve. Bileaflet metallic valves are particularly well seen in this view, enabling detection of both leaflets moving from approximately 25 degrees in the fully closed position to approximately 80 degrees in the fully open position. If one or both leaflets are dysfunctional this can often be noted in the 2D view and is usually associated with a high pressure gradient (PG) across the prosthesis. Separating from bypass in this scenario would more than likely prove difficult.
When visualization of the leaflets is difficult and high gradients are obtained, a thorough search for prosthesis dysfunction must occur if grave perioperative morbidity is to be avoided. Three-dimensional (3D) TEE is becoming more commonplace but is still in its infancy regarding assessment of valve pathology [12]. Postoperatively it also provides excellent views of both bioprosthetic and mechanical valves. The relatively improved spatial and temporal resolution of the matrix array transesophageal transducer provides 3D views of unparalleled quality at acceptable frame rates. It is expected that a combined 2D and 3D TEE examination will become routine for surgical planning and guidance.
Transvalvular velocity
Continuous wave Doppler (CWD) is used to measure flow velocity across the valve. To make an accurate measurement it is very important that the ultrasound wave is parallel to the direction of blood flow. The Doppler equation includes a crossing angle, Θ (theta) which can be used to correct for non-parallel flow.
Equation 1.
The Doppler equation, where v = velocity directed towards the transducer from the scatterer, ?f = the Doppler shift, f0 = the transmitted frequency, cos θ = cosine of the crossing angle theta and c = the speed of propagation in the medium.
When the angle Θ is zero, the cosine of Θ is 1, hence no correction is needed. When Θ is less than 20°, the error is negligible (less than 6%) so most echocardiographers will ignore a slightly off-angle measurement. Non-parallel flow in relation to the Doppler beam will underestimate velocity, hence in search for an accurate measurement, parallel Doppler beam alignment is vital.
Although it is more difficult to align the ultrasound beam correctly with TEE than with transthoracic echocardiography (TTE), this angle can usually be obtained in the transgastric long axis (TG LAX) and deep TG views.
The color Doppler sector is useful to guide the echocardiographer towards the outflow tract and AV. A meticulous search for the maximal aortic flow velocity signal is essential. The maximum velocity will vary with cardiac output (CO), as discussed later. ‘Normal’ measurements associated with prosthetic valves show a wide range of inter-patient variability and it is important to interpret measurements in the light of the information supplied by the manufacturer for the specific type and size of valve [13].
Transvalvular ‘pressure gradient’
There is excellent anatomical and physiological correlation between echocardiography and cardiac catheterisation findings [14]. The ‘peak-to-peak’, ’peak’ and ‘mean’ gradients can be reported from catheterisation data. It is important to distinguish between the maximum instantaneous peak PG (or pressure drop) obtained with echocardiography and the peak-to-peak gradient obtained in the catheter laboratory.
The peak-to-peak gradient is obtained by measuring the difference between peak LV pressure and peak aortic pressure with a pressure transducer at different times in the cardiac cycle.
The maximum instantaneous echo PG is higher than the peak-to-peak gradient. It has been shown that the best correlation is between the mean Doppler gradient and the mean cardiac catheter gradient measured simultaneously [15].
When transvalvular velocity has been measured, the Bernoulli equation is used to convert velocity into a PG.
The Bernoulli equation in its full form has been shown to have many more assumptions than just those referred to in the equation below. It is also assumed that the diameter of the left ventricular outflow tract (LVOT) proximal to the AV constriction is of the same diameter as the ascending aorta, that the inflow shape in AS has a flat, orifice-like shape as opposed to a funnel-shape and that flow through the constriction is laminar not turbulent [16, 17].
Equation 2.
In its original form, the Bernoulli equation has three parts which consider (i) convective acceleration where ρ (rho) refers to blood density, (ii) viscous friction and (iii) the rate of change of flow acceleration.
In the first part of the Bernoulli equation, blood density (ρ) multiplied by 0.5 is 3.98 but is rounded up to 4. The second and third parts of the original equation are then assumed to be constant, hence the modified Bernoulli equation is created:
Equation 3.
The modified Bernoulli equation, where V2 is the maximal veocity across the aortic valve and V1 is the maximal velocity across the LVOT
V1 in the Bernoulli equation refers to the velocity upstream from the constriction (ie the velocity in the LVOT when being applied to AV velocities). If the LVOT velocity is less than 1 m/sec then the value for V1 is assumed to be negligible and also ignored. This allows the simplified Bernoulli equation:
Equation 4.
The simplified Bernoulli equation, where V is the maximal velocity across the aortic valve.
