Abstract
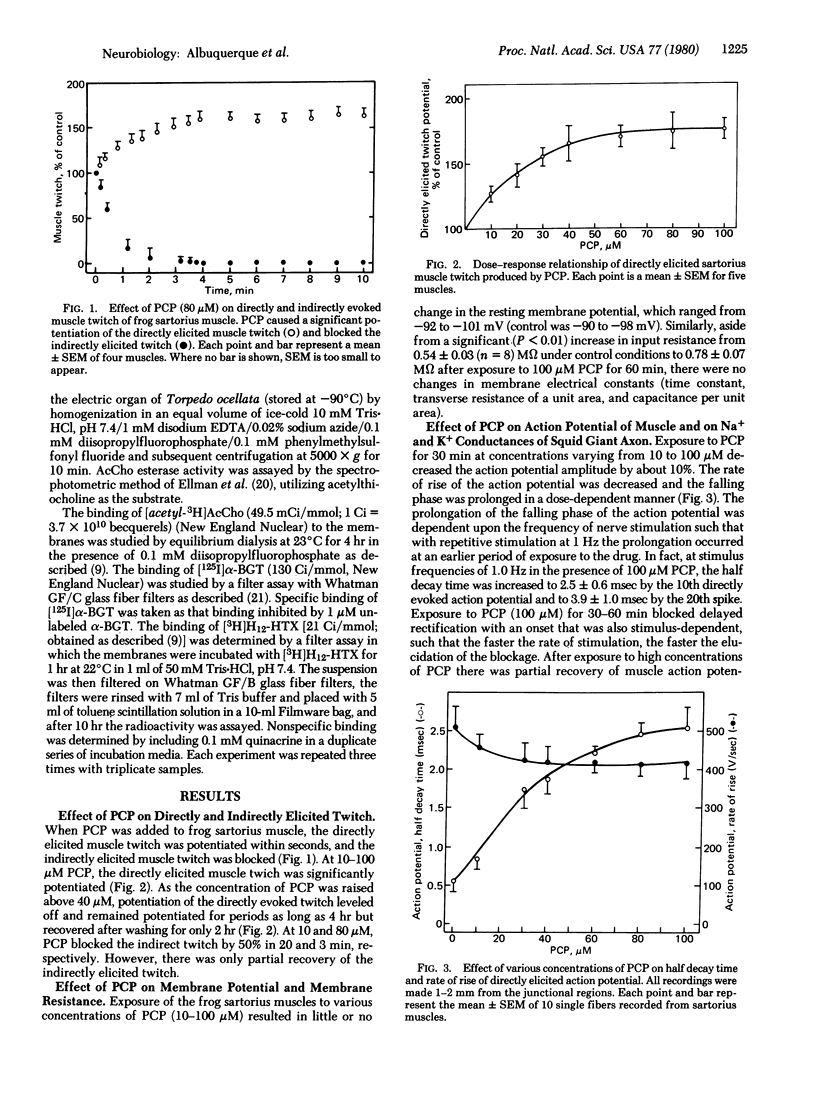
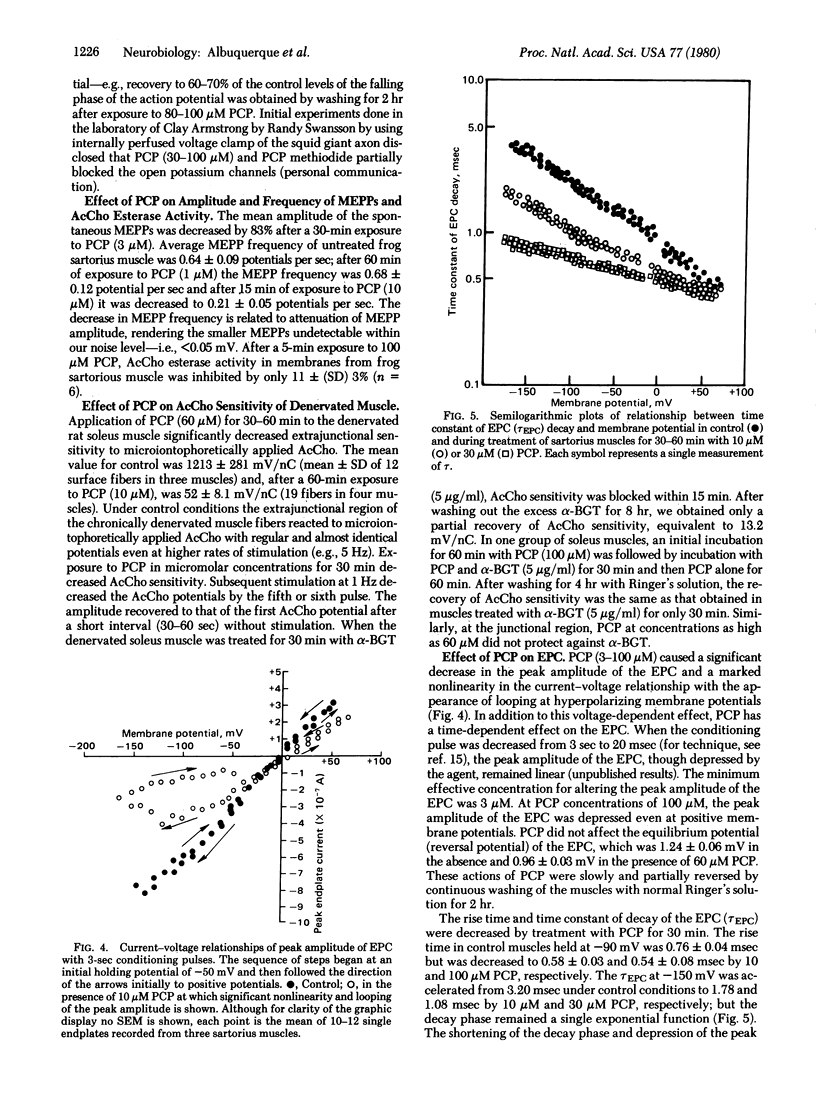
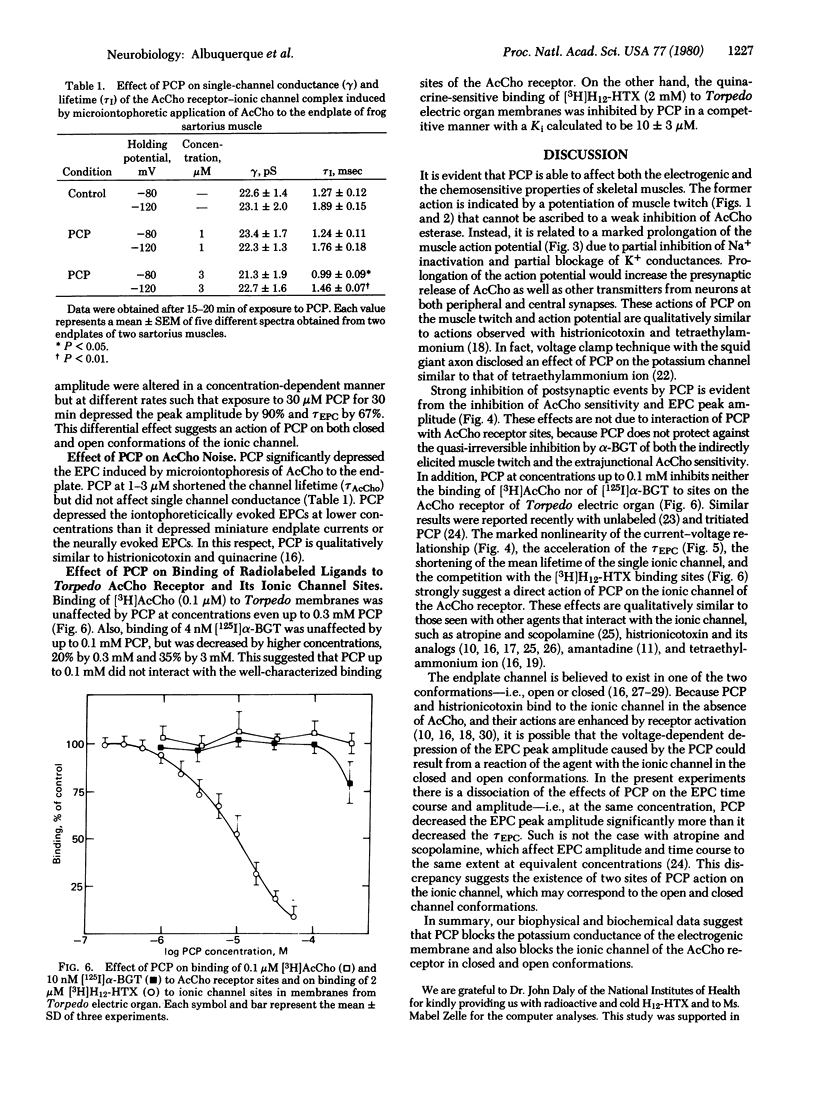
The effects of phencyclidine (PCP) were studied on the electrogenic and chemosensitive properties of the neuromuscular junction of skeletal muscle as well as on the binding sites on the acetylcholine (AcCho) receptor and its ionic channel in the electric organ membranes of the electric ray. The directly elicited muscle twitch was markedly potentiated by prolonging the falling phase of the muscle action potential and blocking delayed rectification. The indirectly elicited muscle twitch was transiently potentiated and then blocked by PCP at concentrations below 60 μM. PCP blocked miniature endplate potentials and AcCho sensitivities at the junctional region of innervated muscle, blocked the extrajunctional sensitivity of the chronically denervated muscle, and significantly depressed the peak amplitude of the endplate current (EPC) in a voltage- and time-dependent manner. PCP also caused acceleration of the time course of EPC decay and shortening of the mean life-time of the open ionic channel. The effects of PCP were not due to inhibition of AcCho receptor sites because PCP did not protect against the quasi-irreversible inhibition of receptor sites by α-bungarotoxin, nor did it inhibit binding of [3H]AcCho or [125I-labeled α-bungarotoxin to the receptor sites. On the other hand, PCP blocked the binding of [3H]perhydrohistrionicotoxin to the sites of the ionic channel of the AcCho receptor. The data suggest that PCP reacts with the electrogenic K+ channel and the ionic channel associated with the AcCho receptor in the open as well as the closed conformation.