Severe AS in a native valve is defined as a flow velocity more than 4.5 m/sec, a mean PG more than 50 mmHg and a peak PG more than 80 mmHg. However in a newly implanted prosthetic aortic valve, a peak velocity of greater than 3.5 m/sec or peak PG of 50 mmHg and mean PG of 30 mmHg would be considered a significantly high gradient. The mean PG is obtained by accurately tracing the outline of the Doppler spectral display during systole when CWD is placed across the AV prosthesis. The mean gradient is more appropriate in reflecting the severity of post-implantation PG as many factors can alter the peak velocity (see later). It is important to include both the heart rate and rhythm when reporting valve gradients, as these will affect CO and flow [9].
In the absence of any detectable functional abnormalities, peak and mean PG across an AV prosthesis should be repeated when the patient is hemodynamically stable and ventricular filling is optimized. A decrease in inotropic drugs is also necessary before assuming that high gradients are pathological in nature [18].
Aortic valve area (AVA)
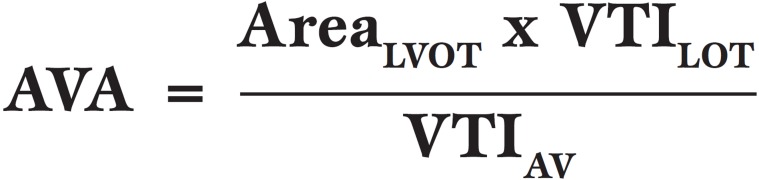
Planimetry has already been mentioned above. In the native AV the continuity equation is most commonly used to calculate AVA and is also used in prosthetic valves. It is based on the conservation of mass and states that blood flow [area x velocity time integral (VTI)] through sequential areas of a continuous, intact system must be equal. Therefore blood flow through the LVOT (AreaLVOT x VTILVOT) must equal blood flow across the AV (AreaAV x VTIAV). Therefore:
AVA.
Aortic valve area (AVA)
Pulsed wave Doppler (PWD) is used to measure the lower flow velocity across the LVOT, while CWD will be necessary to measure the much higher flow velocities across a stenotic AV [19]. The AreaLVOT is obtained by measuring its diameter in the ME AV LAX view during systole, and applying the formula for the area of a circle (πr2 or 0.785 x diameter2).
Measurement of the LVOT diameter can be difficult immediately post-AVR due to poor image quality. The prosthetic valve size can be used in place of the LVOT diameter, but in the knowledge that it may yield a higher value for the effective orifice area (EOA) [20]. Another pitfall of the continuity equation is when the ultrasound beam is not parallel to blood flow and the Doppler measurements will therefore be inaccurate [21]. One of the most common causes of misinterpretation of echocardiography findings in this condition is when a mitral regurgitation (MR) jet is present. When attempting examination of the AV in the TG views, the CWD beam may accidentally cross the MR flow. The orientation and high velocity of a possible MR jet will be similar to that of a high velocity across the AV and produce an erroneous AVA. If a patient is not in sinus rhythm the AV and LVOT flow velocities will vary with each cardiac cycle. Multiple measurements should then be made to obtain an average. Small errors in measurement will result in large errors in calculated values.
Doppler-derived assessments
Doppler-derived values have been shown to provide reliable estimation of the degree of AS compared to catheter-derived values [22]. The following three methods are similar in principle and use the continuity equation to find the ratio of LVOT to AV velocity. The Dimensionless Velocity Index (DVI) has been validated as a method of assessment of the degree of obstruction in St Jude Medical valves placed in the aortic position [23]. The DVI is the ratio of the peak velocity in the LVOT to the peak velocity across the AV. Severe stenosis is suggested by a value of 0.25 or less.
The Dimensionless Severity Index (DSI) is similar in that it uses the ratio of the VTI through the LVOT to the VTI through the AV. PWD is placed in the LVOT and the resultant waveform is traced to calculate the VTI in centimetres. CWD is then used to acquire the higher-velocity envelope across the AV to gain VTIAV, in centimetres. Again, a ratio of less than 0.25 indicates severe stenosis [22].
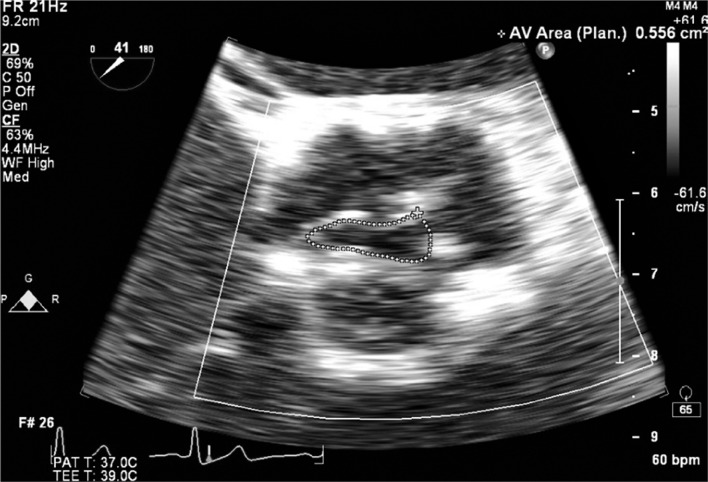
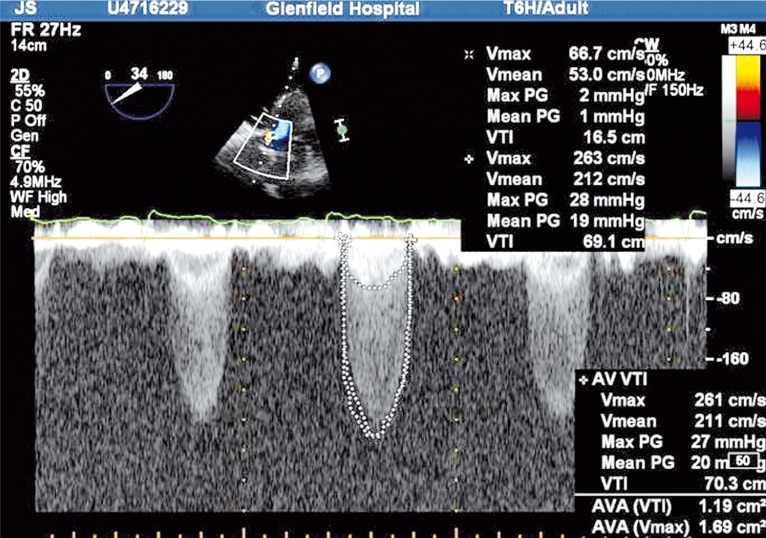
The double-envelope continuity equation technique has been described for use with prosthetic valves [24]. With this technique, the high velocity VTIAV is obtained from the outside edge of the CWD signal. Instead of separately using PWD to obtain the LVOT velocity from a different measurement, the denser low velocity signal present in the same envelope is used to represent VTILVOT. If the ratio is less than 0.25 then stenosis is severe. The advantages of this semi-quantitative estimate of function are simplification of the continuity equation and the avoidance of beat-to-beat variation in measurements, especially if the patient is not in sinus rhythm (Figure 2).
Figure 2.
The double-envelope continuing equation technique.
Many patients with native valve AS also have aortic regurgitation (AR). This leads to increased transaortic blood flow during systole with a higher gradient for a given AV orifice. The AR however does not affect the continuity equation calculations because the increase in systolic flow is measured in both the LVOT and across the AV. The same argument is applicable with a significant paravalvular leak in a prosthetic valve in the aortic position. However, coexisting mitral stenosis will cause a low AV gradient because the fixed CO will lead to a decrease in transvalvular blood flow [25].
Novel methods
Recently, there has been resurgence in publication of research-based and theoretical methods of assessing the AV, many of which were originally described decades ago. These include aortic valve resistance, percentage stroke work loss and the energy loss coefficient.
The aforementioned Doppler-derived techniques of determining severity of AS in both native and prosthetic valves may not be reliable in a patient with a low CO. Hence, interest has arisen in methods which can overcome the flow dependence of many measures of AS.
These methods tend not to be used routinely in clinical practice and even less so to assess prosthetic valves.
Valve resistance was introduced as a ‘stenotic index’ in the 1950’s but did not reach worldwide acceptance. AV resistance is the pressure gradient to flow rate ratio expressed in units of dyne.s.cm-5 and can be calculated using both catheter-derived data and by Doppler-derived data, as shown by the two equations below.
Equation 5.
Valve resistance calculated from cardiac catheter data, where CO is cardiac output (ml/min), HR is heart rate (beats/min), SEP is systolic ejection period (s/beat) and 1.333 is the conversion factor from P (pressure gradient) in mmHg to dyne.s.c-5.
For Doppler echocardiography, the AV resistance can be calculated as follows:
Equation 6.
Valve resistance calculated from Doppler echocardiography data, where V is the maximum velocity recorded across the aortic valve by CWD, area LVOT is the area of the left ventricular outflow tract obtained from the AV long-axis view as πr2 (assuming a circular shape) and velocity LVOT is the maximum velocity recorded in the left ventricular outflow tract by pulsed wave Doppler.