Keywords: perhydrohistrionicotoxin, α-bungarotoxin, endplate current, acetylcholine noise, electric ray
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Adler M., Oliveira A. C., Albuquerque E. X., Mansour N. A., Eldefrawi A. T. Reaction of tetraethylammonium with the open and closed conformations of the acetylcholine receptor ionic channel complex. J Gen Physiol. 1979 Jul;74(1):129–152. doi: 10.1085/jgp.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. A comparison of acetylcholine and stable depolarizing agents. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):362–368. doi: 10.1098/rspb.1957.0017. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Copio D. S., Hudson C. S., Rash J., Mansour N. A., Eldefrawi A. T., Albuguergue E. X. Effects of antibodies to Torpedo acetylcholine receptor on the acetylcholine receptor--ionic channel complex of Torpedo electroplax and rabbit intercostal muscle. Exp Neurol. 1979 May;64(2):428–444. doi: 10.1016/0014-4886(79)90281-4. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., Mansour N. A., Daly J. W., Witkop B., Albuquerque E. X. Acetylcholine receptor and ionic channel of Torpedo electroplax: binding of perhydrohistrionicotoxin to membrane and solubilized preparations. Biochemistry. 1978 Dec 12;17(25):5474–5484. doi: 10.1021/bi00618a023. [DOI] [PubMed] [Google Scholar]
- Kloog Y., Gabrialevitz A., Kalir A., Balderman D., Sokolovsky M. Functional evidence for a second binding site of nicotinic antagonists using phencyclidine derivatives. Biochem Pharmacol. 1979 Apr 15;28(8):1447–1450. doi: 10.1016/0006-2952(79)90456-8. [DOI] [PubMed] [Google Scholar]
- Kloog Y., Rehavi M., Maayani S., Sokolovsky M. Anticholinesterase and antiacetylcholine activity of 1-phenylcyclohexylamine derivatives. Eur J Pharmacol. 1977 Oct 1;45(3):221–227. doi: 10.1016/0014-2999(77)90002-4. [DOI] [PubMed] [Google Scholar]
- Lapa A. J., Albuquerque E. X., Sarvey J. M., Daly J., Witkop B. Effects of histrionicotoxin on the chemosensitive and electrical properties of skeletal muscle. Exp Neurol. 1975 Jun;47(3):558–580. doi: 10.1016/0014-4886(75)90088-6. [DOI] [PubMed] [Google Scholar]
- Maayani S., Weinstein H., Ben-Zvi N., Cohen S., Sokolovsky M. Psychotomimetics as anticholinergic agents. I. 1-Cyclohexylpiperidine derivatives: anticholinesterase activity and antagonistic activity to acetylcholine. Biochem Pharmacol. 1974 Apr 15;23(8):1263–1281. doi: 10.1016/0006-2952(74)90330-x. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa L. M., Albuquerque E. X. Voltage- and time-dependent action of histrionicotoxin on the endplate current of the frog muscle. J Gen Physiol. 1978 Sep;72(3):351–367. doi: 10.1085/jgp.72.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. C., Mansour N. A., Eldefrawi A. T., Eldefrawi M. E., Albuquerque E. X. Mechanism of action of amantadine on neuromuscular transmission. Mol Pharmacol. 1978 Sep;14(5):787–803. [PubMed] [Google Scholar]
- Tsai M. C., Oliveira A. C., Albuquerque E. X., Eldefrawi M. E., Eldefrawi A. T. Mode of action of quinacrine on the acetylcholine receptor ionic channel complex. Mol Pharmacol. 1979 Sep;16(2):382–392. [PubMed] [Google Scholar]
- Vincent J. P., Cavey D., Kamenka J. M., Geneste P., Lazdunski M. Interaction of phencyclidines with the muscarinic and opiate receptors in the central nervous system. Brain Res. 1978 Aug 18;152(1):176–182. doi: 10.1016/0006-8993(78)90145-2. [DOI] [PubMed] [Google Scholar]