Valve resistance is maintained to represent a functional index of hemodynamic impairment rather than an anatomic index, such as valve area, and AV resistance appears to remain more constant as flow varies than calculated AV area, particularly in low-flow states [26].
Percentage Stroke Work Loss (PSWL) was described by Tobin et al. [27] in 1967 and is calculated as below:
Equation 7.
Calculation of Percentage Stroke Work Loss, expressed as a percentage.
PSWL is a pressure-corrected measurement and has recently been compared to other methods of assessment of the AV for its accuracy. It was found to have good diagnostic accuracy in identifying patients with AS, as assessed by cardiac catheterization. The advantage of this method is its absolute simplicity, requiring just the measurement of the Doppler gradient and the cuff systolic blood pressure [28].
The Energy Loss Coefficient has recently been described by Garcia et al. [29] as a new, more accurate index of AS. They used an experimental model of native AV and bioprosthetic valves to calculate the energy loss coefficient and compared this to the standard measurement of EOA. The measurement was then calculated retrospectively in 138 patients with moderate or severe AS. The energy loss coefficient was found to reflect more accurately the degree of stenosis than the EOA in terms of the end-points of death or AVR.
Equation 8.
Calculation of the energy loss coefficient where EOA is effective orifice area and AA is aortic cross-sectional area, where both EOA and AA are measured in cm2.
The authors conclude that prospective studies are now necessary to further document the validity of this new index in the clinical situation.
Causes of high transvalvular pressure gradients post-AVR
A. Functional phenomena
Intraoperative TEE is very useful for immediate postoperative evaluation of newly implanted heart valves. The measurement of a high Doppler flow velocity across a newly implanted mechanical or bioprosthetic AV immediately after the cessation of cardiopulmonary bypass (CPB) can however be misleading. Several factors may contribute to an increased velocity [18]. A more accurate assessment of valve function can be made if the type of valve prosthesis is known. A Doppler measurement made through the smaller central orifice of a bileaflet prosthesis will demonstrate higher peak velocity than through the larger side orifices. Making several measurements can decrease this error. Any paravalvular leak should be critically examined and its long-term impact considered against the risks of a second bypass period. Abnormalities identified in the operating theatre may require immediate surgical correction. High transvalvular velocities on Doppler interrogation immediately post-AVR may be due to a single or a combination of functional issues. These include a dysfunctional prosthesis and subvalvular or supravalvular obstruction.
Dysfunctional prosthesis: an unusually high peak velocity through a new valve must raise suspicion of prosthesis malfunction. In the case of a bileaflet or tilting disc mechanical prosthesis it is of the utmost importance to visualise the full excursion of the leaflets. This is often difficult because of the echogenic shadowing and dropout due to the metal in the valve. If it is not possible to see leaflet motion clearly, fluoroscopy in the catheter suite is indicated to confirm normal function of the valve. Prosthetic valves, even when functioning normally, are to some degree obstructive to blood flow. Knowledge of normal hemodynamic values can assist in interpretation of Doppler data [13,30].
Subvalvular & supravalvular obstruction: the simplified Bernoulli equation can only be used when it is known that the pre-restriction velocity (ie that in the LVOT) is much lower than the post-restriction velocity. Otherwise the modified Bernoulli equation must be used, especially in the unusual situation of more than one lesion in series. If the LVOT velocity is greater than 1 m/s then V1 cannot be ignored and a search for the cause of this increased gradient must be made.
There are several potential causes of subvalvular obstruction in the patient undergoing AVR. It is known that hypertension is strongly associated with degenerative AS with a prevalence of 24-68% [31]. In patients suffering from both AS and hypertension it is common to see symmetrical, concentric hypertrophy of the left ventricular (LV) wall, sometimes to a marked degree. When a prosthetic valve is then inserted in the aortic position, abnormal intracavity flow acceleration can occur, causing increased velocities in the LVOT and LVOT obstruction [32]. Systolic anterior motion (SAM) of the anterior mitral valve leaflet after AVR has been described [33,34]. Thought to be precipitated by small, hyperdynamic and asymmetrically hypertrophied ventricles, the mechanism is probably due to SAM of the mitral valve or the sudden drop in LV systolic pressure causing an unloading effect. This again illustrates the need to assess the LVOT gradient with PWD as a separate measurement to the CWD across the AV.
Supravalvular obstruction may occur due to surgical or technical problems, especially in the more technically demanding insertion of a stentless valve, which is often placed in the supra-annular position. If detected, abnormalities may need intervention. Intraoperative TEE has been shown to be effective in assessing stentless AV prosthesis function [11].
B. Pressure recovery
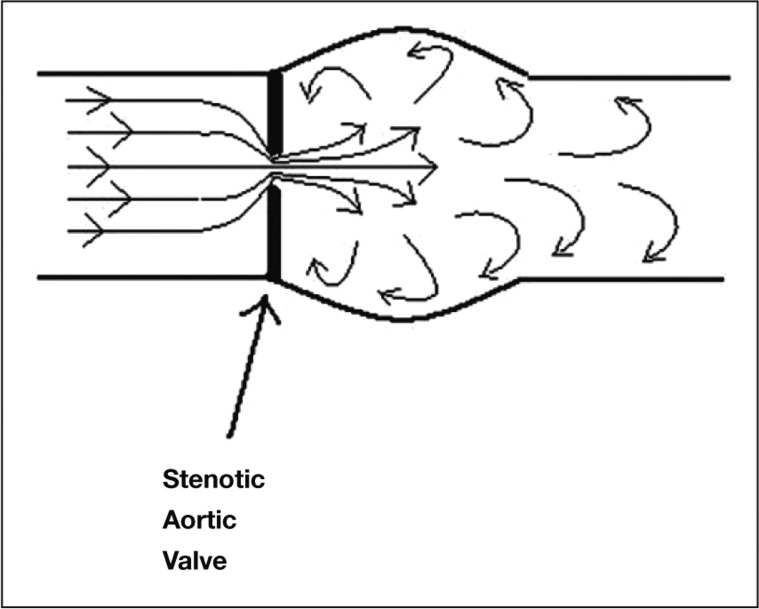
Pressure recovery has been described as ‘the increase of pressure downstream from a stenosis due to reconversion of kinetic energy into potential energy’ and has been the subject of many experimental and clinical studies in AS [35]. The principle has been used to explain the overestimation that can be seen when Doppler transvalvular velocities are used to measure PG compared to catheter gradients in patients with AS [36,37]. This overestimation is by no means consistently present in vivo or in vitro [38] and there has been good correlation in many studies between the two methods of measurement, but rarely a conclusive explanation of why this is so variable (Figure 3).
Figure 3.
Diagrammatic representation of blood flow through a stenosed aortic valve.
When blood flow converges towards a constricting orifice such as a stenotic AV, the velocity increases (kinetic energy increases) and so to keep the total energy constant the pressure energy (potential energy) must decrease. Some energy is dissipated as heat. The source of the energy is the LV. In an AS jet the pressure will be lowest where the velocity is highest. This is at the vena contracta, which corresponds to the minimal cross-sectional valve area. Distal to the stenosis as velocity decreases, pressure will increase. This total amount of pressure recovery is related to the viscous and turbulence energy losses across the stenotic valve. The Doppler gradients that are measured at the vena contracta will be significantly higher than the catheter measurements taken downstream in the ascending aorta after the pressure has completely recovered [39]. The pressure gradient will therefore depend on where the pressure is sampled. In mild to moderate AS the three cusps of the valve form a funnel rather than a diaphragm, as would be found in severe stenosis [40]. This leads to greater pressure recovery. The pressure recovery phenomenon is more significant in prosthetic aortic valves than in native valves. Clinically relevant pressure recovery causing a difference in Doppler gradient compared to catheter-derived gradient will only be seen in cases where the proximal ascending aorta is less than 3cm in diameter [41].
C. Prosthesis-patient mismatch
The problem of prosthesis-patient mismatch (PPM) was recognised over three decades ago and was originally described by Rahimtoola in 1978 [42]. The basic concept is that the EOA of the valve prosthesis, after insertion into the patient, is smaller than expected in relation to the patient’s body surface area (BSA). This consequently leads to high transvalvular gradients as the CO required by the patient necessitates higher flows if the EOA is too small. Stated more simply, if the gradient across the valve is to remain low then the EOA needs to be proportionate to flow requirements. PPM is common (20-70% of AVR’s) and leads to worse hemodynamic function, a lower degree of LV mass regression and higher morbidity and mortality [43]. Often the problem of PPM is more manifest when the patient exercises, when a higher CO is required. Hence it has been suggested that the best strategy to avoid PPM is to divide the EOA for the valve prosthesis by the patient’s BSA to calculate the indexed EOA (EOAi) in cm2/m2. This value has been shown to be the only value that correlates consistently with post-operative gradients, which increase exponentially if the EOAi is <0.85cm2/m2. Severe PPM exists if the EOAi is <0.65 cm2/m2 [44] (Figure 4).
Figure 4.
Effective Orifice Areas (EOA) of prosthetic valves. On the left is a mechanical bileaflet valve and on the right a bioprosthetic trileaflet valve. The orange highlighted line represents the EOA of each valve.
D. Hyperdynamic state
A failing LV resulting in low flow across a valvular stenosis gives rise to a low calculated peak PG [45]. Conversely a high flow such as when inotropes are infused intravenously, results in a high calculated PG on intraoperative TEE. Changes in stroke volume and CO, which occur in the anesthetised, underfilled patient can therefore significantly affect ‘pressure gradients’. Immediately after cessation of CPB patients often require inotropic support which, together with a reduced preload, results in a hyperdynamic circulation. The post-CPB patient will often have a low hematocrit due to hemodilution from the bypass prime fluid. Blood density is considered in the full Bernoulli equation and this situation will therefore contribute to calculation of a high PG across the valve.
E. Afterload mismatch
The prolonged use of CPB is known to create a systemic inflammatory response with a resultant low SVR post-operatively. Recently in clinical practice, a severely low SVR is infrequently encountered, perhaps due to shorter bypass times and the use of more sophisticated extracorporeal circuits. A low SVR in the early post-operative period, combined with inotropic drug infusion, low filling pressures and specifically the stentless supra-annular positioned AV prosthesis, has been suggested as allowing a functional ‘afterload mismatch’ which tends to normalise over time [11].
Conclusions
All the above factors make it important to thoroughly assess a newly implanted prosthetic valve with as many different methods as possible before making decisions regarding the overall function of the valve. Moderate gradients do not necessarily indicate imperfect surgical placement and intraoperative TEE is able to discriminate patients with functional phenomena from those with reversible ‘physiological’ phenomena or potential PPM. The decision to resume CPB and replace a prosthetic valve should never be made on velocity and PG alone as the variety of factors discussed in this article can all cause a transient high PG across a newly implanted prosthesis. Manufacturer’s information should be consulted to determine the expected transvalvular velocities and gradients for that particular model and size of valve. Invariably, in a patient in whom a high PG across an AV prosthesis has been detected immediately postbypass, a repeat examination even hours later, but more usually days later, should reveal lower pressure gradients if there are no clear functional problems. The possibility of PPM should always be considered and an appropriate prosthetic valve with the largest possible EOA should be implanted.
Footnotes
Conflict of interest There are no conflicts of interest to declare.
References
- Nowrangi SK, Connolly HM, Freeman WK, Click RL. Impact of intraoperative transesophageal echocardiography among patients undergoing aortic valve replacement for aortic stenosis. J Am Soc Echocardiogr. 2001;14:863–866. doi: 10.1067/mje.2001.113368. [DOI] [PubMed] [Google Scholar]
- Ionescu AA, West RR, Proudman C. et al. Prospective study of routine perioperative transesophageal echocardiography for elective valve replacement: clinical impact and cost-saving implications. J Am Soc Echocardiogr. 2001;14:659–667. doi: 10.1067/mje.2001.112101. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Vaturi M, Weisemberg DE. et al. Impact of intraoperative transesophageal echocardiography in patients undergoing valve replacement. Ann Thorac Surg. 2004;78:579–583. doi: 10.1016/j.athoracsur.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Rosemberger P, Loffler M. et al. Impact of intraoperative transesophageal echocardiography on surgical decisions in 12,566 patients undergoing cardiac surgery. Ann Thor Surg. 2008;85:845–852. doi: 10.1016/j.athoracsur.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Tingleff J, Joyce FS, Pettersson G. et al. Intraoperative echocardiographic study of air embolism during cardiac operations. Ann Thorac Surg. 1995;60:673–677. doi: 10.1016/0003-4975(95)00577-8. [DOI] [PubMed] [Google Scholar]
- Little SH, Chan KL, Burwash IG. et al. Impact of blood pressure on the doppler echocardiographic assessment of severity of aortic stenosis. Heart. 2005;91:354–361. doi: 10.1136/hrt.2006.098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadem L, Dumesnil JG, Rieu R. et al. Impact of systemic hypertension on the assessment of aortic stenosis. Heart. 2005;91:354–361. doi: 10.1136/hrt.2003.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo J. The effects of hypertension on aortic valve stenosis. Heart. 2005;91:280–282. doi: 10.1136/hrt.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones MA, Otto CM, Stoddard M. et al. Recommendations for quantification of doppler echocardiography: a report from the doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- Bernard Y, Meneveau N, Vuillemenot A. et al. Planimetry of aortic valve area using multiplane transoesophageal echocardiography is not a reliable method for assessing severity of aortic stenosis. Heart. 1997;78:68–73. doi: 10.1136/hrt.78.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morocutti G, Gelsomino S, Spedicato L. et al. Intraoperative transesophageal echo-doppler evaluation of stentless aortic xenografts. Incidence and significance of moderate gradients. Cardiovasc Surg. 2002;10:328–332. doi: 10.1016/s0967-2109(02)00019-4. [DOI] [PubMed] [Google Scholar]
- Sugeng L, Shernan SK, Salgo IS. et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol. 2008;52:446–449. doi: 10.1016/j.jacc.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Rosenhek R, Binder T, Maurer G, Baumgartner H. Normal values for doppler echocardiographic assessment of heart valve prostheses. J Am Soc Echocardiogr. 2003;16:1116–1127. doi: 10.1067/S0894-7317(03)00638-2. [DOI] [PubMed] [Google Scholar]
- Roger VL, Tajik AJ, Reeder GS. et al. Effect of doppler echocardiography on utilization of hemodynamic cardiac catheterization in the preoperative evaluation of aortic stenosis. Mayo Clin Proc. 1996;71:141–149. doi: 10.4065/71.2.141. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Seward JB, Reeder GS. et al. Continuous-wave doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous doppler-catheter correlative study in 100 adult patients. Circulation. 1985;71:1162–1169. doi: 10.1161/01.cir.71.6.1162. [DOI] [PubMed] [Google Scholar]
- Garcia D, Pibarot P, Landry C. et al. Estimation of aortic valve effective orifice area by doppler echocardiography: effects of valve inflow shape and flow rate. J Am Soc Echocardiogr. 2004;17:756–765. doi: 10.1016/j.echo.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Garcia D, Kadem L, Savery D. et al. Analytical modelling of the instantaneous maximal transvalvular pressure gradient in aortic stenosis. J Biomech. 2006;39:3036–3044. doi: 10.1016/j.jbiomech.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Schroeder RA, Mark JB. Is the valve OK or not? Immediate evaluation of a replaced aortic valve. Anesth Analg. 2005;101:1288–1291. doi: 10.1213/01.ANE.0000181339.39448.F0. [DOI] [PubMed] [Google Scholar]
- Carabello BA, Crawford FA. Valvular Heart Disease. New Engl J of Med. 1997;337:32–48. doi: 10.1056/NEJM199707033370107. [DOI] [PubMed] [Google Scholar]
- Pibarot P, Honos GN, Durand LG, Dumesnil JG. Substitution of left ventricular outflow tract diameter with prosthesis size is inadequate for calculation of the aortic prosthetic valve area by the continuity equation. J Am Soc Echocardiogr. 1995;8:511–517. doi: 10.1016/s0894-7317(05)80339-6. [DOI] [PubMed] [Google Scholar]
- Hatle L, Angelsen BA, Tromsdal A. et al. Non-invasive assessment of aortic stenosis by doppler ultrasound. Br Heart J. 1980;43:284–292. doi: 10.1136/hrt.43.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JK, Taliercio CP, Holmes DR Jr. et al. Prediction of the severity of aortic stenosis by doppler aortic valve area determination: prospective doppler-catheterization correlation in 100 patients. J Am Coll Cardiol. 1988;11:1227–1234. doi: 10.1016/0735-1097(88)90286-0. [DOI] [PubMed] [Google Scholar]
- Chafizadeh ER, Zoghbi WA. Doppler echocardiographic assessment of the St Jude Medical prosthetic valve in the aortic position using the continuity equation. Circulation. 1991;83:213–223. doi: 10.1161/01.cir.83.1.213. [DOI] [PubMed] [Google Scholar]
- Maslow AD, Haering JM, Heindel S. et al. An evaluation of prosthetic aortic valves using transesophageal echocardiography: the double-envelope technique. Anesth Analg. 2000;91:509–516. doi: 10.1097/00000539-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Troianos CA. Assessment of the aortic valve. In: Savage RM, Aronson S, eds. Comprehensive textbook of intraoperative transesophageal echocardiography. Lippincott Williams & Wilkins. 2005;15:205–218. [Google Scholar]
- Antonini-Canterin F, Faggiano P, Zanuttini D, Ribichini F. Is aortic valve resistance more clinically meaningful than valve area in aortic stenosis? Heart. 1999;82:9–10. doi: 10.1136/hrt.82.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JR, Rahimtoola SH, Blundell PE, Swan HJ. Percentage of left ventricular stroke work loss. A simple hemodynamic concept for estimation of severity in valvular aortic stenosis. Circulation. 1967;35:868–879. doi: 10.1161/01.cir.35.5.868. [DOI] [PubMed] [Google Scholar]
- Antonini-Canterin F, Huang G, Cervesato E. et al. Reliability of new and old Doppler echocardiographic indexes of the severity of aortic stenosis in patients with a low cardiac output. Ital Heart J. 2002;3:248–255. [PubMed] [Google Scholar]
- Garcia D, Pibarot P, Dumesnil JG. et al. Assessment of aortic valve stenosis severity: a new index based on the energy loss concept. Circulation. 2000;101:765–771. doi: 10.1161/01.cir.101.7.765. [DOI] [PubMed] [Google Scholar]
- Reisner SA, Meltzer RS. Normal values of prosthetic valve Doppler echocardiographic parameters: a review. J Am Soc Echocardiogr. 1988;1:201–210. doi: 10.1016/s0894-7317(88)80076-2. [DOI] [PubMed] [Google Scholar]
- Linhartová K, Filipovský J, Cerbák R. et al. Severe aortic stenosis and its association with hypertension: analysis of clinical and echocardiographic parameters. Blood Pressure. 2007;16:122–128. doi: 10.1080/08037050701343241. [DOI] [PubMed] [Google Scholar]
- Orsinelli DA, Aurigemma GP, Battista S. et al. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. J Am Coll Cardiol. 1993;22:1679–1683. doi: 10.1016/0735-1097(93)90595-r. [DOI] [PubMed] [Google Scholar]
- Bartunek J, Sys SU, Rodrigues AC. et al. Abnormal systolic intraventricular flow velocities after valve replacement for aortic stenosis. Mechanisms, predictive factors, and prognostic significance. Circulation. 1996;93:712–719. doi: 10.1161/01.cir.93.4.712. [DOI] [PubMed] [Google Scholar]
- Routledge T, Nashef SA. Severe mitral systolic anterior motion complicating aortic valve replacement. Interact CardioVasc Thorac Surg. 2005;4:486–487. doi: 10.1510/icvts.2005.111039. [DOI] [PubMed] [Google Scholar]
- Clark C. The fluid mechanics of aortic stenosis - I. Theory and steady flow experiments. J Biomechanics. 1976;9:521–528. doi: 10.1016/0021-9290(76)90068-3. [DOI] [PubMed] [Google Scholar]
- Razzolini R, Manica A, Tarantini G. et al. Discrepancies between catheter and Doppler estimates of aortic stenosis: the role of pressure recovery evaluated \'in vivo\'. J Heart Valve Dis. 2007;16:225–229. [PubMed] [Google Scholar]
- Hegrenaes L, Hatle L. Aortic stenosis in adults. Non-invasive estimation of pressure differences by continuous wave Doppler echocardiography. Br Heart J. Br Heart J. 1985;54:396–404. doi: 10.1136/hrt.54.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger J, Schima H, Maurer G, Baumgartner H. Importance of pressure recovery for the assessment of aortic stenosis by Doppler ultrasound: the role of aortic size, aortic valve area, and direction of the stenotic jet in vitro. Circulation. 1996;94:1934–1940. doi: 10.1161/01.cir.94.8.1934. [DOI] [PubMed] [Google Scholar]
- Popescu WM, Prokop E, Elefteriades JA. et al. Phantom aortic valve pressure gradient: discrepancies between cardiac catheterization and Doppler echocardiography. Anesth Analg. 2005;100:1259–1262. doi: 10.1213/01.ANE.0000151127.36285.8A. [DOI] [PubMed] [Google Scholar]
- Chambers J. Aortic stenosis: is common but often unrecognised. BMJ. 2005;330:801–802. doi: 10.1136/bmj.330.7495.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner H, Stefanelli T, Niederberger J. et al. \'Overestimation\' of catheter gradients by doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol. 1999;33:1655–1661. doi: 10.1016/s0735-1097(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. 1978;58:20–24. doi: 10.1161/01.cir.58.1.20. [DOI] [PubMed] [Google Scholar]
- Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart. 2006;92:1022–1029. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol. 2000;36:1131–1141. doi: 10.1016/s0735-1097(00)00859-7. [DOI] [PubMed] [Google Scholar]
- Bermejo J, Yotti R. Low-gradient aortic valve stenosis: value and limitations of dobutamine stress testing. Heart. 2007;93:298–302. doi: 10.1136/hrt.2005.066860. [DOI] [PMC free article] [PubMed] [Google Scholar]